High-Density Lipoprotein Predicts Intrahospital Mortality in Influenza
Abstract
1. Introduction
2. Methods
3. Results
3.1. Patient Characteristics
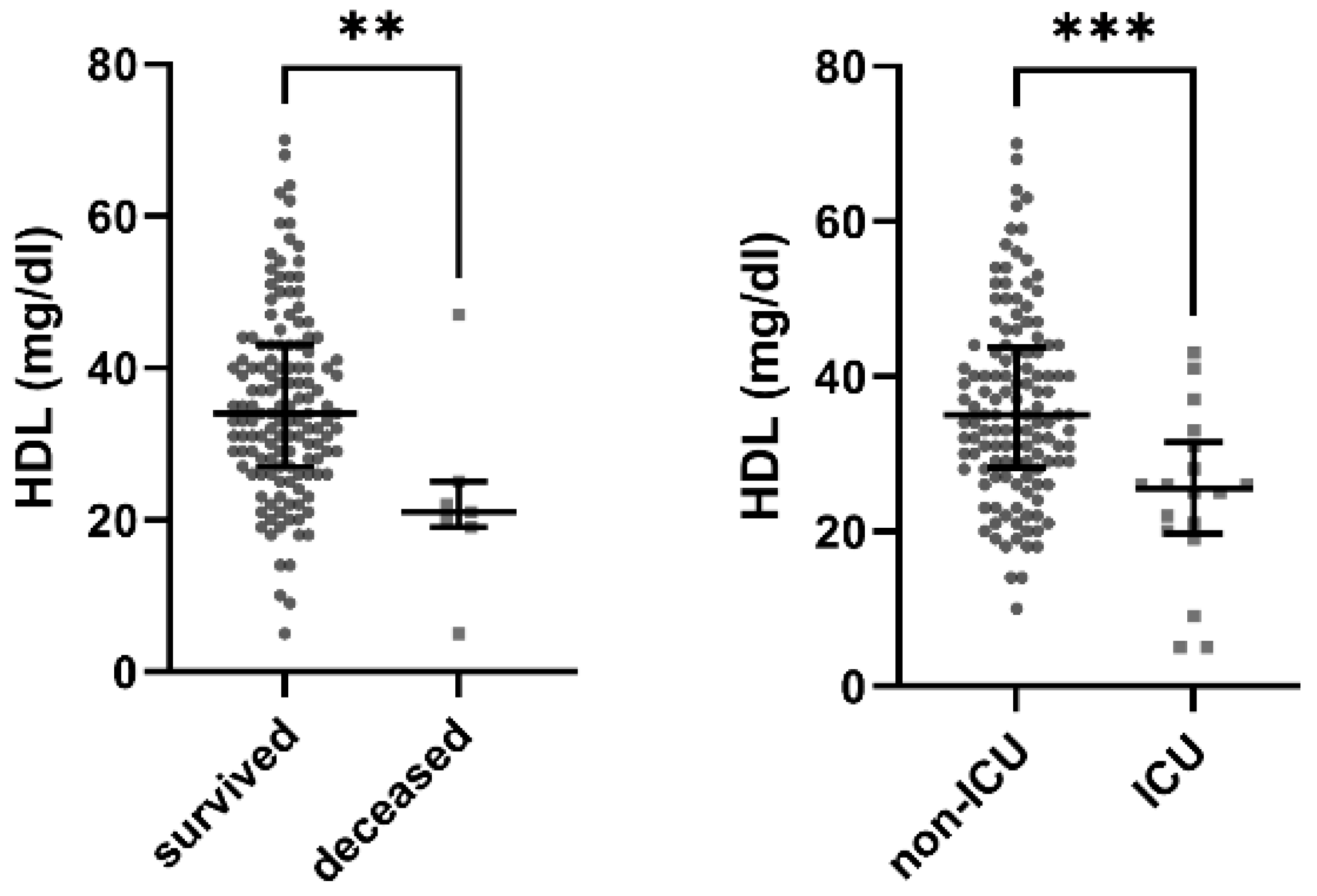
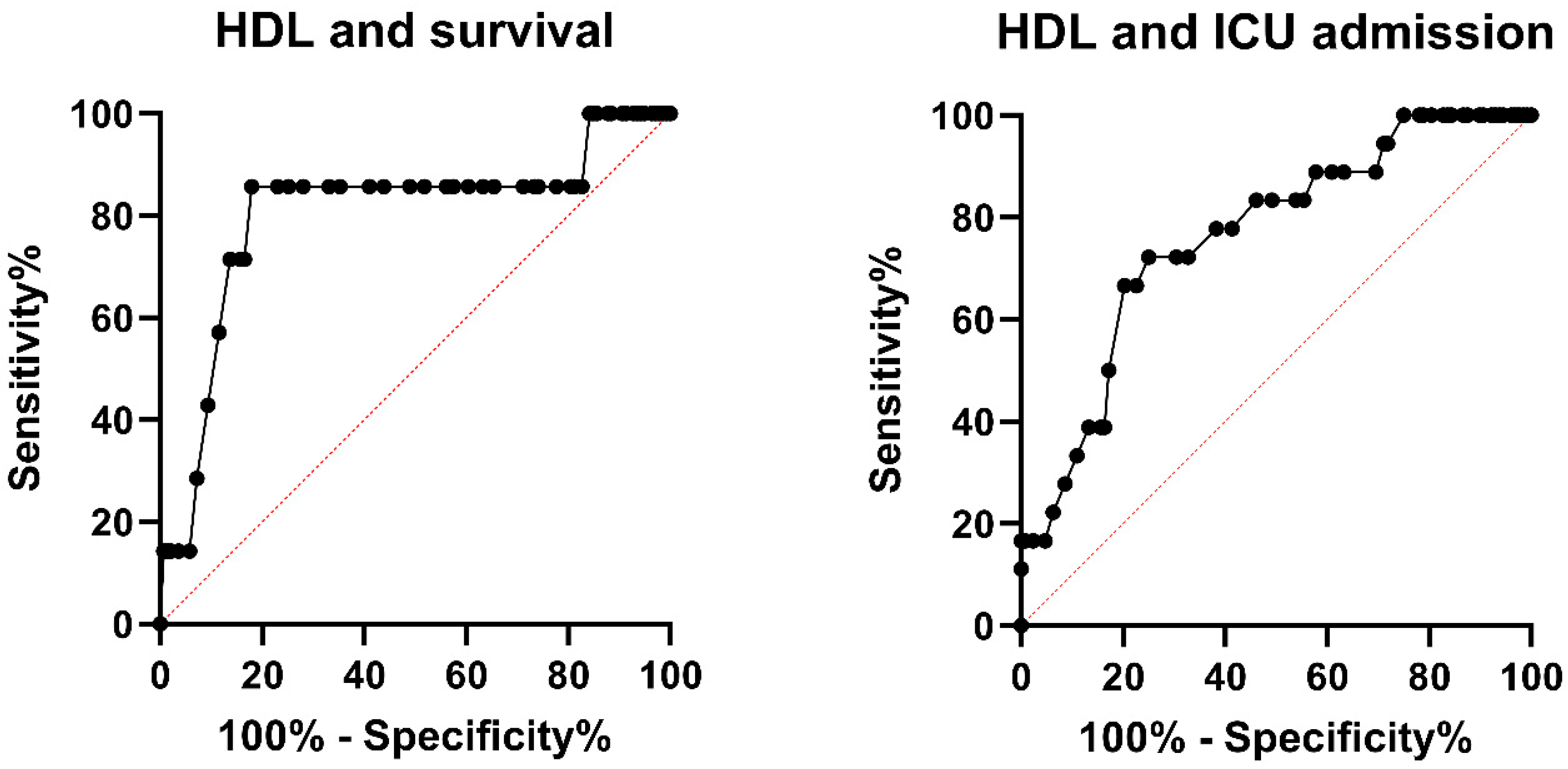
3.2. Low HDL Is a Significant Predictor for Death
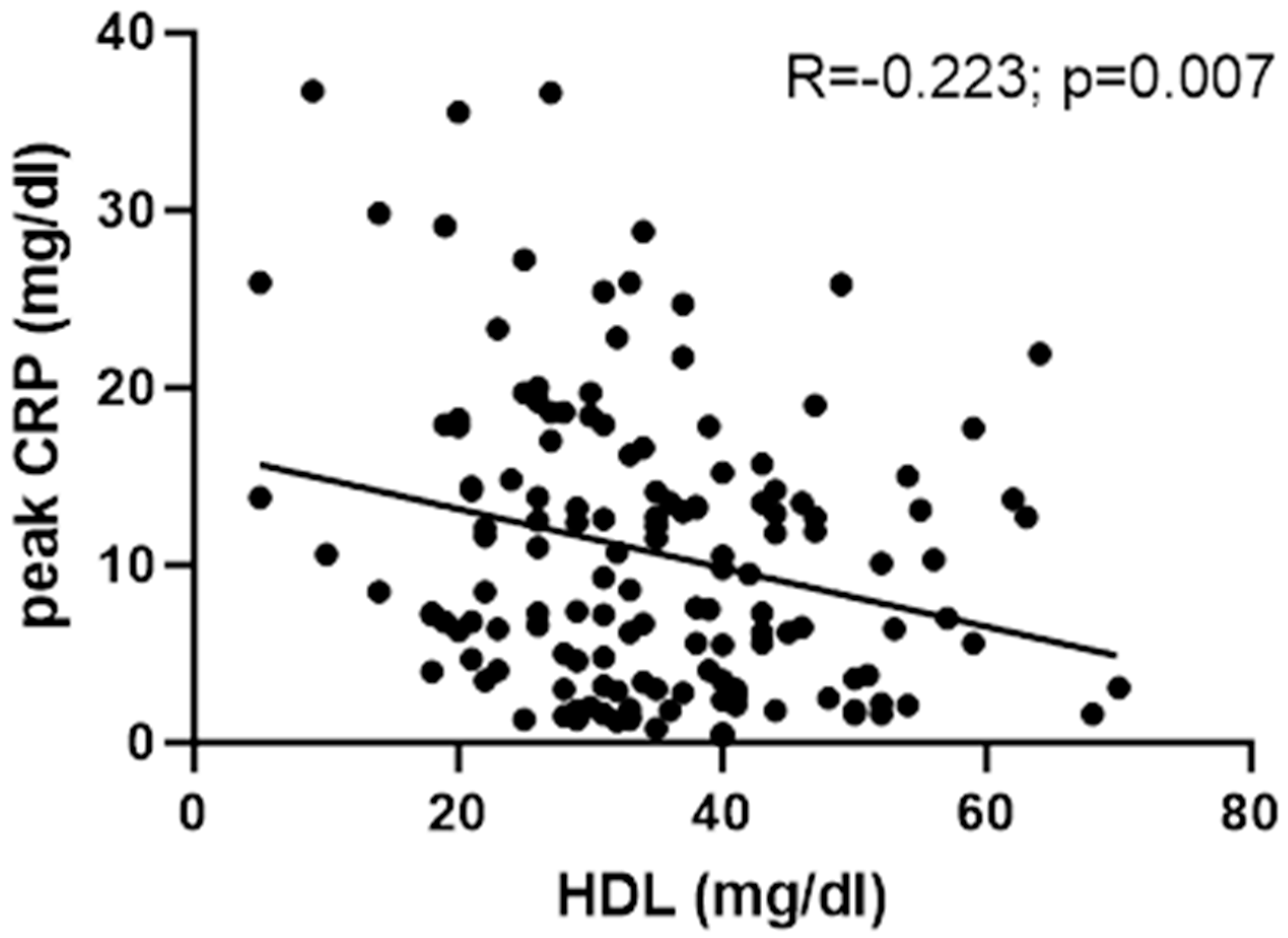
3.3. HDL and Inflammation Parameters
4. Discussion
4.1. HDL as a Prognostic Marker in Influenza
4.2. The 2018/19 and 2019/20 Influenza Seasons and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BNP | B-type natriuretic peptide |
| CRP | C-reactive protein |
| eGFR | Estimated glomerular filtration rate |
| HDL | High-density lipoprotein |
| IQR | Interquartile range |
| ICU | Intensive care unit |
| LDL | Low-density lipoprotein |
| LPS | Lipopolysaccharide |
| LTA | Lipoteichoic acid |
| MDRD | Modification of Diet in Renal Disease |
| OR | Odds ratio |
| PAMPs | Pathogen-associated molecular patterns |
| ROC | Receiver operator characteristic |
| SHR | Stress hyperglycaemia ratio |
References
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. J. Prev. Cardiol. 2022, 29, 5–115. [Google Scholar] [CrossRef]
- Rawshani, A.; Rawshani, A.; Franzén, S.; Sattar, N.; Eliasson, B.; Svensson, A.M.; Zethelius, B.; Miftaraj, M.; McGuire, D.K.; Rosengren, A.; et al. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2018, 379, 633–644. [Google Scholar] [CrossRef]
- Liang, Y.; Vetrano, D.L.; Qiu, C. Serum total cholesterol and risk of cardiovascular and non-cardiovascular mortality in old age: A population-based study. BMC Geriatr. 2017, 17, 294. [Google Scholar] [CrossRef]
- Ravnskov, U.; Diamond, D.M.; Hama, R.; Hamazaki, T.; Hammarskjold, B.; Hynes, N.; Kendrick, M.; Langsjoen, P.H.; Malhotra, A.; Mascitelli, L.; et al. Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: A systematic review. BMJ Open 2016, 6, e010401. [Google Scholar] [CrossRef]
- Kaysen, G.A.; Ye, X.; Raimann, J.G.; Wang, Y.; Topping, A.; Usvyat, L.A.; Stuard, S.; Canaud, B.; van der Sande, F.M.; Kooman, J.P.; et al. Lipid levels are inversely associated with infectious and all-cause mortality: International MONDO study results. J. Lipid Res. 2018, 59, 1519–1528. [Google Scholar] [CrossRef]
- Cao, H.; Huang, W. HDL and Sepsis. Adv. Exp. Med. Biol. 2022, 1377, 129–139. [Google Scholar] [CrossRef]
- Catapano, A.L.; Pirillo, A.; Bonacina, F.; Norata, G.D. HDL in innate and adaptive immunity. Cardiovasc. Res. 2014, 103, 372–383. [Google Scholar] [CrossRef]
- Heinzl, M.W.; Resl, M.; Klammer, C.; Egger, M.; Dieplinger, B.; Clodi, M. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Is Not Induced in Artificial Human Inflammation and Is Not Correlated with Inflammatory Response. Infect. Immun. 2020, 88, e00842-19. [Google Scholar] [CrossRef]
- Walley, K.R.; Francis, G.A.; Opal, S.M.; Stein, E.A.; Russell, J.A.; Boyd, J.H. The Central Role of Proprotein Convertase Subtilisin/Kexin Type 9 in Septic Pathogen Lipid Transport and Clearance. Am. J. Respir. Crit. Care Med. 2015, 192, 1275–1286. [Google Scholar] [CrossRef]
- Birjmohun, R.S.; van Leuven, S.I.; Levels, J.H.; van’t Veer, C.; Kuivenhoven, J.A.; Meijers, J.C.; Levi, M.; Kastelein, J.J.; van der Poll, T.; Stroes, E.S. High-density lipoprotein attenuates inflammation and coagulation response on endotoxin challenge in humans. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1153–1158. [Google Scholar] [CrossRef]
- Madsen, C.M.; Varbo, A.; Tybjaerg-Hansen, A.; Frikke-Schmidt, R.; Nordestgaard, B.G. U-shaped relationship of HDL and risk of infectious disease: Two prospective population-based cohort studies. Eur. Heart J. 2018, 39, 1181–1190. [Google Scholar] [CrossRef]
- Lahoz, C.; Salinero-Fort, M.A.; Cardenas, J.; Rodriguez-Artalejo, F.; Diaz-Almiron, M.; Vich-Perez, P.; San Andres-Rebollo, F.J.; Vicente, I.; Mostaza, J.M. HDL-cholesterol concentration and risk of SARS-CoV-2 infection in people over 75 years of age: A cohort with half a million participants from the Community of Madrid. Clin. Investig. Arterioscler. 2022, 34, 113–119. [Google Scholar] [CrossRef]
- Biller, K.; Fae, P.; Germann, R.; Drexel, H.; Walli, A.K.; Fraunberger, P. Cholesterol rather than procalcitonin or C-reactive protein predicts mortality in patients with infection. Shock 2014, 42, 129–132. [Google Scholar] [CrossRef]
- Barlage, S.; Gnewuch, C.; Liebisch, G.; Wolf, Z.; Audebert, F.X.; Gluck, T.; Frohlich, D.; Kramer, B.K.; Rothe, G.; Schmitz, G. Changes in HDL-associated apolipoproteins relate to mortality in human sepsis and correlate to monocyte and platelet activation. Intensive Care Med. 2009, 35, 1877–1885. [Google Scholar] [CrossRef]
- Chien, J.Y.; Jerng, J.S.; Yu, C.J.; Yang, P.C. Low serum level of high-density lipoprotein cholesterol is a poor prognostic factor for severe sepsis. Crit. Care Med. 2005, 33, 1688–1693. [Google Scholar] [CrossRef]
- Cirstea, M.; Walley, K.R.; Russell, J.A.; Brunham, L.R.; Genga, K.R.; Boyd, J.H. Decreased high-density lipoprotein cholesterol level is an early prognostic marker for organ dysfunction and death in patients with suspected sepsis. J. Crit. Care 2017, 38, 289–294. [Google Scholar] [CrossRef]
- Obendorf, F.; Klammer, C.; Heinzl, M.; Egger-Salmhofer, M.; Resl, M.; Dieplinger, B.; Clodi, M. Correction to: Intrahospital mortality of influenza patients during the 2017–2018 influenza season: Report from a tertiary care hospital in Austria. Wien. Klin. Wochenschr. 2020, 132, 215. [Google Scholar] [CrossRef]
- Roberts, G.W.; Quinn, S.J.; Valentine, N.; Alhawassi, T.; O’Dea, H.; Stranks, S.N.; Burt, M.G.; Doogue, M.P. Relative Hyperglycemia, a Marker of Critical Illness: Introducing the Stress Hyperglycemia Ratio. J. Clin. Endocrinol. Metab. 2015, 100, 4490–4497. [Google Scholar] [CrossRef]
- Walley, K.R.; Thain, K.R.; Russell, J.A.; Reilly, M.P.; Meyer, N.J.; Ferguson, J.F.; Christie, J.D.; Nakada, T.A.; Fjell, C.D.; Thair, S.A.; et al. PCSK9 is a critical regulator of the innate immune response and septic shock outcome. Sci. Transl. Med. 2014, 6, 258ra143. [Google Scholar] [CrossRef]
- Norata, G.D.; Tavori, H.; Pirillo, A.; Fazio, S.; Catapano, A.L. Biology of proprotein convertase subtilisin kexin 9: Beyond low-density lipoprotein cholesterol lowering. Cardiovasc. Res. 2016, 112, 429–442. [Google Scholar] [CrossRef]
- Mostaza, J.M.; Salinero-Fort, M.A.; Cardenas-Valladolid, J.; Rodriguez-Artalejo, F.; Diaz-Almiron, M.; Vich-Perez, P.; San Andres-Rebollo, F.J.; Vicente, I.; Lahoz, C. Pre-infection HDL-cholesterol levels and mortality among elderly patients infected with SARS-CoV-2. Atherosclerosis 2022, 341, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Hament, J.M.; Kimpen, J.L.; Fleer, A.; Wolfs, T.F. Respiratory viral infection predisposing for bacterial disease: A concise review. FEMS Immunol. Med. Microbiol. 1999, 26, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Morens, D.M.; Taubenberger, J.K.; Fauci, A.S. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: Implications for pandemic influenza preparedness. J. Infect. Dis. 2008, 198, 962–970. [Google Scholar] [CrossRef] [PubMed]
- van der Sluijs, K.F.; van der Poll, T.; Lutter, R.; Juffermans, N.P.; Schultz, M.J. Bench-to-bedside review: Bacterial pneumonia with influenza—Pathogenesis and clinical implications. Crit. Care 2010, 14, 219. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Survived | Deceased | Overall | |
|---|---|---|---|---|
| Hospitalised patients | 190 | 9 | 199 | |
| Male (%) | 164 (86.3) | 9 (100) | 173 (86.0) | |
| Female (%) | 26 (13.7) | 0 (0) | 26 (13.1) | |
| Influenza A (%) | 183 (96.3) | 9 (100) | 192 (96.5) | |
| Influenza B (%) | 7 (3.7) | 0 (0) | 7 (3.5) | |
| ICU admission (%) | 16 (8.4) | 7 (77.8) | 23 (11.6) | |
| Endotracheal intubation (%) | 4 (2.1) | 3 (33.3) | 7 (3.5) | |
| Oseltamivir (%) | 79 (73.8) | 2 (40.0) | 81 (72.3) | |
| Antibiotics (%) | 118 (62.1) | 9 (100) | 127 (63.8) | |
| Lipid lowering therapy (%) | 59 (31.1) | 2 (22.2) | 61 (30.7) | |
| Number of comorbidities (diabetes mellitus, arterial hypertension, kidney disease, coronary heart disease, COPD, and malignant disease) | ||||
| 0 comorbidities (%) | 56 (29.5) | 1 (11.1) | 57 (28.6) | p = 0.046 |
| 1 comorbidity (%) | 43 (22.6) | 0 (0) | 43 (21.6) | |
| 2 comorbidities (%) | 40 (21.1) | 5 (55.6) | 45 (22.6) | |
| ≥3 comorbidities (%) | 51 (26.8) | 3 (33.3) | 54 (27.1) | |
| Malignant disease (%) | 37 (19.5) | 3 (33.3) | 40 (20.1) | p = 0.388 |
| BMI (kg/m2) | 26.2 (23.6–29.7) | 29.6 (24.5–32.5) | 26.2 (23.8–29.8) | p = 0.358 |
| Age (years) | 69 (56–80) | 72 (70–84) | 70 (56–80) | p = 0.192 |
| Body temperature (°C) | 37.8 (36.8–38.6) | 37.0 (36.6–37.9) | 37.8 (36.8–38.6) | p = 0.288 |
| CRP (initial, mg/dL) | 3.5 (1.5–7.3) | 6.9 (1.5–14.7) | 3.5 (1.5–7.4) | p = 0.205 |
| CRP (peak, mg/dL) | 7.3 (3.0–13.3) | 19.0 (13.8–27.2) | 7.5 (3.2–13.8) | p < 0.001 |
| Creatinine (initial, mg/dL) | 1.05 (0.89–1.36) | 1.06 (0.97–2.41) | 1.06 (0.89–1.38) | p = 0.182 |
| eGFR-MDRD (initial, ml/min/1.73 m2) | 73 (54–90) | 73 (29–78) | 73 (54–90) | p = 0.219 |
| Troponine-I (initial, ng/L) | 12 (5–28) | 59 (11–143) | 13 (5–30) | p = 0.011 |
| BNP (initial, pg/mL) | 110 (31–280) | 198 (105–361) | 118 (35–290) | p = 0.137 |
| HBA1c (%) | 5.8 (5.4–6.5) | 5.7 (5.3–5.8) | 5.8 (5.4–6.5) | p = 0.523 |
| Admission blood glucose (mg/dL) | 118 (101–146) | 128 (116–146) | 118 (101–146) | p = 0.352 |
| Stress hyperglycaemia ratio (SHR) | 0.97 (0.86–1.20) | 1.13 (1.07–1.39) | 0.99 (0.86–1.20) | p = 0.112 |
| Total cholesterol (mg/dL) | 142 (112–165) | 101 (92–115) | 139 (111–164) | p = 0.027 |
| Triglycerides (mg/dL) | 95 (75–138) | 106 (58–96) | 96 (75–135) | p = 0.966 |
| LDL (mg/dL) | 82 (62–108) | 62 (60–67) | 82 (62–107) | p = 0.120 |
| HDL (mg/dL) | 35 (28–44) | 21 (19–25) | 34 (27–43) | p = 0.005 |
| Duration of stay (days) | 5 (3–10) | 10 (6–16) | 5 (3–10) | p = 0.084 |
| Duration of stay ICU (days) | 0 (0–0) | 2 (1–10) | 0 (0–0) | p < 0.001 |
| Parameters | No ICU Treatment | ICU Treatment | Overall | |
|---|---|---|---|---|
| Hospitalised patients | 176 | 23 | 199 | |
| Male (%) | 151 (85.8) | 22 | 173 (86.0) | |
| Female (%) | 25 (14.2) | 1 (4.3) | 26 (13.1) | |
| Influenza A (%) | 169 (96.0) | 23 (100) | 192 (96.5) | |
| Influenza B (%) | 7 (4.0) | 0 (0) | 7 (3.5) | |
| Endotracheal intubation (%) | 0 (0) | 7 (30.4) | 7 (3.5) | |
| Oseltamivir (%) | 71 (71.2) | 10 (66.7) | 81 (72.3) | |
| Antibiotics (%) | 107 (60.8) | 20 (87.0) | 127 (63.8) | |
| Lipid lowering therapy (%) | 55 (31.3) | 6 (26.1) | 61 (30.7) | |
| Number of comorbidities (diabetes mellitus, arterial hypertension, kidney disease, coronary heart disease, COPD, and malignant disease) | ||||
| 0 comorbidities (%) | 52 (29.5) | 5 (21.7) | 57 (28.6) | p = 0.048 |
| 1 comorbidity (%) | 42 (23.9) | 1 (4.3) | 43 (21.6) | |
| 2 comorbidities (%) | 38 (21.6) | 7 (30.4) | 45 (22.6) | |
| ≥3 comorbidities (%) | 44 (25.0) | 10 (43.5) | 54 (27.1) | |
| Malignant disease (%) | 32 (18.2) | 8 (34.8) | 40 (20.1) | p = 0.092 |
| BMI (kg/m2) | 26.4 (23.9–29.7) | 24.5 (22.7–32.5) | 26.2 (23.8–29.8) | p = 0.672 |
| Age (years) | 70 (56–81) | 69 (59–73) | 70 (56–80) | p = 0.497 |
| Body temperature (°C) | 37.9 (36.9–38.6) | 37.0 (36.4–38.1) | 37.8 (36.8–38.6) | p = 0.056 |
| CRP (initial, mg/dL) | 3.5 (1.5–7.2) | 3.6 (1.4–13.8) | 3.5 (1.5–7.4) | p = 0.347 |
| CRP (peak, mg/dL) | 7.2 (3.0–12.8) | 18.6 (13.3–25.9) | 7.5 (3.2–13.8) | p < 0.001 |
| Creatinine (initial, mg/dL) | 1.05 (0.89–1.35) | 1.15 (0.89–2.415) | 1.06 (0.89–1.38) | p = 0.246 |
| eGFR-MDRD (initial, ml/min/1.73 m2) | 75 (55–90) | 65 (31–90) | 73 (54–90) | p = 0.334 |
| Troponine-I (initial, ng/L) | 12 (5–29) | 15 (9–59) | 13 (5–30) | p = 0.149 |
| BNP (initial, pg/mL) | 122 (33–291) | 98 (58–198) | 118 (35–290) | p = 0.829 |
| HBA1c (%) | 5.9 (5.5–6.5) | 5.6 (5.1–5.9) | 5.8 (5.4–6.5) | p = 0.014 |
| Admission blood glucose (mg/dL) | 117 (101–145) | 132 (101–167) | 118 (101–146) | p = 0.017 |
| Stress hyperglycaemia ratio (SHR) | 0.95 (0.86–1.18) | 1.17 (0.97–1.39) | 0.99 (0.86–1.20) | p = 0.017 |
| Total cholesterol (mg/dL) | 143 (116–167) | 101 (92–133) | 139 (111–164) | p < 0.001 |
| Triglycerides (mg/dL) | 95 (75–131) | 106 (74–148) | 96 (75–135) | p = 0.426 |
| LDL (mg/dL) | 82 (62–112) | 62 (52–82) | 82 (62–107) | p = 0.011 |
| HDL (mg/dL) | 35 (29–44) | 26 (20–33) | 34 (27–43) | p < 0.001 |
| Duration of stay (days) | 5 (3–8) | 16 (10–30) | 5 (3–10) | p < 0.001 |
| Duration of stay ICU (days) | 0 (0–0) | 2 (2–10) | 0 (0–0) | p < 0.001 |
| Parameters | No ICU treatment | ICU treatment | Overall |
| Parameters * | Odds Ratio (OR) | OR 95% Confidence Interval | p-Value |
|---|---|---|---|
| CRP | 1.075 | 0.985–1.173 | 0.104 |
| BMI | 1.058 | 0.906–1.236 | 0.475 |
| Body temperature | 0.752 | 0.434–1.304 | 0.310 |
| Creatinine initial | 1.616 | 0.621–4.206 | 0.326 |
| Glucose | 1 | 0.989–1.012 | 0.985 |
| HbA1c | 0.672 | 0.275–1.643 | 0.384 |
| SHR | 4.785 | 0.451–50.773 | 0.194 |
| LDL | 0.970 | 0.932–1.011 | 0.146 |
| HDL | 0.891 | 0.817–0.972 | 0.009 |
| Total cholesterol | 0.975 | 0.952–0.999 | 0.039 |
| Troponin-I | 1 | 0.999–1.001 | 0.852 |
| BNP | 1 | 0.998–1.002 | 0.856 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heinzl, M.W.; Freudenthaler, M.; Fellinger, P.; Kolenchery, L.; Resl, M.; Klammer, C.; Obendorf, F.; Schinagl, L.; Berger, T.; Egger, M.; et al. High-Density Lipoprotein Predicts Intrahospital Mortality in Influenza. J. Clin. Med. 2024, 13, 7242. https://doi.org/10.3390/jcm13237242
Heinzl MW, Freudenthaler M, Fellinger P, Kolenchery L, Resl M, Klammer C, Obendorf F, Schinagl L, Berger T, Egger M, et al. High-Density Lipoprotein Predicts Intrahospital Mortality in Influenza. Journal of Clinical Medicine. 2024; 13(23):7242. https://doi.org/10.3390/jcm13237242
Chicago/Turabian StyleHeinzl, Matthias Wolfgang, Markus Freudenthaler, Paul Fellinger, Lisa Kolenchery, Michael Resl, Carmen Klammer, Florian Obendorf, Lukas Schinagl, Thomas Berger, Margot Egger, and et al. 2024. "High-Density Lipoprotein Predicts Intrahospital Mortality in Influenza" Journal of Clinical Medicine 13, no. 23: 7242. https://doi.org/10.3390/jcm13237242
APA StyleHeinzl, M. W., Freudenthaler, M., Fellinger, P., Kolenchery, L., Resl, M., Klammer, C., Obendorf, F., Schinagl, L., Berger, T., Egger, M., Dieplinger, B., & Clodi, M. (2024). High-Density Lipoprotein Predicts Intrahospital Mortality in Influenza. Journal of Clinical Medicine, 13(23), 7242. https://doi.org/10.3390/jcm13237242






