Predicting Severe Respiratory Failure in Patients with COVID-19: A Machine Learning Approach
Abstract
1. Introduction
2. Materials and Methods
| True Class | |||
| Positive | Negative | ||
| Predicted class | Positive | True positive | False positive |
| Negative | False negative | True negative | |
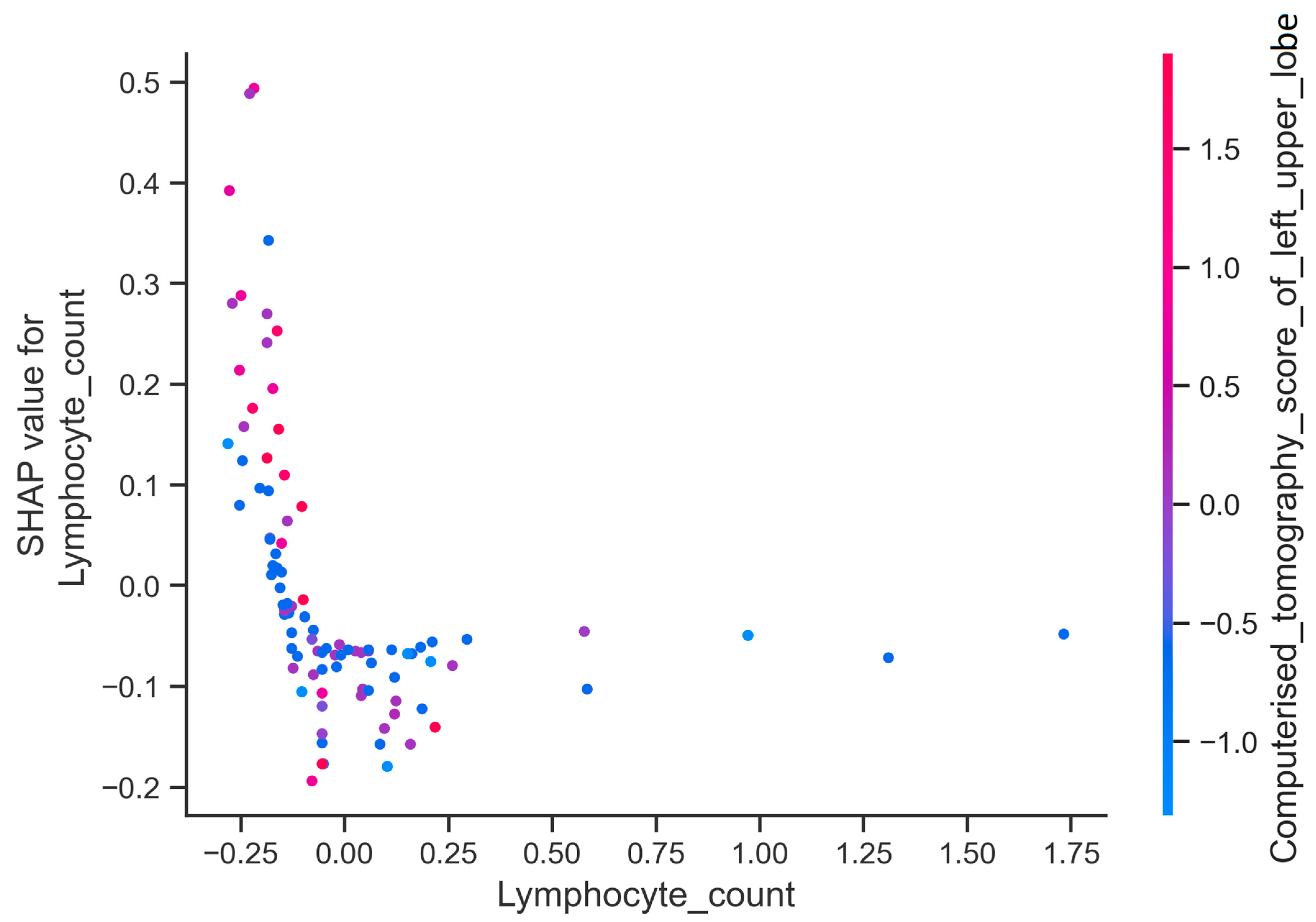
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pandey, S.K.; Mohanta, G.C.; Kumar, V.; Gupta, K. Diagnostic Tools for Rapid Screening and Detection of SARS-CoV-2 Infection. Vaccines 2022, 10, 1200. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Jafari Abarghan, Y.; Heiat, M.; Jahangiri, A.; Hossein Peypar, M.; Abdorrashidi, M.; Tohidinia, A.; Salesi, M.; Tajik, S.; Farzaneh Dehkordi, F.; Sedighian, H. Investigating the impact of Tocilizumab, Sarilumab, and Anakinra on clinical outcomes in COVID-19: A systematic review and meta-analysis. Int. J. Cardiol. Heart Vasc. 2024, 54, 101483. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, F.; Page, J.H.; Weinberg, K.R.; Mishra, A. The Development and Validation of Simplified Machine Learning Algorithms to Predict Prognosis of Hospitalized Patients with COVID-19: Multicenter, Retrospective Study. J. Med. Internet Res. 2022, 24, e31549. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hou, W.; Zhao, Z.; Chen, A.; Li, H.; Duong, T.Q. Machining learning predicts the need for escalated care and mortality in COVID-19 patients from clinical variables. Int. J. Med. Sci. 2021, 18, 1739–1745. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, L.; Halalau, A.; Dalal, B.; Abbas, A.E.; Ivascu, F.; Amin, M.; Nair, G.B. Machine learning methods to predict mechanical ventilation and mortality in patients with COVID-19. PLoS ONE 2021, 16, e0249285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aznar-Gimeno, R.; Esteban, L.M.; Labata-Lezaun, G.; Del-Hoyo-Alonso, R.; Abadia-Gallego, D.; Paño-Pardo, J.R.; Esquillor-Rodrigo, M.J.; Lanas, Á.; Serrano, M.T. A Clinical Decision Web to Predict ICU Admission or Death for Patients Hospitalised with COVID-19 Using Machine Learning Algorithms. Int. J. Environ. Res. Public Health 2021, 18, 8677. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, H.J.; Han, D.; Kim, J.H.; Kim, D.; Ha, B.; Seog, W.; Lee, Y.K.; Lim, D.; Hong, S.O.; Park, M.J.; et al. An Easy-to-Use Machine Learning Model to Predict the Prognosis of Patients with COVID-19: Retrospective Cohort Study. J. Med. Internet Res. 2020, 22, e24225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Ge, P.; Zhu, J.; Li, H.; Graham, J.; Singer, A.; Richman, P.S.; Duong, T.Q. Deep learning prediction of the likelihood of ICU admission and mortality in COVID-19 patients using clinical variables. PeerJ 2020, 8, e10337. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jimenez-Solem, E.; Petersen, T.S.; Hansen, C.; Hansen, C.; Lioma, C.; Igel, C.; Boomsma, W.; Krause, O.; Lorenzen, S.; Selvan, R.; et al. Developing and validating COVID-19 adverse outcome risk prediction models from a bi-national European cohort of 5594 patients. Sci. Rep. 2021, 11, 3246. [Google Scholar] [CrossRef] [PubMed]
- Saadatmand, S.; Salimifard, K.; Mohammadi, R.; Marzban, M.; Naghibzadeh-Tahami, A. Predicting the necessity of oxygen therapy in the early stage of COVID-19 using machine learning. Med. Biol. Eng. Comput. 2022, 60, 957–968. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shamout, F.E.; Shen, Y.; Wu, N.; Kaku, A.; Park, J.; Makino, T.; Jastrzębski, S.; Witowski, J.; Wang, D.; Zhang, B.; et al. An artificial intelligence system for predicting the deterioration of COVID-19 patients in the emergency department. NPJ Digit. Med. 2021, 4, 80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dipaola, F.; Gatti, M.; Giaj Levra, A.; Menè, R.; Shiffer, D.; Faccincani, R.; Raouf, Z.; Secchi, A.; Rovere Querini, P.; Voza, A.; et al. Multimodal deep learning for COVID-19 prognosis prediction in the emergency department: A bi-centric study. Sci. Rep. 2023, 13, 10868. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zakariaee, S.S.; Naderi, N.; Ebrahimi, M.; Kazemi-Arpanahi, H. Comparing machine learning algorithms to predict COVID-19 mortality using a dataset including chest computed tomography severity score data. Sci. Rep. 2023, 13, 11343. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chadaga, K.; Prabhu, S.; Sampathila, N.; Chadaga, R.; Umakanth, S.; Bhat, D.; Shashi Kumar, G.S. Explainable artificial intelligence approaches for COVID-19 prognosis prediction using clinical markers. Sci. Rep. 2024, 14, 1783. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adamidi, E.S.; Mitsis, K.; Nikita, K.S. Artificial intelligence in clinical care amidst COVID-19 pandemic: A systematic review. Comput. Struct. Biotechnol. J. 2021, 19, 2833–2850. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Viderman, D.; Kotov, A.; Popov, M.; Abdildin, Y. Machine and deep learning methods for clinical outcome prediction based on physiological data of COVID-19 patients: A scoping review. Int. J. Med. Inform. 2024, 182, 105308. [Google Scholar] [CrossRef] [PubMed]
- Rasmy, L.; Nigo, M.; Kannadath, B.S.; Xie, Z.; Mao, B.; Patel, K.; Zhou, Y.; Zhang, W.; Ross, A.; Xu, H.; et al. Recurrent neural network models (CovRNN) for predicting outcomes of patients with COVID-19 on admission to hospital: Model development and validation using electronic health record data. Lancet Digit. Health 2022, 4, e415–e425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, K.; Karanth, S.; Patel, B.; Murphy, R.; Jiang, X. A multi-task Gaussian process self-attention neural network for real-time prediction of the need for mechanical ventilators in COVID-19 patients. J. Biomed. Inform. 2022, 130, 104079. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Churpek, M.M.; Gupta, S.; Spicer, A.B.; Hayek, S.S.; Srivastava, A.; Chan, L.; Melamed, M.L.; Brenner, S.K.; Radbel, J.; Madhani-Lovely, F.; et al. Machine Learning Prediction of Death in Critically Ill Patients with Coronavirus Disease 2019. Crit. Care Explor. 2021, 3, e0515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cavallaro, M.; Moiz, H.; Keeling, M.J.; McCarthy, N.D. Contrasting factors associated with COVID-19-related ICU admission and death outcomes in hospitalised patients by means of Shapley values. PLoS Comput. Biol. 2021, 17, e1009121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chi, S.; Guo, A.; Heard, K.; Kim, S.; Foraker, R.; White, P.; Moore, N. Development and Structure of an Accurate Machine Learning Algorithm to Predict Inpatient Mortality and Hospice Outcomes in the Coronavirus Disease 2019 Era. Med. Care 2022, 60, 381–386. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kamran, F.; Tang, S.; Otles, E.; McEvoy, D.S.; Saleh, S.N.; Gong, J.; Li, B.Y.; Dutta, S.; Liu, X.; Medford, R.J.; et al. Early identification of patients admitted to hospital for covid-19 at risk of clinical deterioration: Model development and multisite external validation study. BMJ 2022, 376, e068576. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- König, S.; Pellissier, V.; Leiner, J.; Hohenstein, S.; Ueberham, L.; Meier-Hellmann, A.; Kuhlen, R.; Hindricks, G.; Bollmann, A.; Helios Hospitals. Expected and observed in-hospital mortality in heart failure patients before and during the COVID-19 pandemic: Introduction of the machine learning-based standardized mortality ratio at Helios hospitals. Clin. Cardiol. 2022, 45, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Qi, S.A.; Kuan, L.H.; Sun, W.; Zhang, J.; Greiner, R. Learning accurate personalized survival models for predicting hospital discharge and mortality of COVID-19 patients. Sci. Rep. 2022, 12, 4472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tekcan Sanli, D.E.; Yildirim, D.; Sanli, A.N.; Erozan, N.; Husmen, G.; Altundag, A.; Tuzuner, F.; Dikensoy, O.; Erel Kirisoglu, C. Predictive value of CT imaging findings in COVID-19 pneumonia at the time of first-screen regarding the need for hospitalization or intensive care unit. Diagn. Interv. Radiol. 2021, 27, 599–606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, Y.; Yu, L.; Wang, H.; Shu, Z.; Gong, X. Early Warning Information for Severe and Critical Patients with COVID-19 Based on Quantitative CT Analysis of Lung Segments. Front. Public Health 2021, 9, 596938. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meiler, S.; Schaible, J.; Poschenrieder, F.; Scharf, G.; Zeman, F.; Rennert, J.; Pregler, B.; Kleine, H.; Stroszczynski, C.; Zorger, N.; et al. Can CT performed in the early disease phase predict outcome of patients with COVID 19 pneumonia? Analysis of a cohort of 64 patients from Germany. Eur. J. Radiol. 2020, 131, 109256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gustine, J.N.; Jones, D. Immunopathology of Hyperinflammation in COVID-19. Am. J. Pathol. 2021, 191, 4–17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahnach, M.; Zbiri, S.; Nejjari, S.; Ousti, F.; Elkettani, C. C-reactive protein as an early predictor of COVID-19 severity. J. Med. Biochem. 2020, 39, 500–507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, H.; Ma, Q.; Li, C.; Liu, R.; Zhao, L.; Wang, W.; Zhang, P.; Liu, X.; Gao, G.; Liu, F.; et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 2020, 9, 1123–1130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Engel, H.; Ben Hamouda, N.; Portmann, K.; Delodder, F.; Suys, T.; Feihl, F.; Eggimann, P.; Rossetti, A.O.; Oddo, M. Serum procalcitonin as a marker of post-cardiac arrest syndrome and long-term neurological recovery, but not of early-onset infections, in comatose post-anoxic patients treated with therapeutic hypothermia. Resuscitation 2013, 84, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Zahorec, R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl. Lek. Listy 2021, 122, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062, Erratum in Lancet 2020, 395, 1038. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tan, L.; Wang, Q.; Zhang, D.; Ding, J.; Huang, Q.; Tang, Y.Q.; Wang, Q.; Miao, H. Lymphopenia predicts disease severity of COVID-19: A descriptive and predictive study. Signal Transduct. Target. Ther. 2020, 5, 33, Erratum in Signal Transduct. Target. Ther. 2020, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Jiang, Y.; Yuan, M.; Shen, J.; Yin, H.; Geng, D.; Xu, J.; Hua, Y.; Shi, J.; Shi, Y.; et al. Association of radiologic findings with mortality in patients with avian influenza H7N9 pneumonia. PLoS ONE 2014, 9, e93885. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lowery, S.A.; Sariol, A.; Perlman, S. Innate immune and inflammatory responses to SARS-CoV-2: Implications for COVID-19. Cell Host Microbe 2021, 29, 1052–1062. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liao, Y.C.; Liang, W.G.; Chen, F.W.; Hsu, J.H.; Yang, J.J.; Chang, M.S. IL-19 induces production of IL-6 and TNF-alpha and results in cell apoptosis through TNF-alpha. J. Immunol. 2002, 169, 4288–4297. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral. Sci. 2020, 12, 8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Helal, M.A.; Shouman, S.; Abdelwaly, A.; Elmehrath, A.O.; Essawy, M.; Sayed, S.M.; Saleh, A.H.; El-Badri, N. Molecular basis of the potential interaction of SARS-CoV-2 spike protein to CD147 in COVID-19 associated-lymphopenia. J. Biomol. Struct. Dyn. 2022, 40, 1109–1119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petrie, H.T.; Klassen, L.W.; Kay, H.D. Inhibition of human cytotoxic T lymphocyte activity in vitro by autologous peripheral blood granulocytes. J. Immunol. 1985, 134, 230–234. [Google Scholar] [CrossRef] [PubMed]
- el-Hag, A.; Clark, R.A. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J. Immunol. 1987, 139, 2406–2413. [Google Scholar] [CrossRef] [PubMed]
- Krupski, W.; Gorecki, A.; Kruk-Bachonko, J. Diagnostic value of chest CT scanning for determination of Covid-19 severity in individual lung lobes. Ann. Agric. Environ. Med. 2022, 29, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Friedman, P.J.; Harwood, I.R.; Ellenbogen, P.H. Pulmonary cystic fibrosis in the adult: Early and late radiologic findings with pathologic correlations. AJR Am. J. Roentgenol. 1981, 136, 1131–1144. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422, Erratum in Lancet Respir. Med. 2020, 8, e26. [Google Scholar] [CrossRef] [PubMed]
- González-Jiménez, P.; Méndez, R.; Latorre, A.; Piqueras, M.; Balaguer-Cartagena, M.N.; Moscardó, A.; Alonso, R.; Hervás, D.; Reyes, S.; Menéndez, R. Neutrophil Extracellular Traps and Platelet Activation for Identifying Severe Episodes and Clinical Trajectories in COVID-19. Int. J. Mol. Sci. 2023, 24, 6690. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tuty Kuswardhani, R.A.; Henrina, J.; Pranata, R.; Anthonius Lim, M.; Lawrensia, S.; Suastika, K. Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2020, 14, 2103–2109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, Z.; Peng, F.; Xu, B.; Zhao, J.; Liu, H.; Peng, J.; Li, Q.; Jiang, C.; Zhou, Y.; Liu, S.; et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J. Infect. 2020, 81, e16–e25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Assicot, M.; Gendrel, D.; Carsin, H.; Raymond, J.; Guilbaud, J.; Bohuon, C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993, 341, 515–518. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]



| Patients with Mild Respiratory Failure (n = 206, 64.4%) | Patients with Severe Respiratory Failure (n = 114, 35.6%) | p-Value | ||
|---|---|---|---|---|
| Age | 53.01 ± 14.29 | 57.3 ± 17.63 | 0.025 | |
| Gender (male) | 121 (58.7%) | 78 (68.4%) | 0.087 | |
| Body mass index | 28.25 (17.99–44.08) | 27.76 (17.7–44.9) | 0.696 | |
| Previous COVID-19 vaccination | 53 (25.7%) | 23 (20.2%) | 0.264 | |
| Previous steroid usage | 2 (1%) | 4 (3.5%) | 0.191 | |
| Immunosuppression | 10 (4.9%) | 12 (10.5%) | 0.055 | |
| Pregnancy | 8 (3.9%) | 3 (2.6%) | 0.752 | |
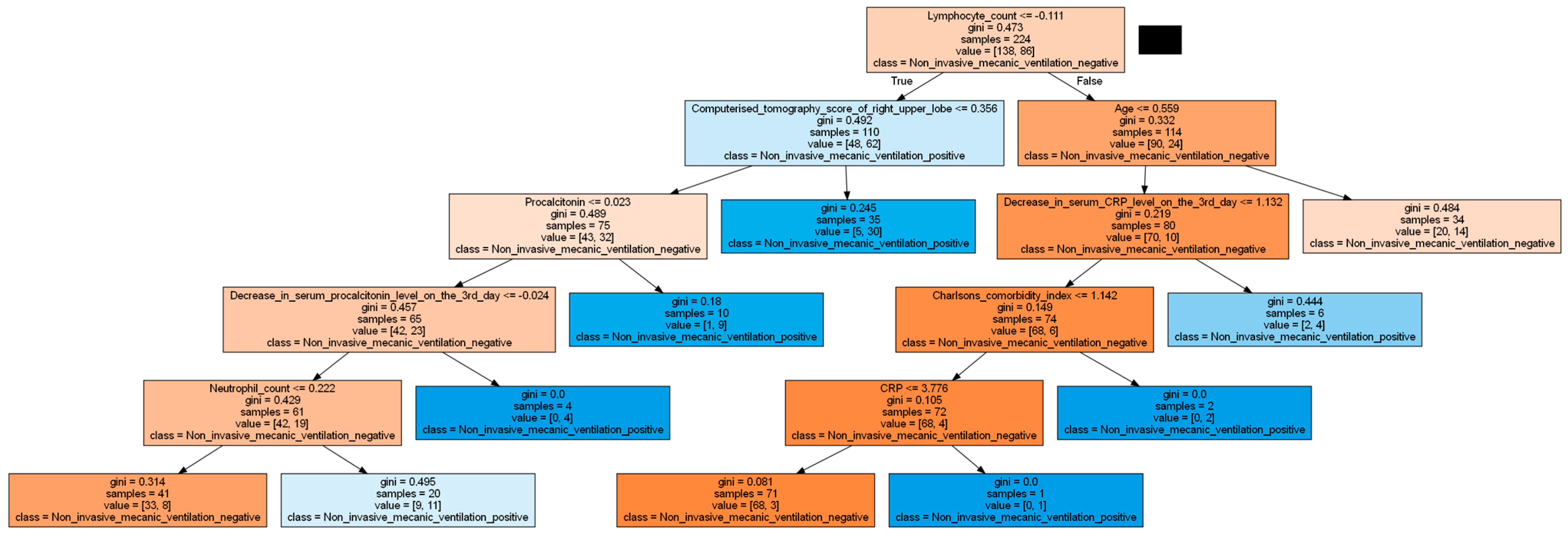
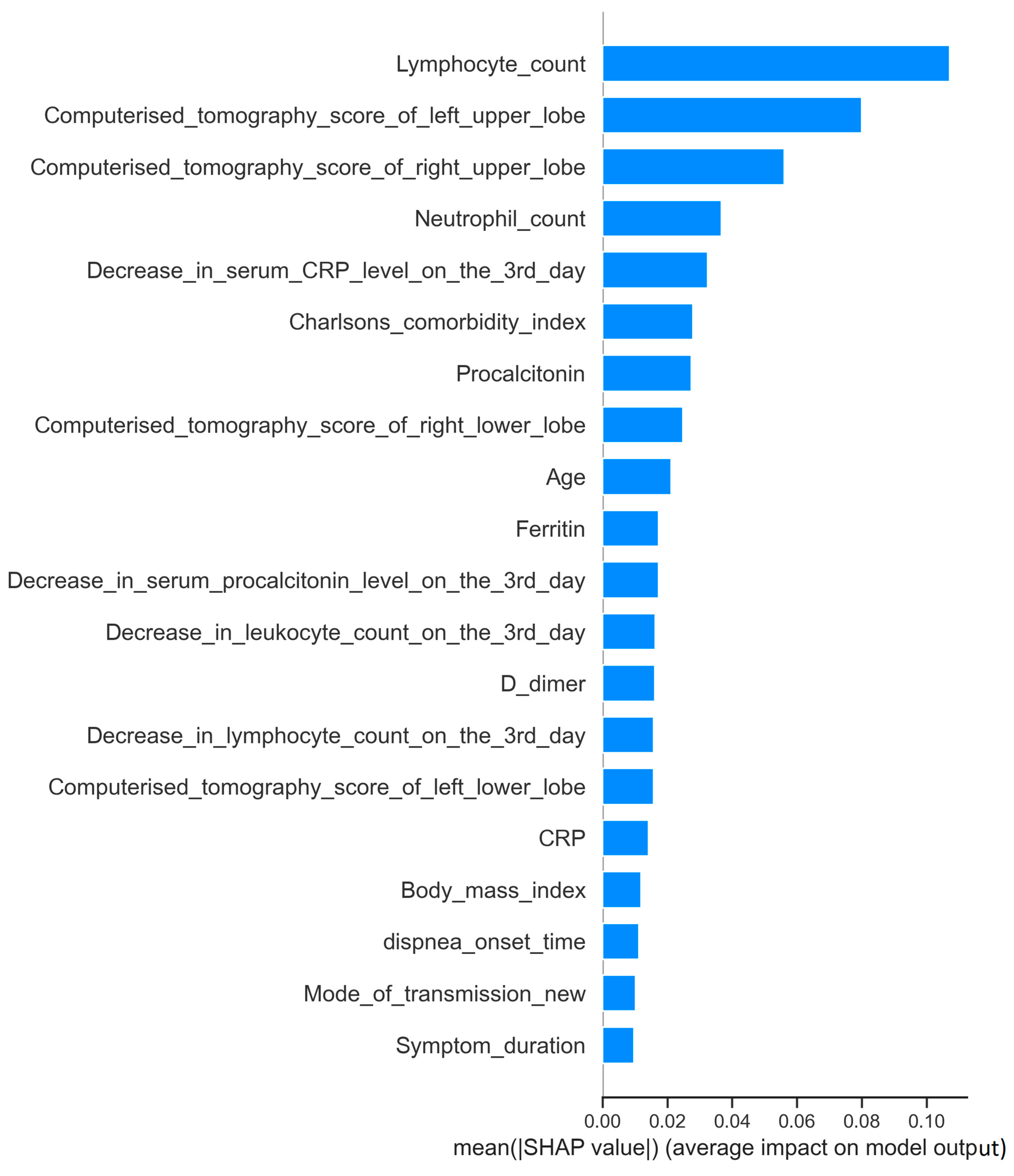
| Charlson comorbidity index | 1 (0–9) | 2 (0–9) | 0.015 | |
| Mode of transmission | Domestic transmission | 97 (47.1%) | 48 (42.1%) | 0.159 |
| Outdoor | 18 (8.7%) | 5 (4.4%) | ||
| Unknown | 91 (44.1%) | 61 (53.5%) | ||
| Smoking status | Smoker | 13 (6.3%) | 5 (4.4%) | 0.412 |
| Nonsmoker | 170 (82.5%) | 91 (79.8%) | ||
| Ex-smoker | 23 (11.2%) | 18 (15.8%) | ||
| Number of cigarettes smoked (package/year) | 0 (0–120) | 0 (0–90) | 0.467 | |
| Angiotensin-converting enzyme inhibitor usage | 44 (21.8%) | 18 (15.8%) | 0.228 | |
| Time from symptom onset to hospital admission | 5 (1–25) | 5 (1–30) | 0.811 | |
| Time from symptom onset to dyspnea onset | 7 (1–19) | 6 (2–14) | 0.153 | |
| Symptoms | Cough | 170 (82.5%) | 91 (79.8%) | 0.551 |
| Weakness | 49 (23.8%) | 34 (29.8%) | 0.238 | |
| High body temperature | 169 (82%) | 84 (73%) | 0.079 | |
| Body pain | 133 (64.6%) | 58 (50.9%) | 0.017 | |
| Sore throat | 7 (3.4%) | 10 (8.8%) | 0.04 | |
| Diarrhea | 21 (10.2%) | 5 (4.4%) | 0.069 | |
| Remdesivir usage | 4 (1.9%) | 12 (10.5%) | 0.001 | |
| Favipiravir usage | 98 (47.6%) | 62 (54.4%) | 0.243 | |
| Comorbidities | Liver transplantation | 0 (0%) | 2 (1.8%) | 0.126 |
| Cancer without metastases | 3 (1.5%) | 3 (2.6%) | 0.365 | |
| Cancer with metastases | 4 (1.9%) | 5 (4.4%) | 0.179 | |
| Heart failure | 5 (2.4%) | 6 (5.3%) | 0.182 | |
| Leukemia | 2 (1%) | 0 (0%) | 0.414 | |
| Myasthenia gravis | 1 (0.5%) | 2 (1.8%) | 0.290 | |
| Lymphoma | 3 (1.5%) | 3 (2.6%) | 0.365 | |
| Rheumatoid arthritis | 1 (0.5%) | 1 (0.9%) | 0.586 | |
| Aplastic anemia | 1 (0.5%) | 0 (0%) | 0.644 | |
| Multiple myeloma | 1 (0.5%) | 0 (0%) | 0.644 | |
| Cerebrovascular diseases | 3 (1.5%) | 2 (1.8%) | 0.585 | |
| Aortic aneurysm | 1 (0.5%) | 0 (0%) | 0.644 | |
| Dementia | 5 (2.4%) | 3 (2.6%) | 0.589 | |
| Parkinson’s disease | 3 (1.5%) | 1 (0.9%) | 0.552 | |
| Hypertension | 69 (33.5%) | 32 (28.1%) | 0.317 | |
| Ischemic heart disease | 13 (6.3%) | 13 (11.4%) | 0.110 | |
| Diabetes mellitus | 52 (25.2%) | 26 (22.8%) | 0.627 | |
| Diabetes mellitus with end-organ damage | 7 (3.4%) | 11 (9.6%) | 0.020 | |
| Chronic obstructive pulmonary disease | 3 (1.5%) | 2 (1.8%) | 0.585 | |
| Bronchiectasis | 1 (0.5%) | 0 (0%) | 0.644 | |
| Asthma | 5 (2.4%) | 5 (4.4%) | 0.260 | |
| Idiopathic pulmonaryfibrosis | 1 (0.5%) | 2 (1.8%) | 0.290 | |
| Severe chronic renal failure | 4 (1.9%) | 4 (3.5%) | 0.305 | |
| Mild chronic renal failure | 2 (1%) | 4 (3.5%) | 0.122 | |
| Renal transplantation | 3 (1.5%) | 2 (1.8%) | 0.585 | |
| Laboratory values | CRP (mg/L) | 62 (3–283) | 86 (3.6–409) | 0.003 |
| Procalcitonin (ng/mL) | 0.18 (0.01–3.82) | 0.21 (0.03–3.62) | 0.013 | |
| D-dimer (ng/mL) | 712 (123–9626) | 802 (136–9746) | 0.245 | |
| Ferritin (ng/mL) | 457 (38–20,598) | 558 (23–5556) | 0.025 | |
| Leukocyte count (/mm³) | 6300 (720–24,410) | 7810 (1860–88,000) | 0.008 | |
| Lymphocyte count (/mm³) | 965 (260–6120) | 685 (120–51,000) | 0.0001 | |
| Neutrophil count (/mm³) | 4885 (220–20,380) | 6340 (1590–24,190) | 0.001 | |
| Decrease in leukocyte count on the third day (%) | 23 [(−47)–(336)] | 34 [(−87)–(339)] | 0.896 | |
| Decrease in neutrophil count on the third day (%) | 36 [(−48)–(509)] | 39 [(−84)–(394)] | 0.666 | |
| Decrease in lymphocyte count on the third day (%) | (−1) [(−73)–(186)] | 0 [(−76)–(148)] | 0.700 | |
| Decrease in CRP level on the third day (%) | (−51) [(−94)–(840)] | (−43) [(−86)–(1682)] | 0.002 | |
| Decrease in procalcitonin level on the third day (%) | (−39) [(−94)–(207)] | (−28) [(−98)–(68,471)] | 0.137 | |
| Computed tomography scores of lung zones | Total lungs | 22 (2–84) | 28 (2–92) | 0.003 |
| Right upper zone | 2 (0–12) | 4 (0–16) | 0.0001 | |
| Left upper zone | 2 (0–16) | 4 (0–16) | 0.001 | |
| Right middle zone | 4 (0–16) | 4 (0–16) | 0.002 | |
| Left middle zone | 4 (0–16) | 4 (0–16) | 0.019 | |
| Right lower zone | 4 (0–16) | 4 (0–16) | 0.072 | |
| Left lower zone | 4 (0–16) | 5 (0–16) | 0.191 | |
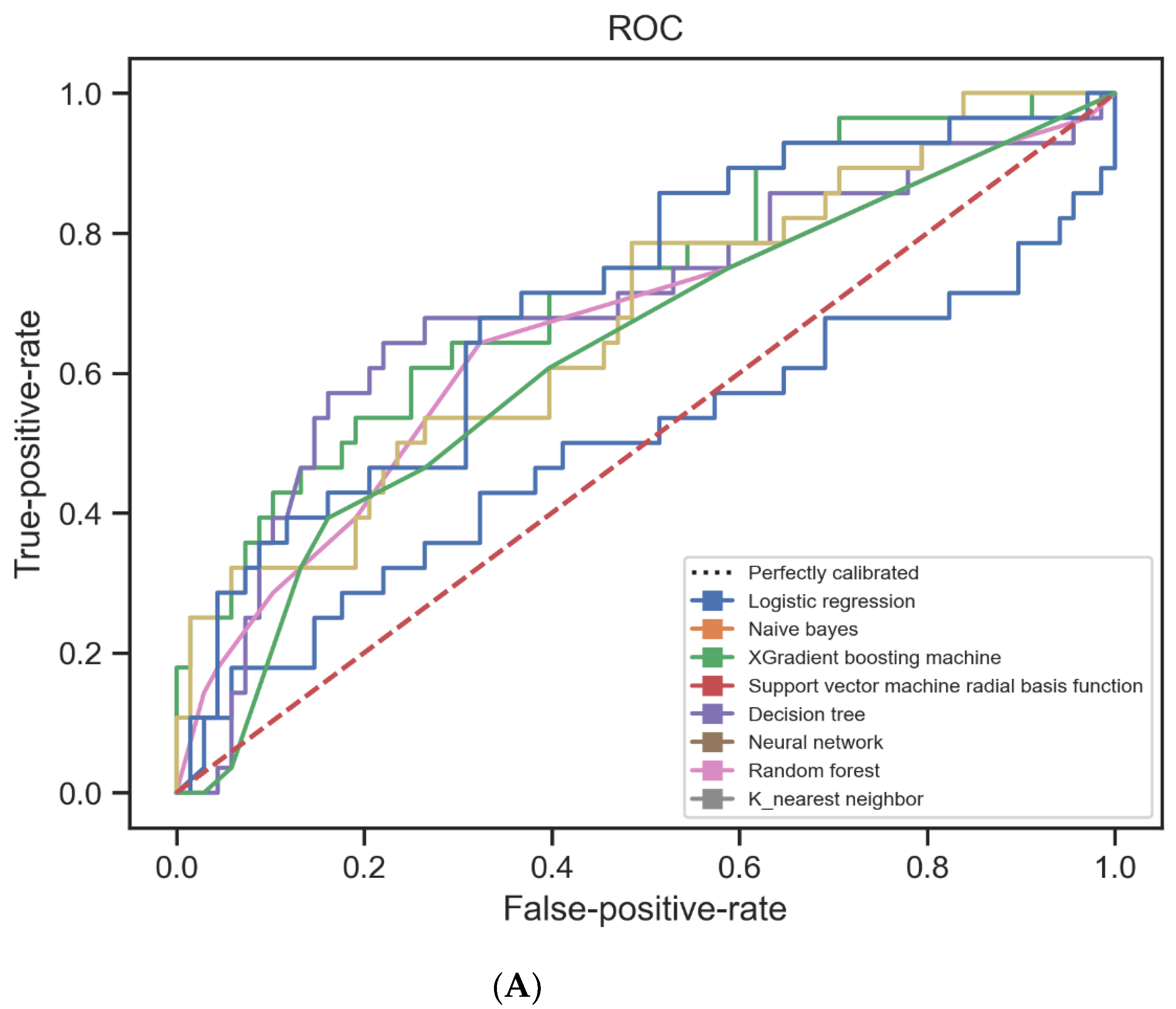
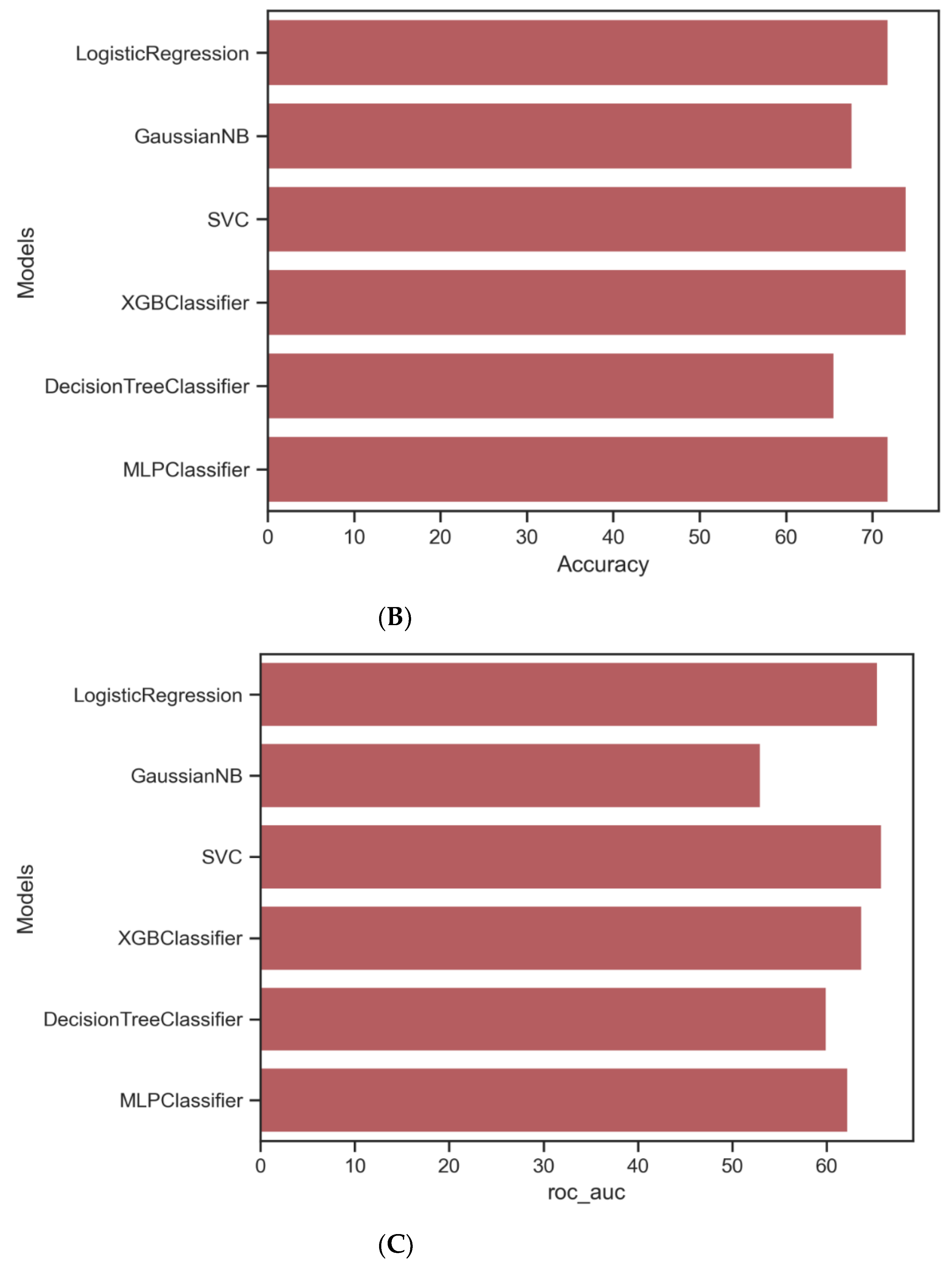
| Algorithm | Tuning Parameter | Accuracy | ROC-AUC * | Precision | Recall | F1 |
|---|---|---|---|---|---|---|
| Logisticregression | - | 0.7187 | 0.7274 | 0.72 | 0.72 | 0.72 |
| Naïve Bayes | - | 0.6770 | 0.5304 | 0.79 | 0.68 | 0.72 |
| K-nearest neighbor | K = 12 | 0.7291 | 0.5672 | 0.89 | 0.73 | 0.79 |
| Radialbasis function support vector machines | Radial basis function, C = 1, gamma = 0.1 | 0.7395 | 0.6586 | 0.76 | 0.74 | 0.75 |
| Neuralnetwork | Activation = relu, alpha = 0.00001, hidden layer size = (10,10,10), solver = sgd | 0.7500 | 0.6764 | 0.76 | 0.75 | 0.76 |
| XGBoost | Learning rate = 0.02, maximum depth = 3, n_estimators = 100, subsample = 0.8 | 0.7395 | 0.6376 | 0.79 | 0.74 | 0.76 |
| Classification and regression tree | Max depth = 5, minimum sample split = 36 | 0.6525 | 0.5997 | 0.65 | 0.66 | 0.65 |
| Randomforests | Maximum depth = 10, maximum features = 2, minimum sample split = 10, n_estimators = 1000 | 0.7187 | 0.6123 | 0.77 | 0.72 | 0.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceylan, B.; Olmuşçelik, O.; Karaalioğlu, B.; Ceylan, Ş.; Şahin, M.; Aydın, S.; Yılmaz, E.; Dumlu, R.; Kapmaz, M.; Çiçek, Y.; et al. Predicting Severe Respiratory Failure in Patients with COVID-19: A Machine Learning Approach. J. Clin. Med. 2024, 13, 7386. https://doi.org/10.3390/jcm13237386
Ceylan B, Olmuşçelik O, Karaalioğlu B, Ceylan Ş, Şahin M, Aydın S, Yılmaz E, Dumlu R, Kapmaz M, Çiçek Y, et al. Predicting Severe Respiratory Failure in Patients with COVID-19: A Machine Learning Approach. Journal of Clinical Medicine. 2024; 13(23):7386. https://doi.org/10.3390/jcm13237386
Chicago/Turabian StyleCeylan, Bahadır, Oktay Olmuşçelik, Banu Karaalioğlu, Şule Ceylan, Meyha Şahin, Selda Aydın, Ezgi Yılmaz, Rıdvan Dumlu, Mahir Kapmaz, Yeliz Çiçek, and et al. 2024. "Predicting Severe Respiratory Failure in Patients with COVID-19: A Machine Learning Approach" Journal of Clinical Medicine 13, no. 23: 7386. https://doi.org/10.3390/jcm13237386
APA StyleCeylan, B., Olmuşçelik, O., Karaalioğlu, B., Ceylan, Ş., Şahin, M., Aydın, S., Yılmaz, E., Dumlu, R., Kapmaz, M., Çiçek, Y., Kansu, A., Duger, M., & Mert, A. (2024). Predicting Severe Respiratory Failure in Patients with COVID-19: A Machine Learning Approach. Journal of Clinical Medicine, 13(23), 7386. https://doi.org/10.3390/jcm13237386






