Role of the Lymphocyte Count-to-C-Reactive Protein Ratio in the Risk Stratification for High EASE Scores After Living Donor Liver Transplantation: A Retrospective Observational Cohort Study
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Living Donor Liver Transplantation
2.3. Early Allograft Failure Simplified Estimation Score
2.4. Early Allograft Failure
2.5. Early Allograft Dysfunction
2.6. Lymphocyte and CRP Measurements
2.7. Recipient and Graft-Donor Variables
2.8. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Comparison of the Prevalence of a High EASE Score and EAD
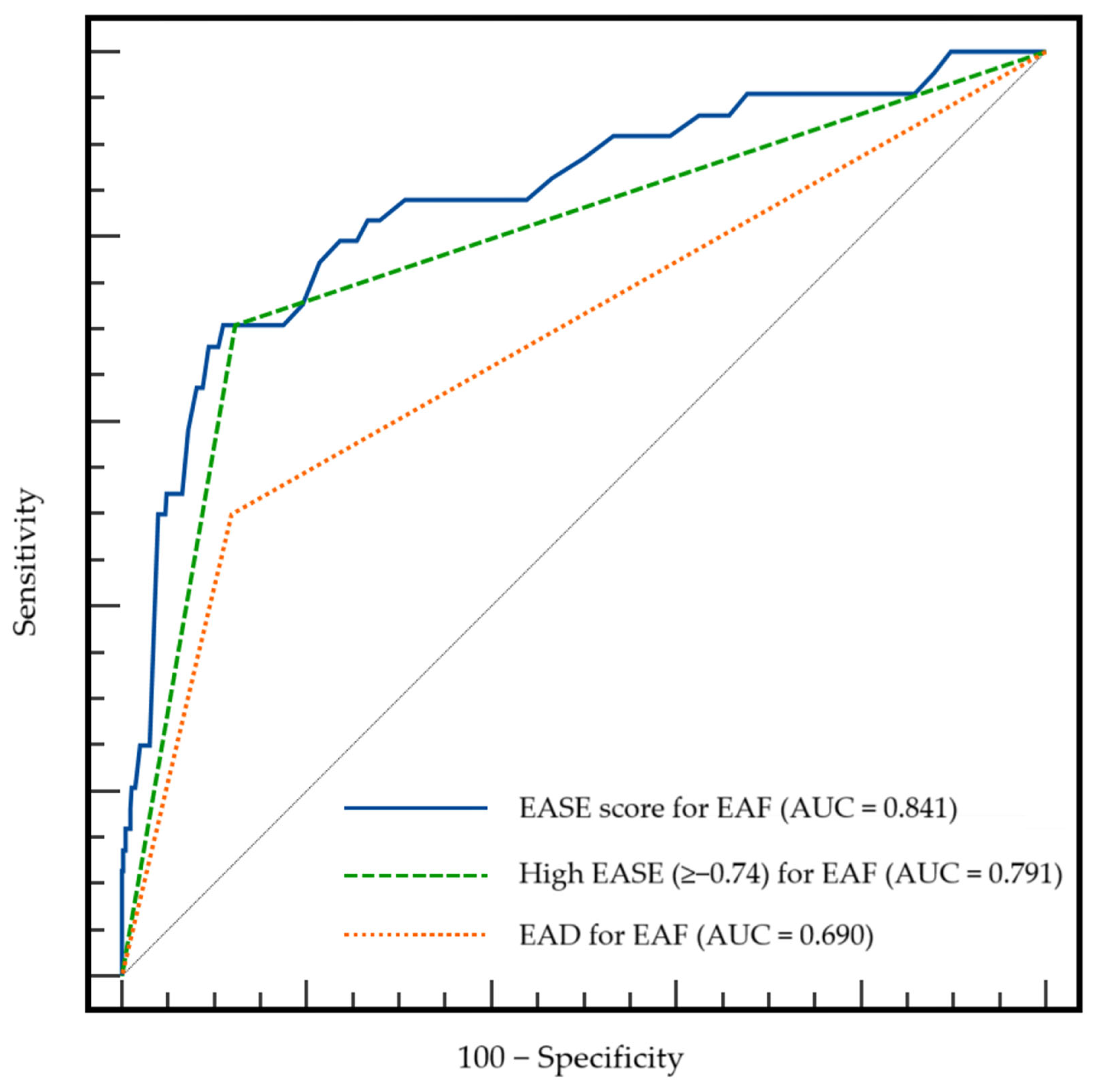
3.3. Comparison of the Predictive Value of the EASE Score, High EASE Score, and EAD for EAF Development
3.4. Comparison of the Predictive Accuracy of a High EASE Score and EAD for Postoperative Outcomes
3.5. Comparison of Perioperative Variables Between Low and High EASE Groups
3.6. Association of Perioperative Variables with a High EASE Score
3.7. Comparison of LCR Levels in the Low and High EASE Groups
3.8. The Predictive Accuracy of LCR for EAF
3.9. Association Between a Low LCR and Inflammatory Factors
3.10. Association of Perioperative Variables with EAF
3.11. Comparison of the Predictive Accuracy of Other Clinical Risk Scores for EAF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, S. Living donor liver transplantation in high Model for End-Stage Liver Disease score patients. Liver Transpl. 2017, 23, S9–S21. [Google Scholar] [CrossRef] [PubMed]
- Agopian, V.G.; Markovic, D.; Klintmalm, G.B.; Saracino, G.; Chapman, W.C.; Vachharajani, N.; Florman, S.S.; Tabrizian, P.; Haydel, B.; Nasralla, D.; et al. Multicenter validation of the liver graft assessment following transplantation (L-GrAFT) score for assessment of early allograft dysfunction. J. Hepatol. 2021, 74, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Agopian, V.G.; Harlander-Locke, M.P.; Markovic, D.; Dumronggittigule, W.; Xia, V.; Kaldas, F.M.; Zarrinpar, A.; Yersiz, H.; Farmer, D.G.; Hiatt, J.R.; et al. Evaluation of Early Allograft Function Using the Liver Graft Assessment Following Transplantation Risk Score Model. JAMA Surg. 2018, 153, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Olthoff, K.M.; Kulik, L.; Samstein, B.; Kaminski, M.; Abecassis, M.; Emond, J.; Shaked, A.; Christie, J.D. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010, 16, 943–949. [Google Scholar] [CrossRef]
- Chen, S.; Wang, T.; Luo, T.; He, S.; Huang, C.; Jia, Z.; Zhan, L.; Wang, D.; Zhu, X.; Guo, Z.; et al. Prediction of Graft Survival Post-liver Transplantation by L-GrAFT Risk Score Model, EASE Score, MEAF Scoring, and EAD. Front. Surg. 2021, 8, 753056. [Google Scholar] [CrossRef]
- Avolio, A.W.; Lai, Q.; Cillo, U.; Romagnoli, R.; De Simone, P. L-GrAFT and EASE scores in liver transplantation: Need for reciprocal external validation and comparison with other scores. J. Hepatol. 2021, 75, 729–731. [Google Scholar] [CrossRef]
- Rocha, N.P.; Fortes, R.C. Total lymphocyte count and serum albumin as predictors of nutritional risk in surgical patients. Arq. Bras. Cir. Dig. 2015, 28, 193–196. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Q.; Mao, W.; Fan, J.; Ye, B. Neutrophil-to-Lymphocyte Ratio Predicts Early Mortality in Patients with HBV-Related Decompensated Cirrhosis. Gastroenterol. Res. Pract. 2016, 2016, 4394650. [Google Scholar] [CrossRef]
- Cervoni, J.P.; Thevenot, T.; Weil, D.; Muel, E.; Barbot, O.; Sheppard, F.; Monnet, E.; Di Martino, V. C-reactive protein predicts short-term mortality in patients with cirrhosis. J. Hepatol. 2012, 56, 1299–1304. [Google Scholar] [CrossRef]
- Zhang, J.N.; Gao, Y.; Wang, X.T.; Li, N.N.; Du, X.; Tang, Y.J.; Lai, Q.Q.; Chen, P.F.; Yue, C.S.; Wu, J.H.; et al. Lymphocyte-C-reactive protein ratio can differentiate disease severity of COVID-19 patients and serve as an assistant screening tool for hospital and ICU admission. Front. Immunol. 2022, 13, 957407. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, T.; Chen, L.; Xu, B.; Wu, G.; Zong, G. Preoperative lymphocyte to C-reactive protein ratio: A new prognostic indicator of post-primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Int. Immunopharmacol. 2023, 114, 109594. [Google Scholar] [CrossRef] [PubMed]
- Okugawa, Y.; Toiyama, Y.; Yamamoto, A.; Shigemori, T.; Ide, S.; Kitajima, T.; Fujikawa, H.; Yasuda, H.; Hiro, J.; Yoshiyama, S.; et al. Lymphocyte-C-reactive Protein Ratio as Promising New Marker for Predicting Surgical and Oncological Outcomes in Colorectal Cancer. Ann. Surg. 2020, 272, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Practice guidelines for perioperative blood management: An updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management*. Anesthesiology 2015, 122, 241–275. [CrossRef] [PubMed]
- Baek, S.D.; Jang, M.; Kim, W.; Yu, H.; Hwang, S.; Lee, S.G.; Hwang, G.S.; Lee, E.K.; Kim, S.M.; Chang, J.W. Benefits of Intraoperative Continuous Renal Replacement Therapy During Liver Transplantation in Patients with Renal Dysfunction. Transplant. Proc. 2017, 49, 1344–1350. [Google Scholar] [CrossRef]
- Douthitt, L.; Bezinover, D.; Uemura, T.; Kadry, Z.; Shah, R.A.; Ghahramani, N.; Janicki, P.K. Perioperative use of continuous renal replacement therapy for orthotopic liver transplantation. Transplant. Proc. 2012, 44, 1314–1317. [Google Scholar] [CrossRef]
- Avolio, A.W.; Franco, A.; Schlegel, A.; Lai, Q.; Meli, S.; Burra, P.; Patrono, D.; Ravaioli, M.; Bassi, D.; Ferla, F.; et al. Development and Validation of a Comprehensive Model to Estimate Early Allograft Failure Among Patients Requiring Early Liver Retransplant. JAMA Surg. 2020, 155, e204095. [Google Scholar] [CrossRef]
- Pomposelli, J.J.; Goodrich, N.P.; Emond, J.C.; Humar, A.; Baker, T.B.; Grant, D.R.; Fisher, R.A.; Roberts, J.P.; Olthoff, K.M.; Gillespie, B.W.; et al. Patterns of Early Allograft Dysfunction in Adult Live Donor Liver Transplantation: The A2ALL Experience. Transplantation 2016, 100, 1490–1499. [Google Scholar] [CrossRef]
- Horoldt, B.S.; Burattin, M.; Gunson, B.K.; Bramhall, S.R.; Nightingale, P.; Hubscher, S.G.; Neuberger, J.M. Does the Banff rejection activity index predict outcome in patients with early acute cellular rejection following liver transplantation? Liver Transpl. 2006, 12, 1144–1151. [Google Scholar] [CrossRef]
- Nicolau-Raducu, R.; Cohen, A.J.; Bokhari, A.; Bohorquez, H.; Bruce, D.; Carmody, I.; Bugeaud, E.; Seal, J.; Sonnier, D.; Nossaman, B.; et al. Predictive model and risk factors associated with a revised definition of early allograft dysfunction in liver transplant recipients. Clin. Transplant. 2017, 31, e13097. [Google Scholar] [CrossRef]
- Wadei, H.M.; Lee, D.D.; Croome, K.P.; Mai, M.L.; Golan, E.; Brotman, R.; Keaveny, A.P.; Taner, C.B. Early Allograft Dysfunction After Liver Transplantation Is Associated With Short- and Long-Term Kidney Function Impairment. Am. J. Transplant. 2016, 16, 850–859. [Google Scholar] [CrossRef]
- Lee, D.D.; Croome, K.P.; Shalev, J.A.; Musto, K.R.; Sharma, M.; Keaveny, A.P.; Taner, C.B. Early allograft dysfunction after liver transplantation: An intermediate outcome measure for targeted improvements. Ann. Hepatol. 2016, 15, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Xu, D.; Li, B.; Xin, S.; Zhang, Z.; Huang, L.; Fu, J.; Yang, Y.; Jin, L.; Zhao, J.M.; et al. Compartmentalization and its implication for peripheral immunologically-competent cells to the liver in patients with HBV-related acute-on-chronic liver failure. Hepatol. Res. 2009, 39, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Berres, M.L.; Schnyder, B.; Yagmur, E.; Inglis, B.; Stanzel, S.; Tischendorf, J.J.; Koch, A.; Winograd, R.; Trautwein, C.; Wasmuth, H.E. Longitudinal monocyte human leukocyte antigen-DR expression is a prognostic marker in critically ill patients with decompensated liver cirrhosis. Liver Int. 2009, 29, 536–543. [Google Scholar] [CrossRef]
- Juvela, S.; Kuhmonen, J.; Siironen, J. C-reactive protein as predictor for poor outcome after aneurysmal subarachnoid haemorrhage. Acta Neurochir. 2012, 154, 397–404. [Google Scholar] [CrossRef]
- Al-Subaie, N.; Reynolds, T.; Myers, A.; Sunderland, R.; Rhodes, A.; Grounds, R.M.; Hall, G.M. C-reactive protein as a predictor of outcome after discharge from the intensive care: A prospective observational study. Br. J. Anaesth 2010, 105, 318–325. [Google Scholar] [CrossRef]
- Silvestre, J.; Povoa, P.; Coelho, L.; Almeida, E.; Moreira, P.; Fernandes, A.; Mealha, R.; Sabino, H. Is C-reactive protein a good prognostic marker in septic patients? Intensive Care Med. 2009, 35, 909–913. [Google Scholar] [CrossRef]
- Ho, K.M.; Lee, K.Y.; Dobb, G.J.; Webb, S.A. C-reactive protein concentration as a predictor of in-hospital mortality after ICU discharge: A prospective cohort study. Intensive Care Med. 2008, 34, 481–487. [Google Scholar] [CrossRef]
- Villacorta, H.; Masetto, A.C.; Mesquita, E.T. C-reactive protein: An inflammatory marker with prognostic value in patients with decompensated heart failure. Arq. Bras. Cardiol. 2007, 88, 585–589. [Google Scholar] [CrossRef]
- Ho, K.M.; Dobb, G.J.; Lee, K.Y.; Towler, S.C.; Webb, S.A. C-reactive protein concentration as a predictor of intensive care unit readmission: A nested case-control study. J. Crit. Care 2006, 21, 259–265. [Google Scholar] [CrossRef]
- Povoa, P.; Almeida, E.; Moreira, P.; Fernandes, A.; Mealha, R.; Aragao, A.; Sabino, H. C-reactive protein as an indicator of sepsis. Intensive Care Med. 1998, 24, 1052–1056. [Google Scholar] [CrossRef]
- Sheldon, J.; Riches, P.; Gooding, R.; Soni, N.; Hobbs, J.R. C-reactive protein and its cytokine mediators in intensive-care patients. Clin. Chem. 1993, 39, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Cousin, F.; Ortega-Deballon, P.; Bourredjem, A.; Doussot, A.; Giaccaglia, V.; Fournel, I. Diagnostic Accuracy of Procalcitonin and C-reactive Protein for the Early Diagnosis of Intra-abdominal Infection After Elective Colorectal Surgery: A Meta-analysis. Ann. Surg. 2016, 264, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.T.; He, S.Y.; Chen, S.L.; Li, L.F.; He, Z.Q.; Zhu, Y.Y.; He, X.; Chen, H. Clinical and prognostic implications of pretreatment albumin to C-reactive protein ratio in patients with hepatocellular carcinoma. BMC Cancer 2019, 19, 538. [Google Scholar] [CrossRef]
- Zhu, S.; Waili, Y.; Qi, X.; Chen, Y.; Lou, Y.; Chen, B. Serum C-reactive protein predicts early mortality in hospitalized patients with HBV-related decompensated cirrhosis. Medicine 2017, 96, e5988. [Google Scholar] [CrossRef]
- Huang, S.S.; Xie, D.M.; Cai, Y.J.; Wu, J.M.; Chen, R.C.; Wang, X.D.; Song, M.; Zheng, M.H.; Wang, Y.Q.; Lin, Z.; et al. C-reactive protein-to-albumin ratio is a predictor of hepatitis B virus related decompensated cirrhosis: Time-dependent receiver operating characteristics and decision curve analysis. Eur. J. Gastroenterol. Hepatol. 2017, 29, 472–480. [Google Scholar] [CrossRef]
- Kwon, J.H.; Jang, J.W.; Kim, Y.W.; Lee, S.W.; Nam, S.W.; Jaegal, D.; Lee, S.; Bae, S.H. The usefulness of C-reactive protein and neutrophil-to-lymphocyte ratio for predicting the outcome in hospitalized patients with liver cirrhosis. BMC Gastroenterol. 2015, 15, 146. [Google Scholar] [CrossRef]
- Chen, W.; Wang, J.B.; Abnet, C.C.; Dawsey, S.M.; Fan, J.H.; Yin, L.Y.; Yin, J.; Taylor, P.R.; Qiao, Y.L.; Freedman, N.D. Association between C-reactive protein, incident liver cancer, and chronic liver disease mortality in the Linxian Nutrition Intervention Trials: A nested case-control study. Cancer Epidemiol. Biomarkers Prev. 2015, 24, 386–392. [Google Scholar] [CrossRef]
- Vanbiervliet, G.; Le Breton, F.; Rosenthal-Allieri, M.A.; Gelsi, E.; Marine-Barjoan, E.; Anty, R.; Piche, T.; Benzaken, S.; Saint-Paul, M.C.; Huet, P.M.; et al. Serum C-reactive protein: A non-invasive marker of alcoholic hepatitis. Scand. J. Gastroenterol. 2006, 41, 1473–1479. [Google Scholar] [CrossRef]
- Di Martino, V.; Coutris, C.; Cervoni, J.P.; Dritsas, S.; Weil, D.; Richou, C.; Vanlemmens, C.; Thevenot, T. Prognostic value of C-reactive protein levels in patients with cirrhosis. Liver Transpl. 2015, 21, 753–760. [Google Scholar] [CrossRef]
- Heikkila, K.; Ebrahim, S.; Rumley, A.; Lowe, G.; Lawlor, D.A. Associations of circulating C-reactive protein and interleukin-6 with survival in women with and without cancer: Findings from the British Women’s Heart and Health Study. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 1155–1159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Albillos, A.; de la Hera, A.; Gonzalez, M.; Moya, J.L.; Calleja, J.L.; Monserrat, J.; Ruiz-del-Arbol, L.; Alvarez-Mon, M. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology 2003, 37, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Rosenbloom, A.J.; Pinsky, M.R.; Bryant, J.L.; Shin, A.; Tran, T.; Whiteside, T. Leukocyte activation in the peripheral blood of patients with cirrhosis of the liver and SIRS. Correlation with serum interleukin-6 levels and organ dysfunction. JAMA 1995, 274, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Hammond, R.; Chan, K.; Chukwuenweniwe, C.; Johnson, R.; Khair, D.; Duck, E.; Olubodun, O.; Barwick, K.; Banya, W.; et al. Comparison of Lymphocyte-CRP Ratio to Conventional Inflammatory Markers for Predicting Clinical Outcomes in COVID-19. J. Pers. Med. 2023, 13, 909. [Google Scholar] [CrossRef]
- Ullah, W.; Basyal, B.; Tariq, S.; Almas, T.; Saeed, R.; Roomi, S.; Haq, S.; Madara, J.; Boigon, M.; Haas, D.C.; et al. Lymphocyte-to-C-Reactive Protein Ratio: A Novel Predictor of Adverse Outcomes in COVID-19. J. Clin. Med. Res. 2020, 12, 415–422. [Google Scholar] [CrossRef]
- Okugawa, Y.; Toiyama, Y.; Yamamoto, A.; Shigemori, T.; Ichikawa, T.; Yin, C.; Suzuki, A.; Fujikawa, H.; Yasuda, H.; Hiro, J.; et al. Lymphocyte-to-C-reactive protein ratio and score are clinically feasible nutrition-inflammation markers of outcome in patients with gastric cancer. Clin. Nutr. 2020, 39, 1209–1217. [Google Scholar] [CrossRef]
- Nakamura, Y.; Shida, D.; Boku, N.; Yoshida, T.; Tanabe, T.; Takamizawa, Y.; Takashima, A.; Kanemitsu, Y. Lymphocyte-to-C-Reactive Protein Ratio Is the Most Sensitive Inflammation-Based Prognostic Score in Patients With Unresectable Metastatic Colorectal Cancer. Dis. Colon Rectum. 2021, 64, 1331–1341. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhang, X.; Zhang, Q.; Ruan, G.T.; Xie, H.L.; Liu, T.; Song, M.M.; Ge, Y.Z.; Deng, L.; Shi, H.P. Lymphocyte-C-reactive protein ratio with calf circumference could better predict survival of patients with non-metastatic cancer. Sci. Rep. 2023, 13, 7217. [Google Scholar]
- Li, X.; Wei, Y.; Xu, Z.; Li, T.; Dong, G.; Liu, X.; Zhu, Z.; Yang, J.; Yang, J. Lymphocyte-to-C-Reactive Protein Ratio as an Early Sepsis Biomarker for Neonates with Suspected Sepsis. Mediators Inflamm. 2023, 2023, 9077787. [Google Scholar] [CrossRef]
- Ye, B.; Ding, Q.; He, X.; Liu, X.; Shen, J. High C-Reactive Protein-to-Lymphocyte Ratio Is Predictive of Unfavorable Prognosis in HBV-Associated Decompensated Cirrhosis. Lab. Med. 2022, 53, e149–e153. [Google Scholar] [CrossRef]
- Lee, D.D.; Li, J.; Wang, G.; Croome, K.P.; Burns, J.M.; Perry, D.K.; Nguyen, J.H.; Hopp, W.J.; Taner, C.B. Looking inward: The impact of operative time on graft survival after liver transplantation. Surgery 2017, 162, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, E.; Pahlavan, S.; Yoeli, D.; Brigham, D.; Sater, A.; Wachs, M.; Bock, M.; Adams, M. Successful use of intra-operative continuous renal replacement therapy in pediatric liver transplant recipients: Single center case series. Pediatr. Transplant. 2022, 26, e14377. [Google Scholar] [CrossRef] [PubMed]
- Pratschke, S.; Bender, A.; Boesch, F.; Andrassy, J.; van Rosmalen, M.; Samuel, U.; Rogiers, X.; Meiser, B.; Kuchenhoff, H.; Driesslein, D.; et al. Association between donor age and risk of graft failure after liver transplantation: An analysis of the Eurotransplant database. Transpl. Int. 2019, 32, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Lichtenegger, P.; Schiefer, J.; Graf, A.; Berlakovich, G.; Faybik, P.; Baron, D.M.; Baron-Stefaniak, J. The association of pre-operative anaemia with survival after orthotopic liver transplantation. Anaesthesia 2020, 75, 472–478. [Google Scholar] [CrossRef]
- Dorantes, R.P.; Boettcher, B.T.; Woehlck, H.J. Calcium Chloride Requirement and Postreperfusion Rebound During Massive Transfusion in Liver Transplantation. J. Cardiothorac. Vasc. Anesth. 2022, 36, 2400–2405. [Google Scholar] [CrossRef]
- Reich, D.L.; Bennett-Guerrero, E.; Bodian, C.A.; Hossain, S.; Winfree, W.; Krol, M. Intraoperative tachycardia and hypertension are independently associated with adverse outcome in noncardiac surgery of long duration. Anesth. Analg. 2002, 95, 273–277. [Google Scholar] [CrossRef]
- Bagley, S.C.; White, H.; Golomb, B.A. Logistic regression in the medical literature: Standards for use and reporting, with particular attention to one medical domain. J. Clin. Epidemiol. 2001, 54, 979–985. [Google Scholar] [CrossRef]
| Group | No EAF | EAF | p |
|---|---|---|---|
| n | 748 | 44 | |
| High EASE Score | 92 (12.3%) | 31 (70.5%) | <0.001 |
| EAD | 89 (11.9%) | 22 (50%) | <0.001 |
| Group | AUC of High EASE | AUC of EAD | p |
|---|---|---|---|
| AKI | 0.521 | 0.547 | 0.185 |
| Graft rejection | 0.504 | 0.510 | 0.798 |
| Infection | 0.525 | 0.543 | 0.233 |
| Graft failure | 0.626 | 0.592 | 0.148 |
| Overall patient mortality | 0.645 | 0.591 | 0.033 |
| Group | Low EASE | High EASE | p |
|---|---|---|---|
| n | 669 | 123 | |
| Age (years) | 54 (49–60) | 53 (44–60) | 0.248 |
| Sex (male) | 468 (70%) | 93 (75.6%) | 0.205 |
| Body mass index (kg/m2) | 24 (22–27) | 24 (21–27) | 0.869 |
| Etiology | |||
| Alcohol | 151 (22.6%) | 26 (21.1%) | 0.307 |
| Hepatitis A | 28 (4.2%) | 6 (4.9%) | |
| Hepatitis B | 367 (54.9%) | 62 (50.4%) | |
| Hepatitis C | 43 (6.4%) | 10 (8.1%) | |
| Autoimmune | 23 (3.4%) | 10 (8.1%) | |
| Drug and Toxin | 12 (1.8%) | 1 (0.8%) | |
| Cryptogenic | 45 (6.7%) | 8 (6.5%) | |
| Comorbidity | |||
| Diabetes mellitus | 172 (25.7%) | 37 (30.1%) | 0.312 |
| Hypertension | 148 (22.1%) | 22 (17.9%) | 0.293 |
| MELD score (points) | 12 (6–22) | 20 (13–32) | <0.001 |
| Hepatic decompensation | |||
| Varix | 167 (25.0%) | 36 (29.3%) | 0.315 |
| Ascites | 299 (44.7%) | 84 (68.3%) | <0.001 |
| Cardiac function | |||
| Ejection fraction (%) | 64 (62–67) | 64 (60–67) | 0.148 |
| Diastolic dysfunction | 291 (43.5%) | 44 (35.8%) | 0.111 |
| Laboratory variables | |||
| Hematocrit (%) | 30 (26–36) | 26 (23–31) | <0.001 |
| WBC count (×109/L) | 4.5 (3.0–6.8) | 4.9 (2.7–9.9) | 0.198 |
| Lymphocyte to CRP ratio | 26.1 (6.0–93.5) | 5.3 (2.2–19.8) | <0.001 |
| Platelet count | 70 (48–109) | 56 (39–82) | <0.001 |
| Albumin (g/dL) | 3.1 (2.7–3.6) | 3.1 (2.7–3.4) | 0.227 |
| Total bilirubin | 2.1 (0.8–9.5) | 7.1 (1.7–25.2) | <0.001 |
| International normalized ratio | 1.4 (1.2–2.0) | 1.8 (1.3–2.4) | <0.001 |
| Sodium (mEq/L) | 139 (135–141) | 138 (134–141) | 0.124 |
| Potassium (mEq/L) | 4 (3.7–4.3) | 4 (3.5–4.4) | 0.563 |
| Calcium (mg/dL) | 8.4 (8.0–8.8) | 8.3 (7.9–8.8) | 0.357 |
| Glucose (mg/dL) | 108 (92–138) | 116 (89–149) | 0.326 |
| Creatinine (mg/dL) | 0.8 (0.7–1.0) | 1.0 (0.7–2.1) | <0.001 |
| Ammonia (μg/dL) | 93 (65–146) | 93 (56–148) | 0.475 |
| Group | Low EASE | High EASE | p |
|---|---|---|---|
| n | 669 | 123 | |
| Operative time (min) | 480 (435–542) | 525 (475–600) | <0.001 |
| Intraoperative CRRT | 54 (8.1%) | 35 (28.5%) | <0.001 |
| Postreperfusion syndrome | 333 (49.8%) | 65 (52.8%) | 0.531 |
| Average of vital signs | |||
| MBP (mmHg) | 76 (70–81) | 76 (69–84) | 0.678 |
| HR (beats/min) | 88 (80–97) | 92 (84–102) | 0.003 |
| CVP (mmHg) | 9 (7.5–10.5) | 9.3 (7.1–11.7) | 0.205 |
| Calcium infusion dose (mg) | 300 (0–788) | 1050 (300–4080) | <0.001 |
| Blood product transfusion (unit) | |||
| Packed red blood cell | 7 (4–11) | 13 (8–22) | <0.001 |
| Fresh frozen plasma | 6 (4–10) | 10 (7–18) | <0.001 |
| Platelet concentrate | 4 (0–6) | 6 (0–12) | <0.001 |
| Hourly fluid infusion (mL/kg/h) | 11.2 (8.4–13.8) | 12.8 (9.0–17.9) | 0.001 |
| Hourly urine output (mL/kg/h) | 1.4 (0.8–2.2) | 1.0 (0.2–1.7) | <0.001 |
| Donor-graft variables | |||
| Age (years) | 33 (27–40) | 35 (32–46) | <0.001 |
| Sex (male) | 422 (63.1%) | 74 (60.2%) | 0.539 |
| GRWR (%) | 1.2 (1.1–1.5) | 1.2 (1.0–1.6) | 0.991 |
| Graft ischemic time (min) | 90 (70–112) | 90 (69–120) | 0.578 |
| Fatty change (%) | 5 (1–5) | 5 (1–5) | 0.686 |
| Univariate Analysis | Multivariate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| β | Odds Ratio | 95% CI | p | β | Odds Ratio | 95% CI | p | |
| Preoperative recipient variables | ||||||||
| Age (years) | −0.014 | 0.986 | 0.967–1.005 | 0.140 | ||||
| Sex (male vs. female) | −0.286 | 0.751 | 0.482–1.170 | 0.206 | ||||
| Body mass index (kg/m2) | 0.007 | 1.007 | 0.959–1.057 | 0.790 | ||||
| Comorbidity | ||||||||
| Diabetes mellitus | 0.218 | 1.243 | 0.815–1.897 | 0.313 | ||||
| Hypertension | −0.266 | 0.767 | 0.467–1.259 | 0.294 | ||||
| Hepatic decompensation | ||||||||
| Varix | 0.218 | 1.244 | 0.812–1.905 | 0.315 | ||||
| Ascites | 0.980 | 2.665 | 1.770–4.013 | <0.001 | ||||
| Cardiac function | ||||||||
| Ejection fraction (%) | −0.032 | 0.968 | 0.926–1.012 | 0.151 | ||||
| Diastolic dysfunction | −0.119 | 0.888 | 0.662–1.191 | 0.427 | ||||
| Laboratory variables | ||||||||
| Hematocrit (%) | −0.081 | 0.922 | 0.893–0.952 | <0.001 | −0.050 | 0.952 | 0.916–0.989 | 0.011 |
| WBC count (x 109/L) | 0.030 | 1.031 | 1.002–1.060 | 0.036 | ||||
| Lymphocyte count-to-CRP ratio | <0.001 | 1.000 | 1.000–1.000 | <0.001 | <0.001 | 1.000 | 1.000–1.000 | <0.001 |
| Lymphocyte count-to-CRP ratio (<12.7) * | 1.530 | 4.617 | 2.997–7.111 | <0.001 | 1.198 | 3.312 | 1.997–5.493 | <0.001 |
| Albumin (g/dL) | −0.229 | 0.796 | 0.579–1.094 | 0.160 | ||||
| International normalized ratio | 0.060 | 1.070 | 0.975–1.157 | 0.170 | ||||
| Sodium (mEq/L) | −0.002 | 0.998 | 0.977–1.018 | 0.818 | ||||
| Potassium (mEq/L) | −0.078 | 0.925 | 0.670–1.278 | 0.637 | ||||
| Calcium (mg/dL) | −0.019 | 0.981 | 0.772–1.247 | 0.875 | ||||
| Glucose (mg/dL) | 0.001 | 1.002 | 0.998–1.005 | 0.446 | ||||
| Creatinine (mg/dL) | 0.267 | 1.306 | 1.152–1.481 | <0.001 | ||||
| Ammonia (μg/dL) | −0.001 | 0.009 | 0.997–1.002 | 0.702 | ||||
| Intraoperative recipient variables | ||||||||
| Operative time (min) | 0.005 | 1.005 | 1.003–1.007 | <0.001 | 0.005 | 1.005 | 1.003–1.007 | <0.001 |
| Interoperative CRRT | 1.511 | 4.530 | 2.802–7.323 | <0.001 | 1.191 | 3.219 | 1.893–5.721 | <0.001 |
| Postreperfusion syndrome | 0.179 | 1.196 | 0.908–1.576 | 0.203 | ||||
| Vital sign averages | ||||||||
| MBP (mmHg) | 0.005 | 1.005 | 0.998–1.011 | 0.147 | ||||
| HR (beats/min) | 0.024 | 1.024 | 1.010–1.039 | 0.001 | 0.022 | 1.022 | 1.006–1.038 | 0.008 |
| CVP (mmHg) | 0.068 | 1.070 | 1.009–1.134 | 0.024 | ||||
| Calcium infusion dose (mg) | 0.001 | 1.001 | 1.000–1.001 | <0.001 | <0.001 | 1.000 | 1.000–1.001 | <0.001 |
| Hourly fluid infusion (mL/kg/h) | 0.019 | 1.019 | 1.000–1.038 | 0.051 | ||||
| Hourly urine output (mL/kg/h) | −0.385 | 0.681 | 0.556–0.833 | <0.001 | ||||
| Donor-graft variables | ||||||||
| Age (years) | 0.023 | 1.024 | 1.007–1.041 | 0.006 | 0.036 | 1.037 | 1.017–1.057 | <0.001 |
| Sex (male) | 0.123 | 1.131 | 0.763–1.677 | 0.539 | ||||
| GRWR (%) | 0.222 | 1.248 | 0.850–1.834 | 0.258 | ||||
| Graft ischemic time (min) | 0.001 | 1.001 | 0.999–1.004 | 0.309 | ||||
| Fatty change (%) | −0.005 | 0.995 | 0.964–1.028 | 0.765 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Park, C.S.; Chae, M.S.; Choi, H.J.; Hong, S.H. Role of the Lymphocyte Count-to-C-Reactive Protein Ratio in the Risk Stratification for High EASE Scores After Living Donor Liver Transplantation: A Retrospective Observational Cohort Study. J. Clin. Med. 2024, 13, 7344. https://doi.org/10.3390/jcm13237344
Park J, Park CS, Chae MS, Choi HJ, Hong SH. Role of the Lymphocyte Count-to-C-Reactive Protein Ratio in the Risk Stratification for High EASE Scores After Living Donor Liver Transplantation: A Retrospective Observational Cohort Study. Journal of Clinical Medicine. 2024; 13(23):7344. https://doi.org/10.3390/jcm13237344
Chicago/Turabian StylePark, Jaesik, Chul Soo Park, Min Suk Chae, Ho Joong Choi, and Sang Hyun Hong. 2024. "Role of the Lymphocyte Count-to-C-Reactive Protein Ratio in the Risk Stratification for High EASE Scores After Living Donor Liver Transplantation: A Retrospective Observational Cohort Study" Journal of Clinical Medicine 13, no. 23: 7344. https://doi.org/10.3390/jcm13237344
APA StylePark, J., Park, C. S., Chae, M. S., Choi, H. J., & Hong, S. H. (2024). Role of the Lymphocyte Count-to-C-Reactive Protein Ratio in the Risk Stratification for High EASE Scores After Living Donor Liver Transplantation: A Retrospective Observational Cohort Study. Journal of Clinical Medicine, 13(23), 7344. https://doi.org/10.3390/jcm13237344






