Association Between Higher Body Mass Index and the Risk of Lumbar Spinal Stenosis in Korean Populations: A Nationwide Cohort Study
Abstract
1. Introduction
2. Methods
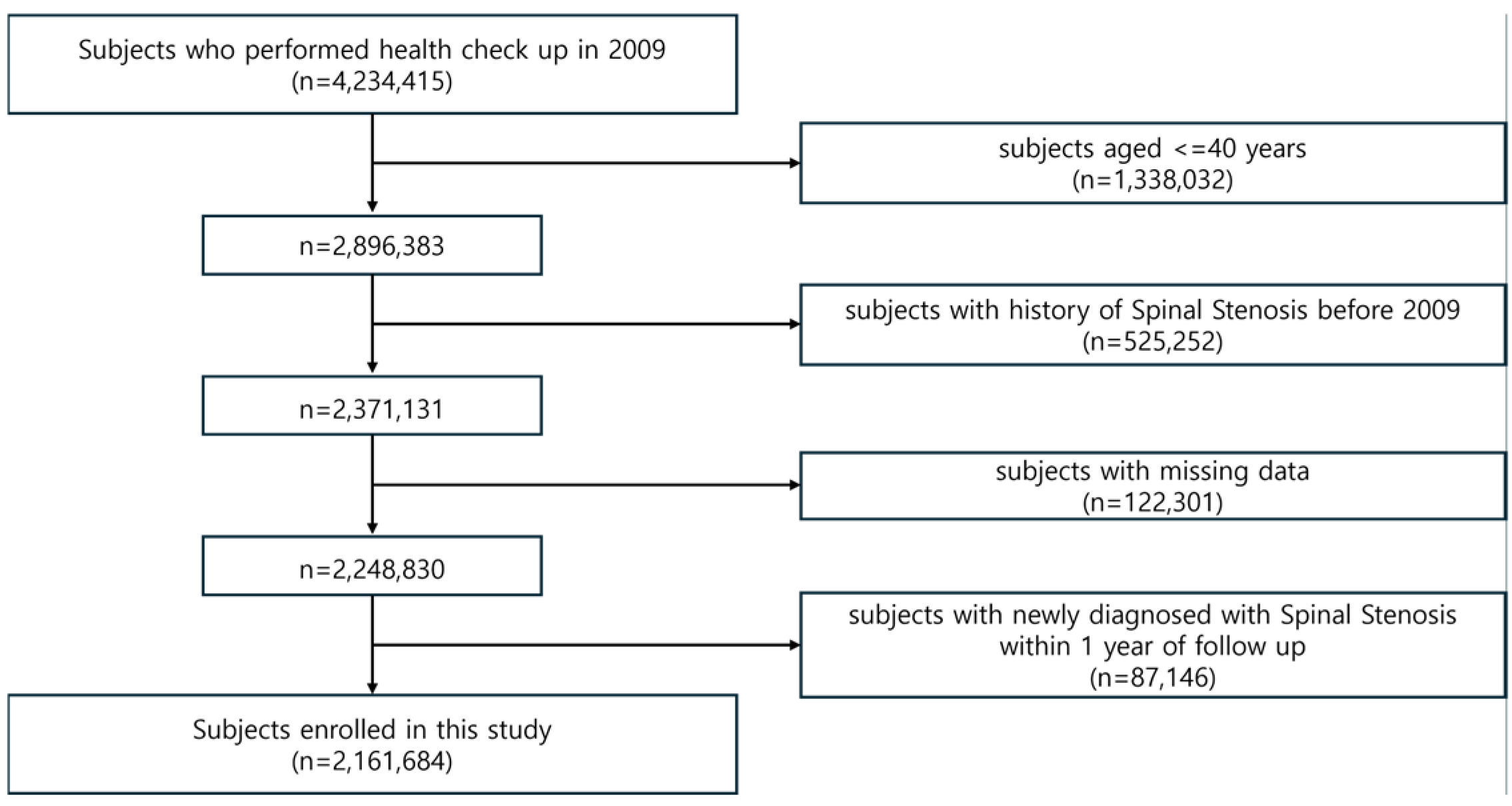
2.1. Data Source and Study Population
2.2. Ethics
2.3. Data Collection and Comorbidities
2.4. Statistical Analysis
2.4.1. Basic Statistical Analysis
2.4.2. Analysis of BMI Categories and Cox Proportional Hazards Model
2.4.3. Analyses of Detailed BMI Classification and Subgroup Effects
3. Results
3.1. Baseline Characteristics
3.2. Spinal Stenosis Risk by BMI Categories
3.3. Detailed BMI Category Analysis
3.4. Subgroup Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katz, J.N.; Harris, M.B. Lumbar Spinal Stenosis. N. Engl. J. Med. 2008, 358, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Otani, K.; Kikuchi, S.; Yabuki, S.; Igarashi, T.; Nikaido, T.; Watanabe, K.; Konno, S. Lumbar spinal stenosis has a negative impact on quality of life compared with other comorbidities: An epidemiological cross-sectional study of 1862 community-dwelling individuals. Sci. World J. 2013, 2013, 590652. [Google Scholar] [CrossRef] [PubMed]
- Myllykangas, H.; Ristolainen, L.; Hurri, H.; Lohikoski, J.; Kautiainen, H.; Puisto, V.; Österman, H.; Manninen, M. Obese people benefit from lumbar spinal stenosis surgery as much as people of normal weight. J. Orthop. Surg. Res. 2021, 16, 550. [Google Scholar] [CrossRef] [PubMed]
- Samartzis, D.; Karppinen, J.; Chan, D.; Luk, K.D.; Cheung, K.M. The association of lumbar intervertebral disc degeneration on magnetic resonance imaging with body mass index in overweight and obese adults: A population-based study. Arthritis Rheum. 2012, 64, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A.; Freeman, B.J.; Morrison, H.P.; Nelson, I.W.; Dolan, P. Mechanical initiation of intervertebral disc degeneration. Spine 2000, 25, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Q.; Cheung, K.M.; Ho, D.W.; Poon, S.C.; Chiba, K.; Kawaguchi, Y.; Hirose, Y.; Alini, M.; Grad, S.; Yee, A.F.; et al. Association of the asporin D14 allele with lumbar-disc degeneration in Asians. Am. J. Hum. Genet. 2008, 82, 744–747. [Google Scholar] [CrossRef]
- Fogelholm, R.R.; Alho, A.V. Smoking and intervertebral disc degeneration. Med. Hypotheses 2001, 56, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Ono, R.; Yamazaki, S.; Takegami, M.; Otani, K.; Sekiguchi, M.; Onishi, Y.; Hayashino, Y.; Kikuchi, S.; Konno, S.; Fukuhara, S. Gender difference in association between low back pain and metabolic syndrome: Locomotive syndrome and health outcome in Aizu cohort study (LOHAS). Spine 2012, 37, 1130–1137. [Google Scholar] [CrossRef]
- Sekiguchi, M.; Yonemoto, K.; Kakuma, T.; Nikaido, T.; Watanabe, K.; Kato, K.; Otani, K.; Yabuki, S.; Kikuchi, S.-i.; Konno, S.-i. Relationship between lumbar spinal stenosis and psychosocial factors: A multicenter cross-sectional study (DISTO project). Eur. Spine J. 2015, 24, 2288–2294. [Google Scholar] [CrossRef]
- Tomkins-Lane, C.C.; Holz, S.C.; Yamakawa, K.S.; Phalke, V.V.; Quint, D.J.; Miner, J.; Haig, A.J. Predictors of walking performance and walking capacity in people with lumbar spinal stenosis, low back pain, and asymptomatic controls. Arch. Phys. Med. Rehabil. 2012, 93, 647–653. [Google Scholar] [CrossRef]
- Gepstein, R.; Shabat, S.; Arinzon, Z.H.; Berner, Y.; Catz, A.; Folman, Y. Does obesity affect the results of lumbar decompressive spinal surgery in the elderly? Clin. Orthop. Relat. Res. 2004, 426, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.H.; Yeh, W.T.; Weng, L.C. Epidemiology of metabolic syndrome in Asia. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. S1), 37–42. [Google Scholar] [PubMed]
- Telfeian, A.E.; Reiter, G.T.; Durham, S.R.; Marcotte, P. Spine surgery in morbidly obese patients. J. Neurosurg. 2002, 97, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Heuch, I.; Hagen, K.; Heuch, I.; Nygaard, Ø.; Zwart, J.A. The impact of body mass index on the prevalence of low back pain: The HUNT study. Spine 2010, 35, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Asadian, L.; Haddadi, K.; Aarabi, M.; Zare, A. Diabetes Mellitus, a New Risk Factor for Lumbar Spinal Stenosis: A Case-Control Study. Clin. Med. Insights Endocrinol. Diabetes 2016, 9, S39035. [Google Scholar] [CrossRef]
- Uesugi, K.; Sekiguchi, M.; Kikuchi, S.; Konno, S. Relationship between lumbar spinal stenosis and lifestyle-related disorders: A cross-sectional multicenter observational study. Spine 2013, 38, E540–E545. [Google Scholar] [CrossRef]
- Liuke, M.; Solovieva, S.; Lamminen, A.; Luoma, K.; Leino-Arjas, P.; Luukkonen, R.; Riihimäki, H. Disc degeneration of the lumbar spine in relation to overweight. Int. J. Obes. 2005, 29, 903–908. [Google Scholar] [CrossRef]
- de Schepper, E.I.; Damen, J.; van Meurs, J.B.; Ginai, A.Z.; Popham, M.; Hofman, A.; Koes, B.W.; Bierma-Zeinstra, S.M. The association between lumbar disc degeneration and low back pain: The influence of age, gender, and individual radiographic features. Spine 2010, 35, 531–536. [Google Scholar] [CrossRef]
- Videman, T.; Gibbons, L.E.; Kaprio, J.; Battié, M.C. Challenging the cumulative injury model: Positive effects of greater body mass on disc degeneration. Spine J. 2010, 10, 26–31. [Google Scholar] [CrossRef]
- Song, S.O.; Jung, C.H.; Song, Y.D.; Park, C.-Y.; Kwon, H.-S.; Cha, B.S.; Park, J.-Y.; Lee, K.-U.; Ko, K.S.; Lee, B.-W. Background and data configuration process of a nationwide population-based study using the Korean national health insurance system. Diabetes Metab. J. 2014, 38, 395–403. [Google Scholar] [CrossRef]
- Stucki, G.; Daltroy, L.; Liang, M.H.; Lipson, S.J.; Fossel, A.H.; Katz, J.N. Measurement properties of a self-administered outcome measure in lumbar spinal stenosis. Spine 1996, 21, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Kalichman, L.; Cole, R.; Kim, D.H.; Li, L.; Suri, P.; Guermazi, A.; Hunter, D.J. Spinal stenosis prevalence and association with symptoms: The Framingham Study. Spine J. 2009, 9, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, Y.; Yoshimura, N.; Muraki, S.; Yamada, H.; Nagata, K.; Hashizume, H.; Takiguchi, N.; Minamide, A.; Oka, H.; Kawaguchi, H.; et al. Prevalence of symptomatic lumbar spinal stenosis and its association with physical performance in a population-based cohort in Japan: The Wakayama Spine Study. Osteoarthr. Cartil. 2012, 20, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Deyo, R.A.; Mirza, S.K. Trends and variations in the use of spine surgery. Clin. Orthop. Relat. Res. 2006, 443, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Gaziano, J.M. Fifth phase of the epidemiologic transition: The age of obesity and inactivity. Jama 2010, 303, 275–276. [Google Scholar] [CrossRef]
- Kelly, T.; Yang, W.; Chen, C.S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef]
- Rao, S.V.; Donahue, M.; Pi-Sunyer, F.X.; Fuster, V. Results of Expert Meetings: Obesity and Cardiovascular Disease. Obesity as a risk factor in coronary artery disease. Am. Heart J. 2001, 142, 1102–1107. [Google Scholar] [CrossRef]
- Melanson, K.J.; McInnis, K.J.; Rippe, J.M.; Blackburn, G.; Wilson, P.F. Obesity and cardiovascular disease risk: Research update. Cardiol. Rev. 2001, 9, 202–207. [Google Scholar] [CrossRef]
- Kauppila, L.I. Atherosclerosis and Disc Degeneration/Low-Back Pain—A Systematic Review. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 661–670. [Google Scholar] [CrossRef]
- Vincent, H.K.; Heywood, K.; Connelly, J.; Hurley, R.W. Obesity and weight loss in the treatment and prevention of osteoarthritis. PM&R 2012, 4, S59–S67. [Google Scholar] [CrossRef]
- Gandhi, R.; Woo, K.M.; Zywiel, M.G.; Rampersaud, Y.R. Metabolic syndrome increases the prevalence of spine osteoarthritis. Orthop. Surg. 2014, 6, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Kalichman, L.; Guermazi, A.; Hunter, D. Association between age, sex, BMI and CT-evaluated spinal degeneration features. J. Back Musculoskelet. Rehabil. 2009, 22, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, Y.; Yoshimura, N.; Muraki, S.; Yamada, H.; Nagata, K.; Hashizume, H.; Takiguchi, N.; Minamide, A.; Oka, H.; Kawaguchi, H.; et al. Associations between radiographic lumbar spinal stenosis and clinical symptoms in the general population: The Wakayama Spine Study. Osteoarthr. Cartil. 2013, 21, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Piscoya, J.L.; Fermor, B.; Kraus, V.B.; Stabler, T.V.; Guilak, F. The influence of mechanical compression on the induction of osteoarthritis-related biomarkers in articular cartilage explants. Osteoarthr. Cartil. 2005, 13, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Vincent, H.K.; Raiser, S.N.; Vincent, K.R. The aging musculoskeletal system and obesity-related considerations with exercise. Ageing Res. Rev. 2012, 11, 361–373. [Google Scholar] [CrossRef]
- Dandona, P.; Aljada, A.; Chaudhuri, A.; Mohanty, P.; Garg, R. Metabolic syndrome: A comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 2005, 111, 1448–1454. [Google Scholar] [CrossRef]
- Berenbaum, F.; Sellam, J. Obesity and osteoarthritis: What are the links? Jt. Bone Spine 2008, 75, 667–668. [Google Scholar] [CrossRef]
- Dumond, H.; Presle, N.; Terlain, B.; Mainard, D.; Loeuille, D.; Netter, P.; Pottie, P. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum. 2003, 48, 3118–3129. [Google Scholar] [CrossRef]
- Griffin, T.M.; Huebner, J.L.; Kraus, V.B.; Guilak, F. Extreme obesity due to impaired leptin signaling in mice does not cause knee osteoarthritis. Arthritis Rheum. 2009, 60, 2935–2944. [Google Scholar] [CrossRef]
- Brunner, A.M.; Henn, C.M.; Drewniak, E.I.; Lesieur-Brooks, A.; Machan, J.; Crisco, J.J.; Ehrlich, M.G. High dietary fat and the development of osteoarthritis in a rabbit model. Osteoarthr. Cartil. 2012, 20, 584–592. [Google Scholar] [CrossRef]
- Griffin, T.M.; Huebner, J.L.; Kraus, V.B.; Yan, Z.; Guilak, F. Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: Effects of short-term exercise. Arthritis Rheum. 2012, 64, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Leino-Arjas, P.; Kaila-Kangas, L.; Solovieva, S.; Riihimäki, H.; Kirjonen, J.; Reunanen, A. Serum Lipids and Low Back Pain: An Association?: A Follow-up Study of a Working Population Sample. Spine 2006, 31, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Leino-Arjas, P.; Kauppila, L.; Kaila-Kangas, L.; Shiri, R.; Heistaro, S.; Heliövaara, M. Serum lipids in relation to sciatica among Finns. Atherosclerosis 2008, 197, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Hemmingsson, E.; Ekelund, U. Is the association between physical activity and body mass index obesity dependent? Int. J. Obes. 2007, 31, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Vincent, H.K.; Lamb, K.M.; Day, T.I.; Tillman, S.M.; Vincent, K.R.; George, S.Z. Morbid obesity is associated with fear of movement and lower quality of life in patients with knee pain-related diagnoses. PM&R 2010, 2, 713–722. [Google Scholar] [CrossRef]
- Vincent, H.K.; Omli, M.R.; Day, T.; Hodges, M.; Vincent, K.R.; George, S.Z. Fear of Movement, Quality of Life, and Self-Reported Disability in Obese Patients with Chronic Lumbar Pain. Pain Med. 2011, 12, 154–164. [Google Scholar] [CrossRef]
- Strain, T.; Wijndaele, K.; Brage, S.; Wareham, N.; Sharp, S.J. Impact of follow-up time and analytical approaches to account for reverse causality on the association between physical activity and health outcomes in UK Biobank. Int. J. Epidemiol. 2020, 49, 162–172. [Google Scholar] [CrossRef]
- Stevens, J.; Juhaeri; Cai, J. Changes in body mass index prior to baseline among participants who are ill or who die during the early years of follow-up. Am. J. Epidemiol. 2001, 153, 946–953. [Google Scholar] [CrossRef][Green Version]

| BMI Level | ||||||
|---|---|---|---|---|---|---|
| <18.5 | 18.5–22.9 | 23.0–24.9 | 25.0–29.9 | ≥30 | p-Value | |
| 50,738 | 803,691 | 574,882 | 665,019 | 67,354 | ||
| Age, ≥65 years | 12,617 (24.87) | 112,562 (14.01) | 80,480 (14) | 93,954 (14.13) | 9018 (13.39) | <0.0001 |
| Sex | ||||||
| Male | 24,123 (47.54) | 375,607 (46.74) | 327,152 (56.91) | 404,400 (60.81) | 32,652 (48.48) | <0.0001 |
| Female | 26,615 (52.46) | 428,084 (53.26) | 247,730 (43.09) | 260,619 (39.19) | 34,702 (51.52) | <0.0001 |
| Income, Lowest Q1 | 11,395 (22.46) | 171,209 (21.3) | 112,350 (19.54) | 128,266 (19.29) | 14,417 (21.4) | <0.0001 |
| Smoking | ||||||
| Non | 31,425 (61.94) | 520,072 (64.71) | 341,419 (59.39) | 377,790 (56.81) | 43,155 (64.07) | <0.0001 |
| Ex | 4867 (9.59) | 102,052 (12.7) | 103,536 (18.01) | 134,275 (20.19) | 10,547 (15.66) | <0.0001 |
| Current | 14,446 (28.47) | 181,567 (22.59) | 129,927 (22.6) | 15,2954 (23) | 13,652 (20.27) | <0.0001 |
| Drinking | ||||||
| Non | 32,493 (64.04) | 471,583 (58.68) | 309,541 (53.84) | 346,741 (52.14) | 39,579 (58.76) | <0.0001 |
| Mild | 15,140 (29.84) | 281,202 (34.99) | 219,693 (38.22) | 253,938 (38.19) | 21,345 (31.69) | <0.0001 |
| Heavy | 3105 (6.12) | 50,906 (6.33) | 45,648 (7.94) | 64,340 (9.67) | 6430 (9.55) | <0.0001 |
| Regular exercise | 6999 (13.79) | 152,185 (18.94) | 123,911 (21.55) | 141,971 (21.35) | 12,315 (18.28) | <0.0001 |
| DM | 3458 (6.82) | 60,460 (7.52) | 61,833 (10.76) | 97,161 (14.61) | 14,550 (21.6) | <0.0001 |
| HTN | 8982 (17.7) | 170,188 (21.18) | 176,144 (30.64) | 274,993 (41.35) | 38,818 (57.63) | <0.0001 |
| Dyslipidemia | 4824 (9.51) | 121,287 (15.09) | 124,586 (21.67) | 181,243 (27.25) | 23,333 (34.64) | <0.0001 |
| CKD | 3585 (7.07) | 53,428 (6.65) | 43,673 (7.6) | 55,183 (8.3) | 6257 (9.29) | <0.0001 |
| Age, years | 54.93 ± 12.64 | 52.31 ± 10.11 | 53.06 ± 9.63 | 53.18 ± 9.57 | 52.48 ± 9.72 | <0.0001 |
| Height, cm | 161.47 ± 8.47 | 161.82 ± 8.25 | 162.8 ± 8.63 | 163.16 ± 8.92 | 161.45 ± 9.6 | <0.0001 |
| Weight, kg | 45.91 ± 5.32 | 55.84 ± 6.55 | 63.62 ± 6.93 | 71.26 ± 8.41 | 83.15 ± 10.53 | <0.0001 |
| BMI, kg/m2 | 17.56 ± 0.83 | 21.27 ± 1.16 | 23.93 ± 0.57 | 26.69 ± 1.28 | 31.82 ± 2.11 | <0.0001 |
| Waist circumference, cm | 67.8 ± 5.93 | 75.06 ± 6.18 | 81.4 ± 5.71 | 87.25 ± 6.21 | 96.44 ± 7.61 | <0.0001 |
| Systolic BP, mmHg | 117.6 ± 15.92 | 120.33 ± 15.15 | 124.16 ± 14.91 | 127.47 ± 14.98 | 131.95 ± 15.79 | <0.0001 |
| Diastolic BP, mmHg | 73.4 ± 10.24 | 74.94 ± 10.02 | 77.3 ± 9.98 | 79.47 ± 10.09 | 82.28 ± 10.7 | <0.0001 |
| Fasting glucose, mg/dL | 95.92 ± 28.35 | 96.72 ± 24.07 | 100.05 ± 25.65 | 103.22 ± 27.27 | 108.24 ± 31.54 | <0.0001 |
| Total cholesterol, mg/dL | 185.6 ± 34.9 | 193.9 ± 35.64 | 200.04 ± 36.82 | 203.32 ± 37.76 | 206.27 ± 39.37 | <0.0001 |
| HDL-C, mg/dL | 62.83 ± 33.56 | 58.74 ± 30.26 | 54.73 ± 27.56 | 52.58 ± 27.36 | 51.93 ± 27.91 | <0.0001 |
| LDL-C, mg/dL | 105.48 ± 39.1 | 113.7 ± 37.63 | 118.27 ± 38.59 | 119.1 ± 39.16 | 119.99 ± 40.37 | <0.0001 |
| Triglyceride, mg/dL | 85.15 (84.79–85.51) | 98.81 (98.69–98.92) | 121.09 (120.92–121.27) | 140.76 (140.57–140.94) | 153.61 (152.98–154.24) | <0.0001 |
| BMI | N | Event | Duration, PY | IR, per 1000 PY | HR (95% C.I) | ||
|---|---|---|---|---|---|---|---|
| Model 1 1 | Model 2 2 | Model 3 3 | |||||
| <18.5 | 50,738 | 13,507 | 412,190.92 | 32.7688 | 0.872 (0.857, 0.887) | 0.799 (0.785, 0.813) | 0.801 (0.787, 0.815) |
| 18.5–22.9 | 803,691 | 253,179 | 6,731,895.55 | 37.6089 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| 23.0–24.9 | 574,882 | 199,344 | 4,744,056.27 | 42.0197 | 1.118 (1.111, 1.124) | 1.136 (1.129, 1.142) | 1.132 (1.126, 1.139) |
| 25.0–29.9 | 665,019 | 247,075 | 5,392,078.62 | 45.8218 | 1.219 (1.213, 1.226) | 1.254 (1.247, 1.261) | 1.245 (1.238, 1.252) |
| ≥30 | 67,354 | 27,268 | 529,408.48 | 51.5065 | 1.372 (1.355, 1.389) | 1.367 (1.350, 1.384) | 1.348 (1.331, 1.366) |
| BMI | N | Event | Duration, PY | IR, per 1000 PY | HR (95% C.I) | ||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||
| <17 | 30,111 | 7920 | 239,932.19 | 33.0093 | 0.863 (0.843, 0.883) | 0.766 (0.749, 0.784) | 0.767 (0.750, 0.785) |
| 18.0–18.9 | 50,274 | 13,674 | 421,877.28 | 32.4123 | 0.847 (0.831, 0.862) | 0.827 (0.812, 0.843) | 0.828 (0.813, 0.844) |
| 19.0–19.9 | 101,371 | 29,457 | 853,988.08 | 34.4935 | 0.901 (0.889, 0.913) | 0.900 (0.888, 0.912) | 0.900 (0.888, 0.913) |
| 20.0–20.9 | 161,958 | 49,136 | 1,364,180.79 | 36.0187 | 0.940 (0.930, 0.951) | 0.942 (0.932, 0.953) | 0.943 (0.932, 0.954) |
| 21.0–21.9 | 232,585 | 74,551 | 1,946,900.94 | 38.2921 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| 22.0–22.9 | 278,130 | 91,948 | 2,317,207.19 | 39.6805 | 1.036 (1.026, 1.046) | 1.052 (1.041, 1.062) | 1.051 (1.041, 1.061) |
| 23.0–23.9 | 296,443 | 101,992 | 2,449,937.68 | 41.6304 | 1.087 (1.077, 1.098) | 1.103 (1.092, 1.113) | 1.101 (1.091, 1.112) |
| 24.0–24.9 | 278,439 | 97,352 | 2,294,118.59 | 42.4355 | 1.109 (1.098, 1.119) | 1.140 (1.129, 1.151) | 1.137 (1.126, 1.148) |
| 25.0–25.9 | 235,430 | 85,167 | 1,924,206.95 | 44.2608 | 1.157 (1.145, 1.168) | 1.195 (1.183, 1.207) | 1.190 (1.179, 1.202) |
| 26.0–26.9 | 180,199 | 66,515 | 1,463,594.33 | 45.4463 | 1.188 (1.175, 1.200) | 1.227 (1.214, 1.240) | 1.221 (1.208, 1.234) |
| 27.0–27.9 | 123,974 | 46,825 | 1,000,338 | 46.8092 | 1.223 (1.209, 1.238) | 1.267 (1.252, 1.282) | 1.259 (1.244, 1.274) |
| 28.0–28.9 | 77,077 | 29,847 | 617,107.43 | 48.366 | 1.264 (1.248, 1.281) | 1.302 (1.284, 1.319) | 1.292 (1.275, 1.310) |
| 29.0–29.9 | 48,339 | 18,721 | 386,831.92 | 48.3957 | 1.265 (1.245, 1.286) | 1.316 (1.295, 1.337) | 1.305 (1.284, 1.326) |
| 30.0–30.9 | 29,393 | 11,628 | 232,806.21 | 49.9471 | 1.306 (1.281, 1.332) | 1.336 (1.310, 1.362) | 1.324 (1.298, 1.350) |
| 31.0–31.9 | 14,824 | 6019 | 116,838.24 | 51.5157 | 1.347 (1.312, 1.383) | 1.354 (1.319, 1.390) | 1.340 (1.305, 1.376) |
| 32.0–32.9 | 10,326 | 4328 | 80,420.21 | 53.8173 | 1.408 (1.366, 1.452) | 1.342 (1.301, 1.383) | 1.328 (1.288, 1.369) |
| 33.0–33.9 | 4895 | 1940 | 38,527.66 | 50.3534 | 1.317 (1.259, 1.378) | 1.341 (1.282, 1.403) | 1.326 (1.267, 1.387) |
| 34.0–34.9 | 3124 | 1294 | 24,300.07 | 53.2509 | 1.393 (1.319, 1.472) | 1.371 (1.297, 1.448) | 1.353 (1.280, 1.429) |
| ≥35 | 4792 | 2059 | 36,516.08 | 56.3861 | 1.477 (1.414, 1.543) | 1.415 (1.355, 1.479) | 1.400 (1.340, 1.462) |
| Obesity | N | Event | Duration | IR | HR (95% C.I) | p for Interaction | ||
|---|---|---|---|---|---|---|---|---|
| Age groups | <65 | No | 1,223,652 | 372,471 | 10,544,323.31 | 35.3243 | 1 (Ref.) | <0.0001 |
| Yes | 629,401 | 219,871 | 5,256,853.22 | 41.8256 | 1.202 (1.195, 1.208) | |||
| ≥65 | No | 205,659 | 93,559 | 1,343,819.43 | 69.6217 | 1 (Ref.) | ||
| Yes | 102,972 | 54,472 | 664,633.88 | 81.9579 | 1.162 (1.150, 1.174) | |||
| Sex | Male | No | 726,882 | 200,044 | 6,187,922.46 | 32.3281 | 1 (Ref.) | <0.0001 |
| Yes | 437,052 | 131,759 | 3,724,987.25 | 35.3717 | 1.142 (1.134, 1.150) | |||
| Female | No | 702,429 | 265,986 | 5,700,220.28 | 46.6624 | 1 (Ref.) | ||
| Yes | 295,321 | 142,584 | 2,196,499.85 | 64.9142 | 1.245 (1.237, 1.253) | |||
| Income | Q2–4 | No | 1,134,357 | 361,613 | 9,489,012.67 | 38.1086 | 1 (Ref.) | 0.6307 |
| Yes | 589,690 | 215,761 | 4,801,151.62 | 44.9394 | 1.197 (1.190, 1.203) | |||
| Q1 | No | 294,954 | 104,417 | 2,399,130.07 | 43.5229 | 1 (Ref.) | ||
| Yes | 142,683 | 58,582 | 1,120,335.48 | 52.2897 | 1.194 (1.181, 1.206) | |||
| Abdominal obesity | No | No | 1,352,033 | 434,929 | 11,302,239.78 | 38.4817 | 1 (Ref.) | <0.0001 |
| Yes | 357,556 | 125,315 | 2,966,052.33 | 42.2498 | 1.162 (1.155, 1.170) | |||
| Yes | No | 77,278 | 31,101 | 585,902.95 | 53.0822 | 1 (Ref.) | ||
| Yes | 374,817 | 149,028 | 2,955,434.78 | 50.4251 | 1.130 (1.116, 1.144) | |||
| Smoking | Non, Ex | No | 1,103,371 | 377,413 | 9,107,575.92 | 41.4395 | 1 (Ref.) | <0.0001 |
| Yes | 565,767 | 226,362 | 4,487,842.7 | 50.4389 | 1.214 (1.207, 1.220) | |||
| Current | No | 325,940 | 88,617 | 2,780,566.81 | 31.8701 | 1 (Ref.) | ||
| Yes | 166,606 | 47,981 | 1,433,644.4 | 33.4679 | 1.121 (1.109, 1.134) | |||
| Drinking | Non, Mild | No | 1,329,652 | 436,664 | 11,052,178.53 | 39.5093 | 1 (Ref.) | <0.0001 |
| Yes | 661,603 | 252,019 | 5,320,662.55 | 47.3661 | 1.201 (1.195, 1.207) | |||
| Heavy | No | 99,659 | 29,366 | 835,964.21 | 35.1283 | 1 (Ref.) | ||
| Yes | 70,770 | 22,324 | 600,824.56 | 37.1556 | 1.132 (1.112, 1.151) | |||
| Regular exercise | No | No | 1,146,216 | 372,009 | 9,531,534.1 | 39.0293 | 1 (Ref.) | 0.0061 |
| Yes | 578,087 | 216,835 | 4,669,042.71 | 46.441 | 1.200 (1.194, 1.207) | |||
| Yes | No | 283,095 | 94,021 | 2,356,608.63 | 39.8967 | 1 (Ref.) | ||
| Yes | 154,286 | 57,508 | 1,252,444.39 | 45.9166 | 1.181 (1.169, 1.193) | |||
| DM | No | No | 1,303,560 | 421,946 | 10,922,212.62 | 38.6319 | 1 (Ref.) | 0.0011 |
| Yes | 620,662 | 230,084 | 5,058,632.16 | 45.4834 | 1.200 (1.194, 1.206) | |||
| Yes | No | 125,751 | 44,084 | 965,930.12 | 45.6389 | 1 (Ref.) | ||
| Yes | 111,711 | 44,259 | 862,854.94 | 51.2937 | 1.172 (1.156, 1.187) | |||
| HTN | No | No | 1,073,997 | 334,071 | 9,134,951.36 | 36.5706 | 1 (Ref.) | 0.0546 |
| Yes | 418,562 | 146,017 | 3,488,030.06 | 41.8623 | 1.201 (1.193, 1.208) | |||
| Yes | No | 355,314 | 131,959 | 2,753,191.38 | 47.9295 | 1 (Ref.) | ||
| Yes | 313,811 | 128,326 | 2,433,457.04 | 52.734 | 1.189 (1.180, 1.198) | |||
| Dyslipidemia | No | No | 1,178,614 | 371,943 | 9,893,708.69 | 37.5939 | 1 (Ref.) | 0.0046 |
| Yes | 527,797 | 190,414 | 4,322,291.81 | 44.0539 | 1.201 (1.194, 1.208) | |||
| Yes | No | 250,697 | 94,087 | 1,994,434.05 | 47.1748 | 1 (Ref.) | ||
| Yes | 204,576 | 83,929 | 1,599,195.29 | 52.482 | 1.182 (1.171, 1.193) | |||
| CKD | No | No | 1,328,625 | 431,119 | 11,101,547.59 | 38.8341 | 1 (Ref.) | 0.2218 |
| Yes | 670,933 | 249,009 | 5,457,433.55 | 45.6275 | 1.195 (1.189, 1.201) | |||
| Yes | No | 100,686 | 34,911 | 786,595.15 | 44.3824 | 1 (Ref.) | ||
| Yes | 61,440 | 25,334 | 464,053.55 | 54.5928 | 1.208 (1.188, 1.227) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, J.-H.; Han, K.; Kim, J.-Y. Association Between Higher Body Mass Index and the Risk of Lumbar Spinal Stenosis in Korean Populations: A Nationwide Cohort Study. J. Clin. Med. 2024, 13, 7397. https://doi.org/10.3390/jcm13237397
Ryu J-H, Han K, Kim J-Y. Association Between Higher Body Mass Index and the Risk of Lumbar Spinal Stenosis in Korean Populations: A Nationwide Cohort Study. Journal of Clinical Medicine. 2024; 13(23):7397. https://doi.org/10.3390/jcm13237397
Chicago/Turabian StyleRyu, Ji-Hyun, Kyungdo Han, and Ju-Yeong Kim. 2024. "Association Between Higher Body Mass Index and the Risk of Lumbar Spinal Stenosis in Korean Populations: A Nationwide Cohort Study" Journal of Clinical Medicine 13, no. 23: 7397. https://doi.org/10.3390/jcm13237397
APA StyleRyu, J.-H., Han, K., & Kim, J.-Y. (2024). Association Between Higher Body Mass Index and the Risk of Lumbar Spinal Stenosis in Korean Populations: A Nationwide Cohort Study. Journal of Clinical Medicine, 13(23), 7397. https://doi.org/10.3390/jcm13237397







