Immune Response to an Adjuvanted Recombinant Zoster Vaccine in Japanese Patients with Rheumatoid Arthritis Receiving Upadacitinib (End Zoster-J Study): Study Protocol for an Exploratory Parallel Triple-Arm Prospective Trial
Abstract
1. Introduction
- (1)
- MTX monotherapy (6–12 mg/week).
- (2)
- Upadacitinib monotherapy (15 mg/day).
- (3)
- MTX (6–12 mg/week) + upadacitinib (15 mg/day) (primary evaluation target) combination therapy.
2. Methods
2.1. Study Setting
2.2. Trial Registration
2.3. Eligibility Criteria
2.4. Who Will Take Informed Consent?
2.5. Additional Consent Provisions for Collection and Use of Participant Data and Biological Specimens
3. Interventions
3.1. Explanation for the Choice of Comparators
3.2. Intervention Description
3.3. Criteria for Discontinuing or Modifying Allocated Interventions
3.3.1. Criteria for Discontinuing Allocated Interventions
- (1)
- When the patient withdraws the informed consent.
- (2)
- When a principal investigator or co-investigator judges that it is difficult to continue the research due to worsening of the primary disease, complications, or the occurrence of serious adverse events.
- (3)
- When there is a need to administer contraindicated drugs.
- (4)
- When serious deviations from the research protocol occur, such as violations of the Clinical Research Act and its implementation regulations, inclusion, or exclusion criteria. Examples of serious deviations are listed below, but are not limited to these events:
- Medication non-compliance: interruption of oral medication for 14 consecutive days.
- Patients who did not receive at least one dose of Shingrix® as scheduled.
- Patients who received other vaccines between obtaining informed consent and 4 weeks after the second Shingrix® dose.
- (5)
- When pregnancy of the study subject is discovered.
- (6)
- When the study is discontinued.
- (7)
- When the drugs being administered (MTX and Upadacitinib) are discontinued or are found to have been discontinued prior to intervention.
- (8)
- When the principal investigator or co-investigator determines that it is difficult to continue the study.
3.3.2. Criteria for Modifying Allocated Interventions
3.4. Strategies to Improve Adherence to Interventions
3.5. Relevant Concomitant Care Permitted or Prohibited During the Trial
3.6. Provisions for Post-Trial Care
4. Outcomes
4.1. Primary Endpoint
4.2. Secondary Endpoints
- (1)
- Presence or absence of RA flares from enrollment to 12 weeks after the second Shingrix® dose.
- (2)
- Proportion of participants with positive anti-gE antibodies at 4 weeks after the first Shingrix® dose.
- (3)
- Antibody titre of anti-gE antibodies at 4 weeks after the first and second Shingrix® doses.
- (4)
- Proportion of gE-specific CD4+ T-cells at 4 weeks after the first and second Shingrix® doses.
- (5)
- Antibody titre of VZV-specific antibodies at 4 weeks after the first and second Shingrix® doses.
- (6)
- Changes in RA disease activity.
4.3. Evaluation of Primary and Secondary Endpoints
4.3.1. Positive Anti-gE Antibodies
4.3.2. RA Flare
4.3.3. Proportion of gE-Specific CD4+ T-Cells
4.3.4. Antibody Titre of VZV-Specific Antibodies
4.3.5. Changes in RA Disease Activity
4.4. Sample Size
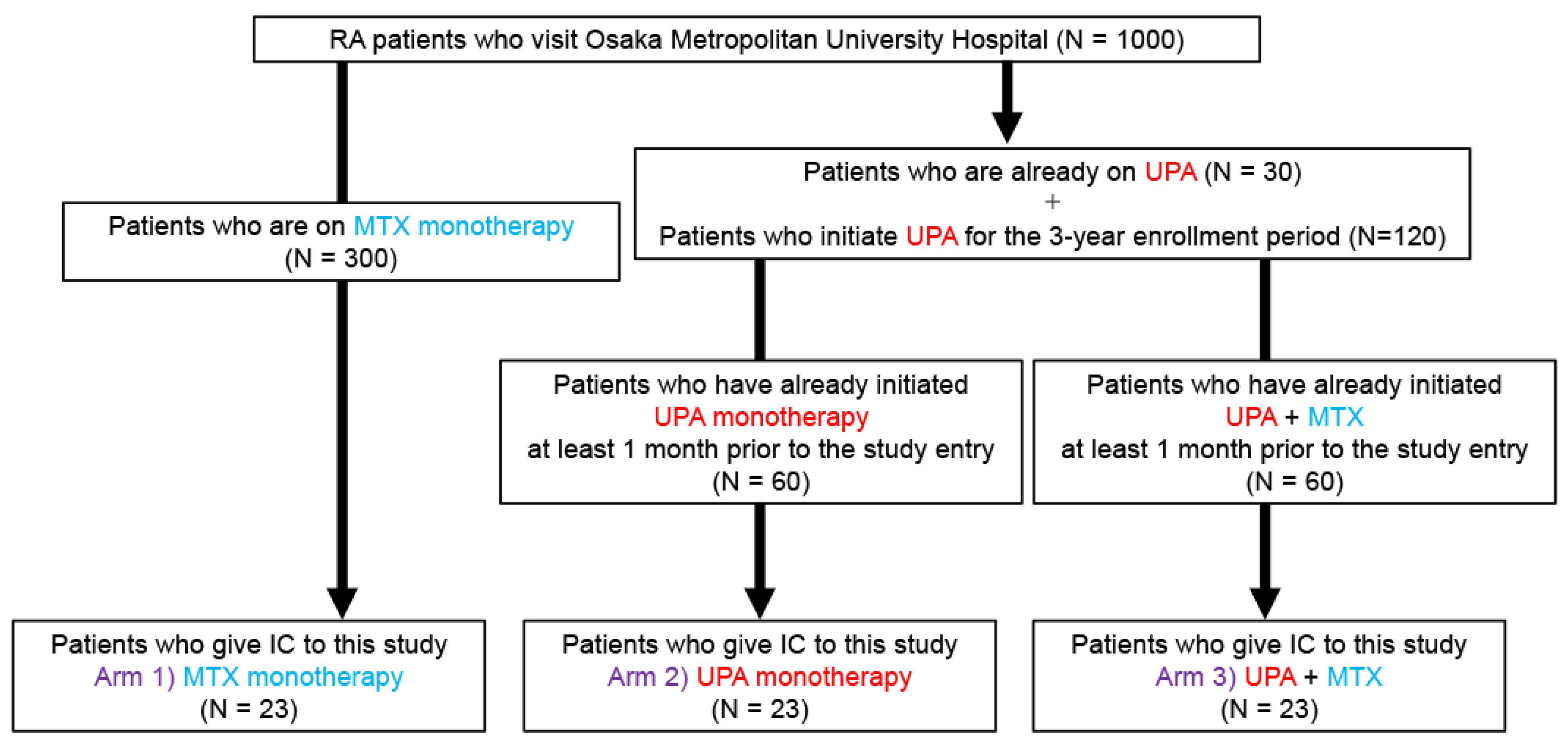
4.5. Recruitment
5. Assignment of Interventions: Allocation
5.1. Sequence Generation
5.2. Concealment Mechanism
5.3. Implementation
5.4. Who Will Be Blinded
5.5. Procedure for Unblinding if Needed
6. Data Collection and Management
6.1. Plans for Assessment and Collection of Outcomes
6.2. Plans to Promote Participant Retention and Complete Follow-Up
6.3. Data Management
6.4. Confidentiality
6.5. Plans for Collection, Laboratory Evaluation, and Storage of Biological Specimens for Genetic or Molecular Analysis in This Trial/Future Use
7. Statistical Methods
7.1. Statistical Methods for Primary and Secondary Outcomes
7.1.1. Full Analysis Set (FAS)
7.1.2. Per Protocol Set (PPS)
- Violation of the inclusion criteria.
- Exclusion criteria violations.
- Medication compliance violation (interruption of oral medication for 14 consecutive days).
- Failure to receive Shingrix® injections.
- Injection of other vaccines between the time of consent and 4 weeks after the second Shingrix® dose.
- Dose changes of anti-rheumatic drugs (e.g., MTX, glucocorticoids, upadacitinib) between the time of consent and 4 weeks after the second Shingrix® dose.
7.1.3. Safety Analysis Set (SAS)
7.2. Primary Endpoint
7.3. Secondary Endpoints
- (1)
- Presence or absence of RA flares from enrollment to 12 weeks after the second Shingrix® dose.
- (2)
- Proportion of positive anti-gE antibodies at 4 weeks after the first Shingrix® dose.
- (3)
- Antibody titre of anti-gE antibodies at 4 weeks after the first and second Shingrix® doses.
- (4)
- Proportion of gE-specific CD4+ T-cells at 4 weeks after the first and second Shingrix® doses.
- (5)
- Antibody titer of VZV-specific antibodies at 4 weeks after first and second Shingrix® doses.
- (6)
- Changes in RA disease activity.
7.4. Interim Analyses
7.5. Methods for Additional Analyses (e.g., Subgroup Analyses)
7.6. Methods in Analysis to Handle Protocol Non-Adherence and Any Statistical Methods to Handle Missing Data
7.6.1. Handling of Patients Enrolled in the Study
7.6.2. Handling of Outliers or Abnormal Values
7.6.3. Handling of Missing Values
7.7. Plans to Give Access to the Full Protocol, Participant Level-Data, and Statistical Code
8. Oversight and Monitoring
8.1. Composition of the Coordinating Centre and Trial Steering Committee
8.2. Composition of the Data Monitoring Committee, Its Role and Reporting Structure
8.3. Adverse Event Reporting and Harms
8.3.1. Definition of Adverse Events
8.3.2. Evaluation for Severity
- (1)
- Mild: when administration can be continued without treatment.
- (2)
- Moderate: when administration can be continued with some kind of treatment.
- (3)
- Severe: when discontinuation is required for the patient.
8.3.3. Evaluation for Seriousness
- (1)
- Serious
- 1.
- Death.
- 2.
- A condition that may lead to death.
- 3.
- A condition that requires hospitalization at a medical institution or an extension of hospitalization for treatment.
- 4.
- Disability.
- 5.
- A condition that may lead to disability.
- 6.
- A condition that is serious according to criteria 1–5.
- 7.
- Congenital diseases or abnormalities in later generations.
- (2)
- Non-Serious
8.3.4. Illness Related to the Study
- (1)
- Definition of illness
- (2)
- Predictability
- (3)
- Causality
8.3.5. Subjects and Reporting Period for Reporting Illness
8.3.6. Procedures for Reporting Illnesses: Procedures in the Event of Illness
- (1)
- Co-investigators report the event to the principal investigator if they become aware of the illness. The principal investigator and co-investigators will then take appropriate measures for the study subject, take the best care, and record it in the medical records.
- (2)
- If the illness is subject to reporting within the deadlines listed in Table 1, the investigators must submit a report to the accredited clinical research review board within the specified reporting deadline.
8.3.7. Procedures for Reporting Illnesses: Procedures of Regular Reporting
- (1)
- The principal investigator will use the jRCT system to report the number of illnesses to the Minister of Health, Labor, and Welfare.
- (2)
- The principal investigator will report the number of illnesses to the administrator of the medical institution and to the accredited clinical research review board within the period specified in Table 1.
- (3)
- With the approval of the accredited clinical research review committee, the principal investigator will use the jRCT system to report to the Regional Bureau of Health and Welfare within the period specified in Table 1.
8.3.8. Observation of Research Subjects After Occurrence of Adverse Events
8.4. Frequency and Plans for Auditing Trial Conduct
8.5. Plans for Communicating Important Protocol Amendments to Relevant Parties (E.G., Trial Participants, Ethical Committees)
- (1)
- When amending the study protocol and informed consent form, the principal investigator must obtain a review from an accredited clinical research review committee prior to the amendment and report the details to the administrator of the implementing medical institution. In the case of changes related to the implementation system or other significant changes, approval from the administrator of the implementing medical institution should be obtained, as necessary. The principal investigator or co-investigator must not conduct the study using a modified research protocol or informed consent form before obtaining approval from the committee or the administrator of the implementing medical institution.
- (2)
- When changes in the study protocol involve changes in the implementation plan, the principal investigator must submit a notification of changes to the Minister of Health, Labor, and Welfare after obtaining approval from the accredited clinical research review committee. The principal investigator or co-investigators must not conduct the study using the modified study protocol and informed consent form before submitting the notification of modification to the Minister of Health, Labor, and Welfare, and before such modification is published in the jRCT.
8.6. Dissemination Plans
9. Discussion
10. Trial Status
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AST | aspartate aminotransferase |
| ALT | alanine aminotransferase |
| BUN | blood urea nitrogen |
| CDAI | clinical disease activity index |
| CRP | C-reactive protein |
| DAS | disease activity score |
| ESR | erythrocyte sedimentation rate |
| gE | glycoprotein E |
| JAK | Janus kinase |
| jRCT | Japan Registry of Clinical Trials |
| RA | rheumatoid arthritis |
| SDAI | simplified disease activity index |
| VZV | varicella-zoster virus |
References
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewe, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 2023, 82, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Roodenrijs, N.M.T.; Welsing, P.M.; Kedves, M.; Hamar, A.; van der Goes, M.C.; Kent, A.; Bakkers, M.; Blaas, E.; Senolt, L.; et al. EULAR definition of difficult-to-treat rheumatoid arthritis. Ann. Rheum. Dis. 2021, 80, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Okano, T.; Gon, T.; Yoshida, N.; Fukumoto, K.; Yamada, S.; Hashimoto, M. Difficult-to-treat rheumatoid arthritis: Current concept and unsolved problems. Front. Med. 2022, 9, 1049875. [Google Scholar] [CrossRef]
- Harigai, M.; Honda, S. Selectivity of Janus Kinase Inhibitors in Rheumatoid Arthritis and Other Immune-Mediated Inflammatory Diseases: Is Expectation the Root of All Headache? Drugs 2020, 80, 1183–1201. [Google Scholar] [CrossRef]
- Lal, H.; Cunningham, A.L.; Godeaux, O.; Chlibek, R.; Diez-Domingo, J.; Hwang, S.J.; Levin, M.J.; McElhaney, J.E.; Poder, A.; Puig-Barbera, J.; et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N. Engl. J. Med. 2015, 372, 2087–2096. [Google Scholar] [CrossRef]
- Kallmark, H.; Einarsson, J.T.; Nilsson, J.A.; Olofsson, T.; Saxne, T.; Geborek, P.; C. Kapetanovic, M. Sustained Remission in Patients With Rheumatoid Arthritis Receiving Triple Therapy Compared to Biologic Therapy: A Swedish Nationwide Register Study. Arthritis Rheumatol. 2021, 73, 1135–1144. [Google Scholar] [CrossRef]
- Stevens, E.; Weinblatt, M.E.; Massarotti, E.; Griffin, F.; Emani, S.; Desai, S. Safety of the Zoster Vaccine Recombinant Adjuvanted in Rheumatoid Arthritis and Other Systemic Rheumatic Disease Patients: A Single Center’s Experience With 400 Patients. ACR Open Rheumatol. 2020, 2, 357–361. [Google Scholar] [CrossRef]
- Dagnew, A.F.; Rausch, D.; Herve, C.; Zahaf, T.; Levin, M.J.; Schuind, A.; group, Z.O.E.s. Efficacy and serious adverse events profile of the adjuvanted recombinant zoster vaccine in adults with pre-existing potential immune-mediated diseases: A pooled post hoc analysis on two parallel randomized trials. Rheumatology 2021, 60, 1226–1233. [Google Scholar] [CrossRef]
- Lenfant, T.; Jin, Y.; Kirchner, E.; Hajj-Ali, R.A.; Calabrese, L.H.; Calabrese, C. Safety of recombinant zoster vaccine: A retrospective study of 622 rheumatology patients. Rheumatology 2021, 60, 5149–5157. [Google Scholar] [CrossRef]
- Vink, P.; Ramon Torrell, J.M.; Sanchez Fructuoso, A.; Kim, S.J.; Kim, S.I.; Zaltzman, J.; Ortiz, F.; Campistol Plana, J.M.; Fernandez Rodriguez, A.M.; Rebollo Rodrigo, H.; et al. Immunogenicity and Safety of the Adjuvanted Recombinant Zoster Vaccine in Chronically Immunosuppressed Adults Following Renal Transplant: A Phase 3, Randomized Clinical Trial. Clin. Infect. Dis. 2020, 70, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.J.; Oxman, M.N.; Zhang, J.H.; Johnson, G.R.; Stanley, H.; Hayward, A.R.; Caulfield, M.J.; Irwin, M.R.; Smith, J.G.; Clair, J.; et al. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J. Infect. Dis. 2008, 197, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.J.; Smith, J.G.; Kaufhold, R.M.; Barber, D.; Hayward, A.R.; Chan, C.Y.; Chan, I.S.; Li, D.J.; Wang, W.; Keller, P.M.; et al. Decline in varicella-zoster virus (VZV)-specific cell-mediated immunity with increasing age and boosting with a high-dose VZV vaccine. J. Infect. Dis. 2003, 188, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Cavanagh, M.M.; Le Saux, S.; Wagar, L.E.; Mackey, S.; Hu, J.; Maecker, H.; Swan, G.E.; Davis, M.M.; Dekker, C.L.; et al. Defective T Memory Cell Differentiation after Varicella Zoster Vaccination in Older Individuals. PLoS Pathog. 2016, 12, e1005892. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Heineman, T.C.; Lal, H.; Godeaux, O.; Chlibek, R.; Hwang, S.J.; McElhaney, J.E.; Vesikari, T.; Andrews, C.; Choi, W.S.; et al. Immune Responses to a Recombinant Glycoprotein E Herpes Zoster Vaccine in Adults Aged 50 Years or Older. J. Infect. Dis. 2018, 217, 1750–1760. [Google Scholar] [CrossRef]
- Ishihara, R.; Watanabe, R.; Shiomi, M.; Katsushima, M.; Fukumoto, K.; Yamada, S.; Okano, T.; Hashimoto, M. Exploring the Link between Varicella-Zoster Virus, Autoimmune Diseases, and the Role of Recombinant Zoster Vaccine. Biomolecules 2024, 14, 739. [Google Scholar] [CrossRef]
- Nakayama, Y.; Watanabe, R.; Yamamoto, W.; Ebina, K.; Hirano, T.; Kotani, T.; Shiba, H.; Katayama, M.; Son, Y.; Amuro, H.; et al. IL-6 inhibitors and JAK inhibitors as favourable treatment options for patients with anaemia and rheumatoid arthritis: ANSWER cohort study. Rheumatology 2023, 63, 349–357. [Google Scholar] [CrossRef]
- Watanabe, R.; Hashimoto, M.; Murata, K.; Murakami, K.; Tanaka, M.; Ohmura, K.; Ito, H.; Matsuda, S. Prevalence and predictive factors of difficult-to-treat rheumatoid arthritis: The KURAMA cohort. Immunol. Med. 2022, 45, 35–44. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; McShane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Yndestad, A.; Henrohn, D.; Danese, S.; Marsal, S.; Galindo, M.; Woolcott, J.C.; Jo, H.; Kwok, K.; Shapiro, A.B.; et al. Influenza Adverse Events in Patients with Rheumatoid Arthritis, Ulcerative Colitis, or Psoriatic Arthritis in the Tofacitinib Clinical Development Programs. Rheumatol. Ther. 2023, 10, 357–373. [Google Scholar] [CrossRef] [PubMed]
- Hastie, A.; Catteau, G.; Enemuo, A.; Mrkvan, T.; Salaun, B.; Volpe, S.; Smetana, J.; Rombo, L.; Schwarz, T.; Pauksens, K.; et al. Immunogenicity of the Adjuvanted Recombinant Zoster Vaccine: Persistence and Anamnestic Response to Additional Doses Administered 10 Years After Primary Vaccination. J. Infect. Dis. 2021, 224, 2025–2034. [Google Scholar] [CrossRef] [PubMed]
- Strezova, A.; Diez-Domingo, J.; Al Shawafi, K.; Tinoco, J.C.; Shi, M.; Pirrotta, P.; Mwakingwe-Omari, A.; Zoster-049 Study, G. Long-term Protection Against Herpes Zoster by the Adjuvanted Recombinant Zoster Vaccine: Interim Efficacy, Immunogenicity, and Safety Results up to 10 Years After Initial Vaccination. Open Forum Infect. Dis. 2022, 9, ofac485. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.L.; Lal, H.; Kovac, M.; Chlibek, R.; Hwang, S.J.; Diez-Domingo, J.; Godeaux, O.; Levin, M.J.; McElhaney, J.E.; Puig-Barbera, J.; et al. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N. Engl. J. Med. 2016, 375, 1019–1032. [Google Scholar] [CrossRef]
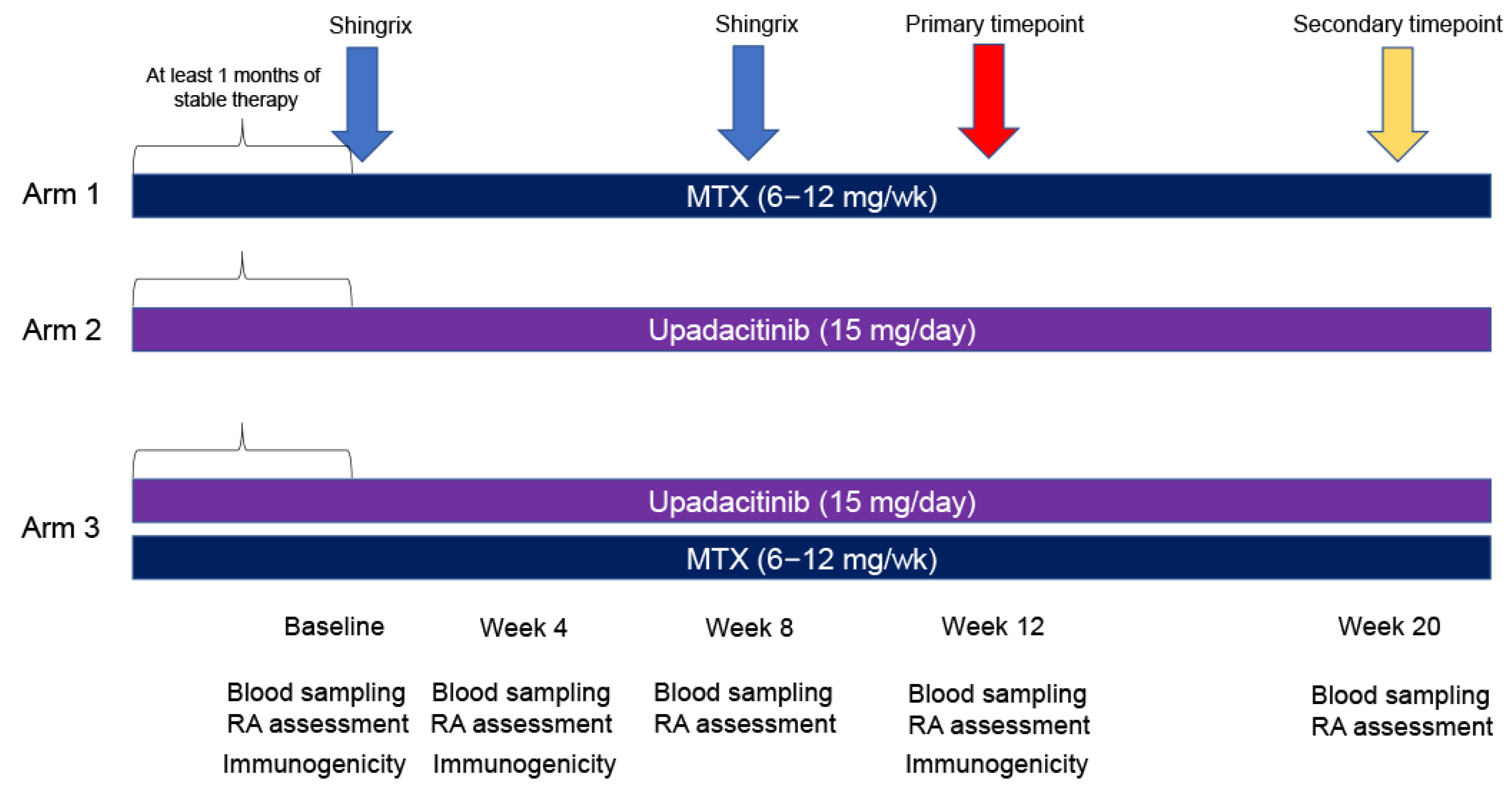
| Before Enrollment | At Enrollment | Week 4 | Week 8 | Week 12 | Week 20 | |
|---|---|---|---|---|---|---|
| Visit | 0 | 1 | 2 | 3 | 4 | 5 |
| Allowance | +1 week | +2 week | +3 week * 1 | −2~+4 week * 2 | ||
| Informed consent | X | |||||
| Eligibility screen | X | |||||
| Shingrix® injection | X | X | ||||
| Disease activity of RA | X | X | X | X | X | X |
| Assessment of adverse events | X | X | X | X | X | |
| Blood tests * 3 Urine tests * 4 Serum storage | X | X | X | X | X | X |
| Immunogenicity * 5 | X | X | X | |||
| Additional blood collection | X | X | X | |||
| Confirmation of concomitant drug | X | X | X | X | X | X |
| RA flare | X |
| Regional Bureau of Health and Welfare | Accredited Clinical Research Review Board | ||||
|---|---|---|---|---|---|
| Type of Report | Regular Report | On-Time Report | Regular Report | ||
| Pharmaceuticals | Known | Death | 〇 | 15 days | 〇 |
| Serious condition other than death | 〇 | 15 days | 〇 | ||
| Non-serious | 〇 | ― | 〇 | ||
| Unknown | Death | 〇 | 15 days | 〇 | |
| Serious condition other than death | 〇 | 30 days | 〇 | ||
| Non-serious | 〇 | ― | 〇 | ||
| Infection | Known | Death/Serious | 〇 | 15 days | 〇 |
| Non-serious | 〇 | 15 days | 〇 | ||
| Unknown | Death/Serious | 〇 | 15 days | 〇 | |
| Non-serious | 〇 | ― | 〇 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, R.; Fujii, H.; Imai, T.; Furusawa, Y.; Katsushima, M.; Fukumoto, K.; Yamada, Y.; Mamoto, K.; Okano, T.; Yamada, S.; et al. Immune Response to an Adjuvanted Recombinant Zoster Vaccine in Japanese Patients with Rheumatoid Arthritis Receiving Upadacitinib (End Zoster-J Study): Study Protocol for an Exploratory Parallel Triple-Arm Prospective Trial. J. Clin. Med. 2024, 13, 7321. https://doi.org/10.3390/jcm13237321
Watanabe R, Fujii H, Imai T, Furusawa Y, Katsushima M, Fukumoto K, Yamada Y, Mamoto K, Okano T, Yamada S, et al. Immune Response to an Adjuvanted Recombinant Zoster Vaccine in Japanese Patients with Rheumatoid Arthritis Receiving Upadacitinib (End Zoster-J Study): Study Protocol for an Exploratory Parallel Triple-Arm Prospective Trial. Journal of Clinical Medicine. 2024; 13(23):7321. https://doi.org/10.3390/jcm13237321
Chicago/Turabian StyleWatanabe, Ryu, Hisako Fujii, Takumi Imai, Yuki Furusawa, Masao Katsushima, Kazuo Fukumoto, Yutaro Yamada, Kenji Mamoto, Tadashi Okano, Shinsuke Yamada, and et al. 2024. "Immune Response to an Adjuvanted Recombinant Zoster Vaccine in Japanese Patients with Rheumatoid Arthritis Receiving Upadacitinib (End Zoster-J Study): Study Protocol for an Exploratory Parallel Triple-Arm Prospective Trial" Journal of Clinical Medicine 13, no. 23: 7321. https://doi.org/10.3390/jcm13237321
APA StyleWatanabe, R., Fujii, H., Imai, T., Furusawa, Y., Katsushima, M., Fukumoto, K., Yamada, Y., Mamoto, K., Okano, T., Yamada, S., & Hashimoto, M. (2024). Immune Response to an Adjuvanted Recombinant Zoster Vaccine in Japanese Patients with Rheumatoid Arthritis Receiving Upadacitinib (End Zoster-J Study): Study Protocol for an Exploratory Parallel Triple-Arm Prospective Trial. Journal of Clinical Medicine, 13(23), 7321. https://doi.org/10.3390/jcm13237321







