Prognostic Value of Baseline Serum Pro-Inflammatory Cytokines in Severe Multisystem Inflammatory Syndrome in Children
Abstract
1. Introduction
2. Materials and Methods
2.1. MIS-C Patients and Clinical Controls
2.2. Laboratory Analyses
2.3. Statistical Analyses
3. Results
3.1. Baseline Characteristics of MIS-C Patients
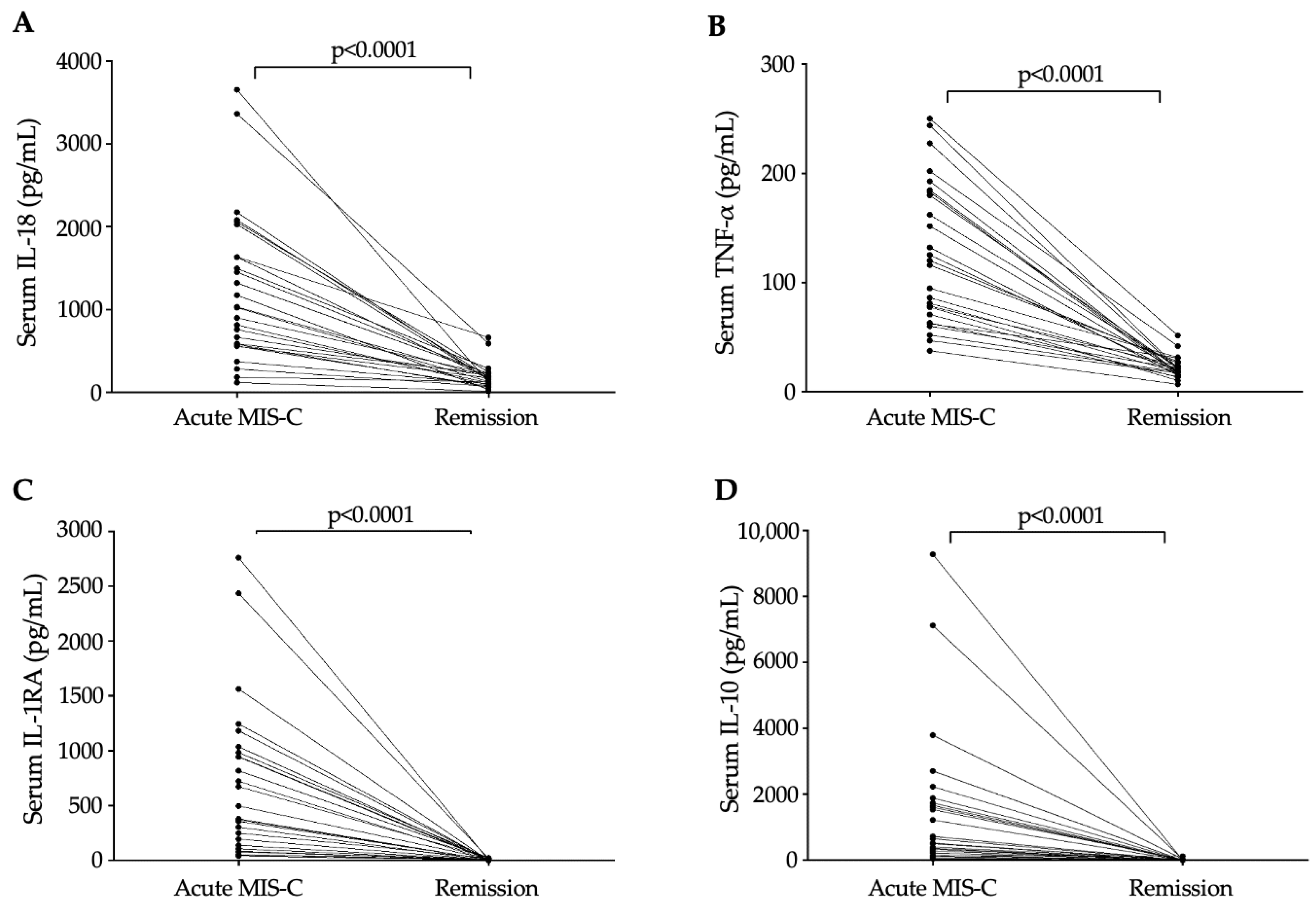
3.2. Alteration in Pro-Inflammatory Cytokines in MIS-C with Different Disease Severity
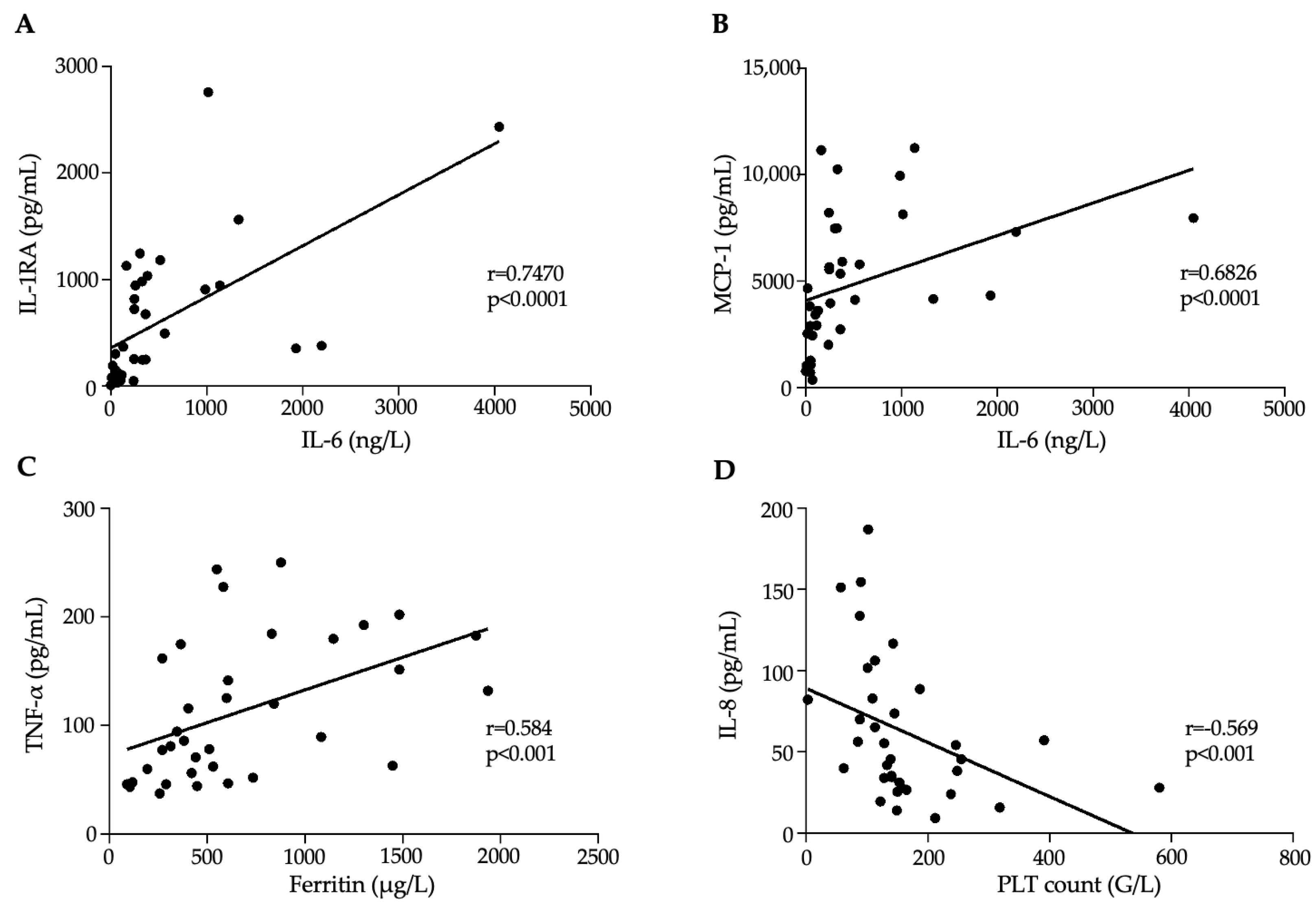
3.3. Association Between Inflammation Dependent Biomarkers and Disease Progression in MIS-C
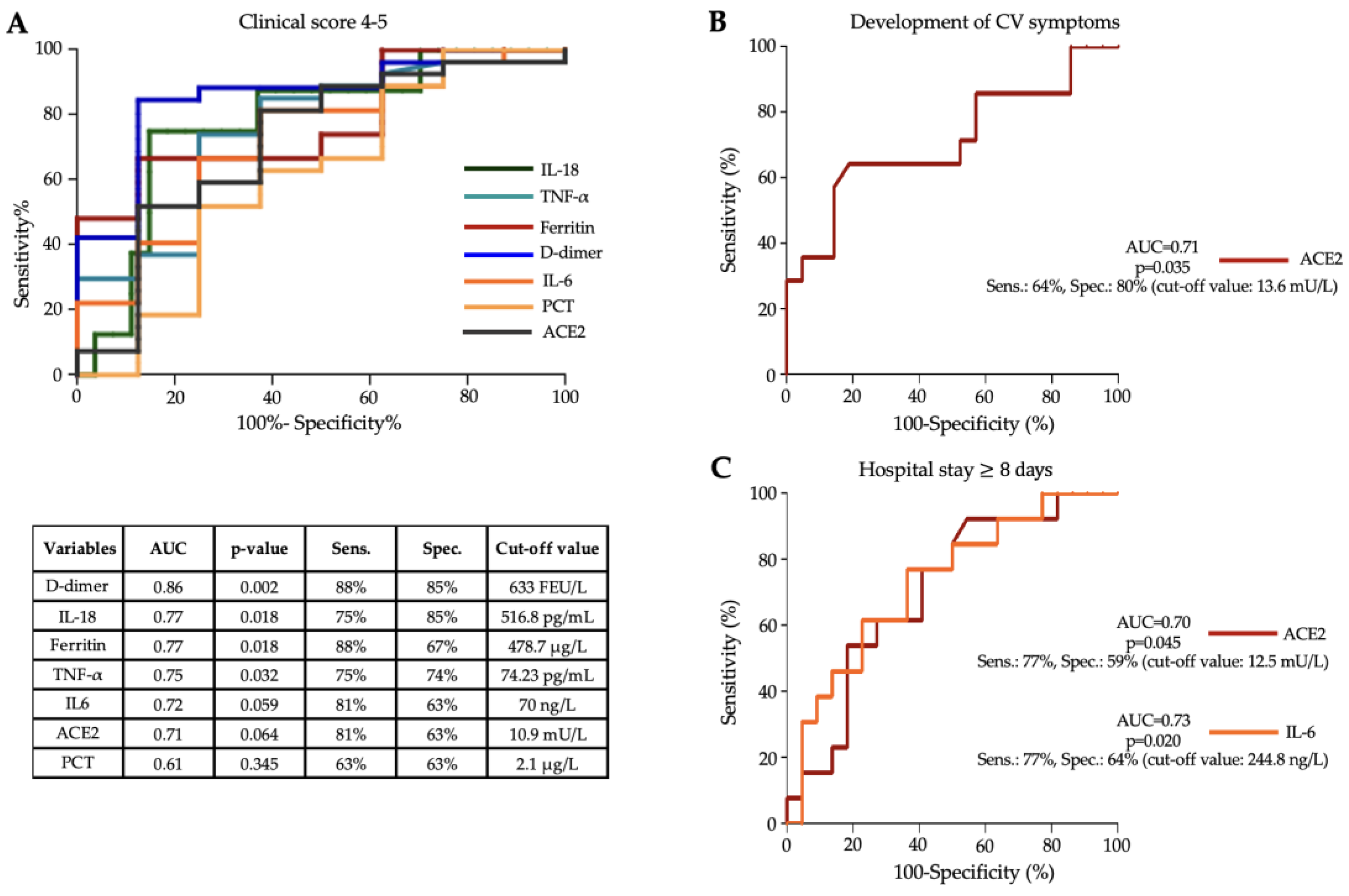
3.4. Diagnostic Efficacy of Serum Cytokines to Assess Disease Severity in MIS-C
4. Discussion
5. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajebhosale, P.P.; Mohamed, M.Y.; Swilem, M.; Abdelmogheth, A.; Nabawi, M.I.; Farahat, A.S.A.; Alsabbagh, W.M.; Lanqawi, N.J.; Addas, H. Clinical profile and immediate outcome of the multisystem inflammatory syndrome in children: Retrospective observational single center study from the United Arab Emirates. J. Pediatr. Crit. Care 2022, 9, 116–123. [Google Scholar] [CrossRef]
- Molloy, M.J.; Auger, K.A.; Hall, M.; Shah, S.S.; Schondelmeyer, A.C.; Parikh, K.; Kazmier, K.M.; Katragadda, H.; Jacob, S.A.; Jerardi, K.E.; et al. Epidemiology and Severity of Illness of MIS-C and Kawasaki Disease During the COVID-19 Pandemic. Pediatrics 2023, 152, e2023062101. [Google Scholar] [CrossRef] [PubMed]
- Abrams, J.Y.; Oster, M.E.; Godfred-Cato, S.E.; Bryant, B.; Datta, S.D.; Campbell, A.P.; Leung, J.W.; Tsang, C.A.; Pierce, T.J.; Kennedy, J.L.; et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: A retrospective surveillance study. Lancet Child Adolesc Health 2021, 5, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Ganigara, M.; Galeotti, C.; Burns, J.; Berganza, F.M.; Hayes, D.A.; Singh-Grewal, D.; Bharath, S.; Sajjan, S.; Bayry, J. Multisystem inflammatory syndrome in children and Kawasaki disease: A critical comparison. Nat. Rev. Rheumatol. 2021, 17, 731–748. [Google Scholar] [CrossRef] [PubMed]
- WHO Brief Report. Multisystem Inflammatory Syndrome in Children and Adolescents with COVID-19. Available online: https://www.who.int/publications/i/item/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 (accessed on 15 January 2022).
- Taus, F.; Salvagno, G.; Canè, S.; Fava, C.; Mazzaferri, F.; Carrara, E.; Petrova, V.; Barouni, R.M.; Dima, F.; Dalbeni, A.; et al. Platelets Promote Thromboinflammation in SARS-CoV-2 Pneumonia. Arter. Thromb. Vasc. Biol. 2020, 40, 2975–2989. [Google Scholar] [CrossRef] [PubMed]
- Hawerkamp, H.C.; Dyer, A.H.; Patil, N.D.; McElheron, M.; O’dowd, N.; O’doherty, L.; Mhaonaigh, A.U.; George, A.M.; O’halloran, A.M.; Reddy, C.; et al. Characterisation of the pro-inflammatory cytokine signature in severe COVID-19. Front. Immunol. 2023, 14, 1170012. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhu, Z.; Tan, C.; Zhou, H.; Hu, Y.; Shen, G.; Zhu, P.; Yang, G.; Xie, X. Changes of serum IL-10, IL-1β, IL-6, MCP-1, TNF-α, IP-10 and IL-4 in COVID-19 patients. Int. J. Clin. Pract. 2021, 75, e14462. [Google Scholar] [CrossRef]
- Moreews, M.; Le Gouge, K.; Khaldi-Plassart, S.; Pescarmona, R.; Mathieu, A.L.; Malcus, C.; Djebali, S.; Bellomo, A.; Dauwalder, O.; Perret, M.; et al. Polyclonal expansion of TCR Vbeta 21.3+ CD4+ and CD8+ T cells is a hallmark of Multisystem Inflammatory Syndrome in Children. Sci. Immunol. 2021, 6, eabh1516. [Google Scholar] [CrossRef] [PubMed]
- Isaza-Correa, J.; Ryan, L.; Kelly, L.; Allen, J.; Melo, A.; Jones, J.; Huggard, D.; Ryan, E.; Maoldomhnaigh, C.; Geoghehan, S.; et al. Innate immune dysregulation in multisystem inflammatory syndrome in children (MIS-C). Sci. Rep. 2023, 13, 16463. [Google Scholar] [CrossRef]
- Lazova, S.; Dimitrova, Y.; Hristova, D.; Tzotcheva, I.; Velikova, T. Cellular, Antibody and Cytokine Pathways in Children with Acute SARS-CoV-2 Infection and MIS-C—Can We Match the Puzzle? Antibodies 2022, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Ge, Y.; Wu, B.; Zhang, W.; Wu, T.; Wen, T.; Liu, J.; Guo, X.; Huang, C.; Jiao, Y.; et al. Serum Cytokine and Chemokine Profile in Relation to the Severity of Coronavirus Disease 2019 in China. J. Infect. Dis. 2020, 222, 746–754. [Google Scholar] [CrossRef] [PubMed]
- CDC Information for Healthcare Providers about Multisystem Inflammatory Syndrome in Children (MIS C). Available online: https://www.cdc.gov/mis/mis-c/hcp_cstecdc/index.html (accessed on 7 February 2024).
- World Health Organization (WHO). Multisystem Inflammatory Syndrome in Children and Adolescents Temporally Related to COVID-19. Available online: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 (accessed on 15 May 2020).
- Fagyas, M.; Fejes, Z.; Sütő, R.; Nagy, Z.; Székely, B.; Pócsi, M.; Ivády, G.; Bíró, E.; Bekő, G.; Nagy, A.; et al. Circulating ACE2 activity predicts mortality and disease severity in hospitalized COVID-19 patients. Int. J. Infect. Dis. 2022, 115, 8–16. [Google Scholar] [CrossRef]
- Nakra, N.A.; Blumberg, D.A.; Herrera-Guerra, A.; Lakshminrusimha, S. Multi-System Inflammatory Syndrome in Children (MIS-C) Following SARS-CoV-2 Infection: Review of Clinical Presentation, Hypothetical Pathogenesis, and Proposed Management. Children 2020, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, A.; Brodsky, N.N.; Sumida, T.S.; Comi, M.; Asashima, H.; Hoehn, K.B.; Li, N.; Liu, Y.; Shah, A.; Ravindra, N.G.; et al. Immune dysregulation and autoreactivity correlate with disease severity in SARS-CoV-2-associated multisystem inflammatory syndrome in children. Immunity 2021, 54, 1083–1095.e7. [Google Scholar] [CrossRef] [PubMed]
- Sinkovits, G.; Schnur, J.; Hurler, L.; Kiszel, P.; Prohászka, Z.Z.; Sík, P.; Kajdácsi, E.; Cervenak, L.; Maráczi, V.; Dávid, M.; et al. Evidence, detailed characterization and clinical context of complement activation in acute multisystem inflammatory syndrome in children. Sci. Rep. 2022, 12, 19759. [Google Scholar] [CrossRef]
- Hoste, L.; Van Paemel, R.; Haerynck, F. Multisystem inflammatory syndrome in children related to COVID-19: A systematic review. Eur. J. Pediatr. 2021, 180, 2019–2034. [Google Scholar] [CrossRef]
- McArdle, A.J.; Vito, O.; Patel, H.; Seaby, E.G.; Shah, P.; Wilson, C.; Broderick, C.; Nijman, R.; Tremoulet, A.H.; Munblit, D.; et al. Treatment of Multisystem Inflammatory Syndrome in Children. N. Engl. J. Med. 2021, 385, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Chiotos, K.; Bassiri, H.; Behrens, E.M.; Blatz, A.M.; Chang, J.; Diorio, C.; Fitzgerald, J.C.; Topjian, A.; John, A.R.O. Multisystem Inflammatory Syndrome in Children During the Coronavirus 2019 Pandemic: A Case Series. J. Pediatr. Infect. Dis. Soc. 2020, 9, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Varga, P.; Balajthy, A.; Biró, E.; Bíró, B.; Reiger, Z.; Szikszay, E.; Mogyorósy, G.; Káposzta, R. Multicolored MIS-C, a single-centre cohort study. BMC Pediatr. 2023, 23, 190. [Google Scholar] [CrossRef] [PubMed]
- Alkan, G.; Sert, A.; Oz, S.K.T.; Emiroglu, M.; Yılmaz, R. Clinical features and outcome of MIS-C patients: An experience from Central Anatolia. Clin. Rheumatol. 2021, 40, 4179–4189. [Google Scholar] [CrossRef]
- Zhao, Y.; Yin, L.; Patel, J.; Tang, L.; Huang, Y. The inflammatory markers of multisystem inflammatory syndrome in children (MIS-C) and adolescents associated with COVID-19: A meta-analysis. J. Med. Virol. 2021, 93, 4358–4369. [Google Scholar] [CrossRef]
- Zhao, Y.; Patel, J.; Huang, Y.; Yin, L.; Tang, L. Cardiac markers of multisystem inflammatory syndrome in children (MIS-C) in COVID-19 patients: A meta-analysis. Am. J. Emerg. Med. 2021, 49, 62–70. [Google Scholar] [CrossRef]
- Abo-Haded, H.M.; Alshengeti, A.M.; Alawfi, A.D.; Khoshhal, S.Q.; Al-Harbi, K.M.; Allugmani, M.D.; El-Agamy, D.S. Cytokine Profiling among Children with Multisystem Inflammatory Syndrome versus Simple COVID-19 Infection: A Study from Northwest Saudi Arabia. Biology 2022, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Satış, H.; Özger, H.S.; Yıldız, P.A.; Hızel, K.; Gulbahar, Ö.; Erbaş, G.; Aygencel, G.; Tunccan, O.G.; Öztürk, M.A.; Dizbay, M.; et al. Prognostic value of interleukin-18 and its association with other inflammatory markers and disease severity in COVID-19. Cytokine 2021, 137, 155302. [Google Scholar] [CrossRef] [PubMed]
- Rajamanickam, A.; Kumar, N.P.; Venkataraman, A.; Varadarjan, P.; Selladurai, E.; Sankaralingam, T.; Thiruvengadam, K.; Selvam, R.; Thimmaiah, A.; Natarajan, S.; et al. Sex-specific differences in systemic immune responses in MIS-C children. Sci. Rep. 2024, 14, 1720. [Google Scholar] [CrossRef]
- Tóth, E.L.; Orbán-Kálmándi, R.; Bagoly, Z.; Lóczi, L.; Deli, T.; Török, O.; Molnár, S.; Baráth, S.; Singh, P.; Hevessy, Z.; et al. Case report: Complex evaluation of coagulation, fibrinolysis and inflammatory cytokines in a SARS-CoV-2 infected pregnant woman with fetal loss. Front. Immunol. 2024, 15, 1329236. [Google Scholar] [CrossRef]
- Kumar, N.P.; Venkataraman, A.; Nancy, A.; Selvaraj, N.; Moideen, K.; Ahamed, S.F.; Renji, R.M.; Sasidaran, K.; Kumar, S.; Periyakuppan, M.; et al. Immune Profiles in Multisystem Inflammatory Syndrome in Children with Cardiovascular Abnormalities. Viruses 2023, 15, 2162. [Google Scholar] [CrossRef] [PubMed]
- Diorio, C.; Henrickson, S.E.; Vella, L.A.; McNerney, K.O.; Chase, J.; Burudpakdee, C.; Lee, J.H.; Jasen, C.; Balamuth, F.; Barrett, D.M.; et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J. Clin. Investig. 2020, 130, 5967–5975. [Google Scholar] [CrossRef] [PubMed]
- Hui, W.F.; Chan, R.W.Y.; Wong, C.K.; Kwok, K.H.A.; Cheung, W.L.; Chung, F.S.; Leung, K.K.Y.; Hon, K.L.; Ku, S.W. The Sequential Use of Extracorporeal Cytokine Removal Devices in an Adolescent With COVID-19 Receiving Continuous Renal Replacement Therapy. Asaio J. 2022, 68, e230–e234. [Google Scholar] [CrossRef]
- Gonçalves, G.S.; Correa-Silva, S.; Zheng, Y.; Avelar, I.; Montenegro, M.M.; Ferreira, A.E.; Bain, V.; Fink, T.T.; Suguita, P.; Astley, C.; et al. Circulating sTREM-1 as a predictive biomarker of pediatric multisystemic inflammatory syndrome (MIS-C). Cytokine 2023, 161, 156084. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, J.; Zhang, D.; Xu, Z.; Ji, J.; Wen, C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front. Immunol. 2020, 11, 1708. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Loretelli, C.; Biganzoli, D.; Abdelsalam, A.; Marano, G.; Carelli, S.; Fiori, L.; Mannarino, S.; D’auria, E.; Verduci, E.; et al. Long-term cytokine profile in multisystem inflammatory disease among children. Cytokine 2024, 183, 156744. [Google Scholar] [CrossRef] [PubMed]

| Patient Parameters | Clinical Controls (n = 11) | Total MIS-C Patients (n = 35) | MIS-C Patients with Score 4–5 (n = 27) | MIS-C Patients with Score 1–3 (n = 8) | p-Value (Score 4–5 vs. Score 1–3) |
|---|---|---|---|---|---|
| Sex (M/F) | 7/4 | 21/14 | 18/9 | 3/5 | 0.220 |
| Age (years) | 8.4 ± 5.2 | 8.4 ± 4.1 | 8.9 ± 3.6 | 6.5 ± 5 | 0.210 |
| WBC count (G/L) | 7.8 (7.1–8.6) | 10.11 (7.07–13.6) | 9.77 (6.8–12.7) | 11.87 (8.9–22.7) | 0.080 |
| Hemoglobin (g/L) | 137 (119–142) * | 116 (107–123) | 115 (105–123) | 116 (112.5–145.8) | 0.360 |
| PLT count (G/L) | 327 (282–374) *** | 140 (102–187) | 133 (101–153) | 229 (133.5–372.8) | 0.030 |
| Lymphocyte count (G/L) | 2.8 (2.2–3.4) *** | 1 (0.6–1.6) | 0.8 (0.4–1.4) | 2.36 (1.5–4.5) | <0.001 |
| CRP (mg/L) | 0.6 (0.5–0.6) *** | 152.4 (112.1–215.3) | 164.8 (117.6–244.4) | 99.69 (20.72–167) | 0.030 |
| PCT (µg/L) | n.m. | 2.64 (0.8–7.6) | 3.19 (0.9–7.6) | 1.82 (0.3–7.6) | 0.350 |
| IL-6 (ng/L) | n.m. | 244.8 (50.9–515.2) | 255.8 (102.3–561.2) | 60.32 (26.4–290) | 0.058 |
| Ferritin (µg/L) | 49.3 (20.7–51.1) *** | 529.2 (312.7–876.6) | 599.4 (344.8–1146) | 392.5 (108–445.8) | 0.010 |
| LDH (U/L) | 222 (217–266.5) | 390 (220.8–566.3) | 402.5 (262.8–609.3) | 322.5 (116.8–466) | 0.240 |
| AST (U/L) | 23 (22.5–26) * | 33 (25–80) | 35 (26–80) | 28.5 (18–83.2) | 0.430 |
| ALT (U/L) | 16 (7.2–18.2) * | 28 (16–58) | 32 (20–58) | 13.17 (11.2–85) | 0.140 |
| cTnT (ng/L) | n.m. | 24 (9.2–54.5) | 28.15 (9.2–49.8) | 10 (4.7–153.4) | 0.760 |
| D-dimer (FEU/L) | n.m. | 1102 (18–2684) | 1779 (856.5–2831) | 4.6 (1.2–352) | 0.001 |
| NT-proBNP (ng/L) | n.m. | 2087 (573.4–8309) | 2454 (490–12460) | 1440 (611.1–4285) | 0.410 |
| Anti-Spike-SARS-Cov2 total Ig (BAU/mL) | n.m. | 500 (194.9–854.6) | 500 (194.9–802.5) | 611.2 (160.4–19110) | 0.490 |
| Variables | Clinical Controls (n = 11) | Total MIS-C Patients (n = 35) | MIS-C Patients with Score 4–5 (n = 27) | MIS-C Patients with Score 1–3 (n = 8) | p-Value (Score 4–5 vs. Score 1–3) |
|---|---|---|---|---|---|
| IFNγ (pg/mL) | 5.1 (3.9–5.9) | 47.49 (19.7–76.5) *** | 40.38 (16.1–76.5) | 48.68 (21.4–179.8) | 0.961 |
| IL1-α (pg/mL) | 136 (122.1–170.1) | 155.2 (132.1–202.5) | 166.4 (136–202.5) | 131.1 (103.6–196.6) | 0.102 |
| IL1-RA (pg/mL) | 8.93 (4.9–12.1) | 357.4 (107.3–947.3) *** | 380.4 (139.8–985) | 120 (36.9–744.2) | 0.053 |
| IL-8 (pg/mL) | 19.38 (13.1–22.5) | 45.65 (28–82.9) ** | 45.65 (34.1–101.8) | 41.18 (18.3–56.8) | 0.138 |
| IL-10 (pg/mL) | 42.7 (16.9–81.7) | 519.3 (152.7–1668) *** | 654 (282.9–1740) | 265.3 (23–1434) | 0.110 |
| IL-17A (pg/mL) | 3.85 (2.1–6.8) | 10.49 (8–17.9) ** | 11.1 (8–17.9) | 9.41 (6.2–17) | 0.410 |
| IL-18 (pg/mL) | 158.8 (121.3–401.6) | 760 (469.9–1557) ** | 1025 (581.4–1637) | 446 (307–672.3) | 0.016 |
| IP-10 (pg/mL) | 61.43 (35.39–101) | 14713 (1666–47008) *** | 15707 (6578–53,166) | 1481 (1047–38,934) | 0.100 |
| MCP-1 (pg/mL) | 811.2 (641.3–924) | 4174 (2542–7486) *** | 4336 (2937–7317) | 2721 (909.9–10,180) | 0.558 |
| TNF-α (pg/mL) | 20.57 (15.4–27.3) | 89.55 (56.3–174.8) *** | 116 (63.15–182) | 52.08 (44.84–123.8) | 0.031 |
| ACE2 activity (mU/L) | 11.62 (9.53–16.25) | 12.34 (9.6–15.9) | 13.26 (11.41–16.83) | 9.92 (7.72–12.53) | 0.066 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartha-Tatár, A.; Sinkovits, G.; Schnur, J.; Maráczi, V.; Dávid, M.; Zsigmond, B.; Rimanóczy, É.; Szalay, B.; Biró, E.; Bekő, G.; et al. Prognostic Value of Baseline Serum Pro-Inflammatory Cytokines in Severe Multisystem Inflammatory Syndrome in Children. J. Clin. Med. 2024, 13, 7177. https://doi.org/10.3390/jcm13237177
Bartha-Tatár A, Sinkovits G, Schnur J, Maráczi V, Dávid M, Zsigmond B, Rimanóczy É, Szalay B, Biró E, Bekő G, et al. Prognostic Value of Baseline Serum Pro-Inflammatory Cytokines in Severe Multisystem Inflammatory Syndrome in Children. Journal of Clinical Medicine. 2024; 13(23):7177. https://doi.org/10.3390/jcm13237177
Chicago/Turabian StyleBartha-Tatár, Anita, György Sinkovits, János Schnur, Veronika Maráczi, Máté Dávid, Borbála Zsigmond, Éva Rimanóczy, Balázs Szalay, Edina Biró, Gabriella Bekő, and et al. 2024. "Prognostic Value of Baseline Serum Pro-Inflammatory Cytokines in Severe Multisystem Inflammatory Syndrome in Children" Journal of Clinical Medicine 13, no. 23: 7177. https://doi.org/10.3390/jcm13237177
APA StyleBartha-Tatár, A., Sinkovits, G., Schnur, J., Maráczi, V., Dávid, M., Zsigmond, B., Rimanóczy, É., Szalay, B., Biró, E., Bekő, G., Varga, P., Szabó, T., Fagyas, M., Fejes, Z., Kappelmayer, J., & Nagy Jr., B. (2024). Prognostic Value of Baseline Serum Pro-Inflammatory Cytokines in Severe Multisystem Inflammatory Syndrome in Children. Journal of Clinical Medicine, 13(23), 7177. https://doi.org/10.3390/jcm13237177






