Abstract
Bladder cancer (BC) currently ranks as the 9th most common cancer worldwide. It is characterised by very high rates of recurrence and metastasis. Most cases of BC are of urothelial origin, and due to its ability to penetrate muscle tissue, BC is divided into non-muscle-invasive BC (NMIBC) and muscle-invasive BC (MIBC). The current diagnosis of BC is still based primarily on invasive cystoscopy, which is an expensive and invasive method that carries a risk of various complications. Urine sediment cytology is often used as a complementary test, the biggest drawback of which is its very low sensitivity concerning the detection of BC at early stages, which is crucial for prompt implementation of appropriate treatment. Therefore, there is a great need to develop innovative diagnostic techniques that would enable early detection and accurate prognosis of BC. Great potential in this regard is shown by epigenetic changes, which are often possible to observe long before the onset of clinical symptoms of the disease. In addition, these changes can be detected in readily available biological material, such as urine or blood, indicating the possibility of constructing non-invasive diagnostic tests. Over the past few years, many studies have emerged using epigenetic alterations as novel diagnostic and prognostic biomarkers of BC. This review provides an update on promising diagnostic biomarkers for the detection and prognosis of BC based on epigenetic changes such as DNA methylation and expression levels of selected non-coding RNAs (ncRNAs), taking into account the latest literature data.
1. Introduction
1.1. Epidemiology
Currently, bladder cancer (BC) is positioned as the 9th most common cancer worldwide. In 2022, there were approximately 614,000 new cases of BC and 220,000 deaths attributed to the disease were recorded worldwide. BC is a particular issue for European populations. The highest incidence rate among women is observed in the Netherlands while the highest incidence rate among men is observed in Spain [1]. The occurrence of BC is primarily associated with external risk factors, and up to 50% of patients have the disease due to smoking tobacco products [2]. Other risk factors identified by the International Agency for Research on Cancer (IARC) include occupational exposure during the production of aluminium and rubber products, working with synthetic dyes, ingestion of opium, environmental exposure to X or gamma radiation and arsenic, taking drugs such as cyclophosphamide and chlornafazine, and infection with Schistosoma bacteria. Additionally, a higher risk of BC is observed in men than in women [2,3].
BC is characterised by high rates of recurrence and metastasis, which are primarily due to the late diagnosis of patients who are at an intermediate or advanced stage of this cancer, which is often due to the lack of clear symptoms of BC in its early stages or the presence of symptoms such as haematuria, which may be misinterpreted as other less serious disorders such as cystitis, urolithiasis, and others [4,5]. Crucial to prolonging the survival of patients struggling with BC are studies on tools for early detection, prediction of treatment effects, as well as prognostic techniques [6].
1.2. Etiology
Approximately 90% of BC cases are of urothelial origin, that is, originating in the epithelial tissue lining, among others, of the inner surface of the bladder. The remaining cases are squamous cell carcinoma, adenocarcinoma, or neuroendocrine carcinoma [7,8]. Due to the ability of the tumour to penetrate muscle tissue, BC is divided into muscle-invasive bladder cancer (MIBC) (stages T2–T4) and non-muscle-invasive bladder cancer (NMIBC), which includes approximately 75% (stages Tis, Ta, and T1) of all diagnosed cases [9].
The exact genetic basis of BC is not yet known and there are still many questions concerning its pathogenesis. It is also unclear whether specific genetic burdens have an impact on the increased risk of BC, and research is still underway to determine the exact genetic risk factors [9]. Deletion of chromosome 9 is a frequently observed genetic mutation in both NMIBC and MIBC. Due to this mutation, it is observed that there is a loss of the CDKN2A gene encoding two proteins: p16 and p14ARF, which regulate the RB and p53 pathways, and TSC1, which regulates mTOR signalling and is associated with an increase in the expression of mTOR molecular targets, such as telomerase reverse transcriptase TERT. An increase in the level of TERT plays a key role in maintaining telomere integrity and contributes to tumour progression in BC [10,11,12]. According to currently available information about the molecular characteristics of BC, point mutations within the FGFR3 gene are known to occur in about 60% of patients diagnosed with NMIBC. Mutations within the RAS genes are also common but are mutually exclusive with mutations within FGFR3. Mutations in one or the other of these genes occur in about 90% of cases in stage Ta [9,10]. A study using human urothelial cells with mutated FGFR3 showed increased growth, suggesting that it may play a very important role in the growth of cancerous tumours [13]. About 30% of cases also have mutations within the PIK3CA genes, often coexisting with FGFR3 or RAS mutations, suggesting that NMIBC involves both activation of RAS–MAPK signalling as well as PI3K signalling [14]. Mutations in the ERBB3 genes, leading to PI3K activation, are also observed at the T1 stage [15]. In NMIBC, there is also inactivation of some genes that encode chromatin regulatory proteins, such as STAG2, KDM6A, KMT2D, KMD3C, CREBBP, EP300, and ARID1A, but their function in BC development is not yet fully understood [14]. In the case of MIBC, the vast majority of cases have been found to involve the loss of cell cycle checkpoints due to mutations within TP53, RB1, or ATM, or due to changes in the activity of their regulators. In addition, the DNA damage response is impaired through the loss of ATM or ERCC2 function [16,17]. As for activating FGFR3 point mutations, they are less frequently observed than in NMIBC, even though increased expression of this gene is common [18]. In about 70% of MIBC cases, activation of the RAS–MAPK and PI3K pathways is observed, probably through positive regulation or mutation within the ERBB2/3 genes [9,16]. In this type of BC, activation of MET77 signalling and the NOTCH pathway as well as activation of the AKT–mTOR pathway are also observed [9,19,20].
The epigenetic mechanisms responsible for regulating the expression levels of selected genes in a manner unrelated to changes in the DNA nucleotide sequence also play a very important role in the pathogenesis of BC [10]. DNA methylation is a common epigenetic modification involving the regulation of gene expression through the methylation of CpG dinucleotides within the 5′ regulatory region of a gene. This reaction is catalysed by DNA methyltransferases. The methylation profiles of genes can be analysed both from so-called free circulating DNA (cfDNA) and in tumour cells excreted in urine [7]. Significantly, DNA methylation does not affect the continuity of base pairs within genes and is only observed in their promoter regions where it can affect protein–DNA interactions, thus regulating the expression of a given gene [21]. Molecules involved in epigenetic regulation of gene expression are, in addition, non-coding RNAs (ncRNAs), i.e., molecules that do not form a matrix for protein synthesis. These include microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs). MiRNAs are a family of short RNAs of approximately 18–25 nucleotides in length that regulate gene expression at the post-transcriptional level by interacting with 3′ mRNA fragments that are not translated [22]. Through this action, miRNAs can inhibit mRNA translation, preventing the formation of protein products. In addition, these molecules can inhibit the translation of both suppressor genes and oncogenes, making their role in carcinogenesis very important [7]. Another type of ncRNAs are lncRNAs, which, unlike miRNAs, are long molecules consisting of more than 200 nucleotides [23]. These molecules play an important role in the epigenetic regulation of gene expression. Their mechanism of action varies widely and may be based, for example, on chromatin modification and remodelling, histone modification, changes in nucleosome positioning, or direct effects on the regulation of the expression of selected genes [7,23]. An interesting example of ncRNAs are circRNA molecules, which have a different structure in that they do not possess the 3′ and 5′ ends, which makes them resistant to degradation by RNA exonucleases [24,25]. It has been suggested that they play an important role in processes such as proliferation, invasion, migration, and apoptosis of cancer cells [26]. Moreover, epigenetic changes, such as DNA methylation, and expression levels of miRNAs, lncRNAs, and circRNAs can be easily detected in tissues and, most importantly, in readily available biological material such as blood and urine, often long before the clinical manifestations of cancer. Therefore, they are being investigated both to explain the molecular mechanisms involved in BC pathogenesis and as potential biomarkers and molecular targets for the development of modern diagnostic and therapeutic techniques [7,8].
1.3. Treatment
The treatment of patients with BC is usually multimodal and can combine surgery, chemotherapy, radiation therapy, and targeted therapy including immunotherapy [9]. A frequently used diagnostic method and, in the case of NMIBC, a therapeutic method, is transurethral resection of the bladder tumour (TURBT). It allows determining the stage of the tumor and, in the case of NMIBC, allows complete resection of the tumour [9,27]. TURBT is the primary standard of care for NMIBC, both in combination with and without single dose intravesical chemotherapy, but it is a major procedure and can place a heavy burden on the patient [9]. In the case of NMIBC, immunotherapy with the BCG vaccine administered intravesically is also a common treatment [28]. It is used to stimulate the inflammatory response and activate macrophages. However, this therapy is associated with the risk of side effects such as bladder and prostate inflammation/infection, sepsis, fever, and general malaise [29]. For patients intolerant of BCG therapy, intravesical maintenance chemotherapy is also being introduced, leading to a reduction of about 44% in recurrences occurring at one year [30]. For MIBC and NMIBC patients in whom BCG treatment has failed to elicit a response, the standard of care is the so-called radical cystectomy [31]. In men, it involves removal of the bladder, prostate gland, and seminal vesicles and distal ureters, while in women, it involves removal of the bladder, entire urethra, anterior vaginal wall, uterus, and distal ureteral segments [31]. An alternative treatment for MIBC is trimodal therapy (TMT), which combines TURBT followed by simultaneous radio- and chemotherapy. TMT allows for bladder preservation and is another option for patients ineligible for radical cystectomy. In addition, for some patients, partial cystectomy is suggested as a less burdensome method. This is mainly true for patients with lower grade MIBC [31,32]. In the 1990s, combination chemotherapy based on cisplatin for metastatic BC became the mainstay of BC treatment [9]. Although it is the standard of care for BC, not all patients qualify for its use, so it is often replaced by gemcitabine in combination with carboplatin [33]. Additionally, in recent years, several new treatment strategies have been proposed to be incorporated into standard treatments. The first is treatment with erdafitinibum, a small-molecule inhibitor of fibroblast growth factor 3, which has seen a response rate of 42% in phase 2 trials in patients previously treated with platinum-based chemotherapy [34]. The next new therapy is based on an antibody-drug conjugate called enfortumab venodine, which is directed against nectin-4 [35,36]. Another antibody-drug conjugate is sacituzumab govitecan, in which a monoclonal antibody targets TROP2 combined with a topoisomerase I inhibitor. In this case, the response rate was 27% in patients previously treated with platinum-based chemotherapy [37]. It is worth mentioning that immunotherapy is an important aspect in the treatment of BC, but due to the difficulties associated with the immunosuppressive tumour microenvironment, the effectiveness of immune checkpoint inhibitors is significantly limited [38]. Therefore, it seems necessary to develop new independent biomarkers based on integrated genomic analysis to detect BC at a much earlier stage of progression, better predict clinical outcomes, and tailor treatment to patients at high risk of progression [39].
Innovations in medicine are developing at an extremely fast pace. However, it should be emphasised that many of the new developments are still in the phase of verification studies that will allow to assess their effectiveness and safety. In particular, epigenetic biomarkers are becoming an increasingly promising tool in predicting the course of disease and early diagnosis [40]. They have wide potential as multifunctional indicators to revolutionise treatment modalities and personalise therapy for patients with bladder cancer, which is crucial in the development of modern medicine [41].
1.4. Prognosis
A crucial issue from the perspective of clinical practice is the accurate assessment of the survival chances of patients diagnosed with BC. Properly determined patient prognosis is necessary to select the best possible individualised treatment regimen that is optimal for a given patient. Determining the chance of survival is based primarily on an assessment of the stage of the tumor, the size of the cancerous tumour, the type of BC (NMIBC or MIBC), the patient’s demographic and clinical factors, and the patient’s overall health. Statistics published by the National Cancer Institute (NCI) indicate that the 5-year relative survival rate for BC in situ is 91%, for localised BC it is 71%, for BC that has spread to nearby lymph nodes and organs it is 39%, and for metastatic BC it is 8% [42]. Many of the currently conducted analyses focus on the search for new biomarkers that could provide accurate prognostic indicators, given that the currently used criteria, due to the heterogeneity of BC, genomic instability, and differences in sensitivity to chemotherapy or immunotherapy, are not effective enough [43,44].
The following section will provide a review of selected recent literature reports indicating epigenetic changes with high potential for use as biomarkers for BC detection and a summary of the diagnostic tests developed.
2. Methodology
This paper is based on a literature review of epigenetic biomarkers for bladder cancer focusing on the potential usefulness of selected alterations for diagnosis and prognosis in BC. This narrative review focuses primarily on data on epigenetic alterations, such as methylation levels of specific genes or combinations of genes, and expression levels of ncRNA molecules, such as microRNAs, lncRNAs, and circRNAs.
Articles were searched in databases such as “Pubmed” and “Google Scholar” in order to access scientific papers related to the topics covered. The search for relevant publications was based on several keywords and their combinations. The main keywords used in the search included “bladder cancer”, “biomarkers”, “epigenetics”, “early detection”, “prognosis”, and “epigenetic biomarkers”. The selection of relevant articles focused on works published in the last eight years. The articles included in the bibliography published earlier than 2016 were used for the introductory section, where the current state of knowledge on the epidemiology, etiology, treatment, prognosis, and issues concerning the classical approach to the diagnosis of BC was described. The main goal was to collect the latest discoveries in the field on the subjects under discussion and update the state of knowledge and, therefore, the focus was particularly on results published in recent years. In order to perform this review, we looked for papers that included an assessment of the diagnostic usefulness of epigenetic biomarkers or their combinations. As for biomarkers used for BC detection, indicators such as sensitivity and specificity were chosen (with only biomarkers with sensitivity and specificity of no less than 60% being considered). As for biomarkers with potential for prognostic use, we chose articles with prognostic indicators such as OS (overall survival), PFS (progression-free survival), CSS (cancer-specific survival), DSS (disease-specific survival), or RFS (recurrence-free survival). Publications that did not include the aforementioned data of diagnostic or prognostic utility were not included in this review.
The selected scientific papers were analysed, and the data contained within them were grouped to clearly and transparently present potential epigenetic biomarkers of BC and their diagnostic and/or prognostic role. Through such analysis, it was possible to draw conclusions about the latest trends and most promising epigenetic changes that could be used as novel diagnostic and prognostic tools in BC patients.
3. Diagnosis of Bladder Cancer
3.1. Classic Approach
Currently, invasive cystoscopy remains the diagnostic gold standard for BC, but in addition to the heavy burden it carries, it is also a highly expensive method. Standard white light cystoscopy (WLC) is an inaccurate method, so improvements have begun to be made using techniques such as laser-induced fluorescence (LIF), autofluorescence cystoscopy (AFC), and photodynamic diagnosis (PDD). PDD involves infusing a photosensitiser into the bladder prior to cystoscopy, which causes cancer cells to exhibit red fluorescence in the presence of blue light [45]. Additionally, a 2021 meta-analysis by Veeratterapillay et al. showed that the use of PDD increased recurrence-free survival (RFS) in patients with NMIBC compared to WLC [46]. Urine sediment cytology is also frequently used as a complementary test, demonstrating a sensitivity and specificity of 48% and 86%, respectively. However, despite a fairly high sensitivity for high grade tumours of 84%, the sensitivity for low grade tumours was only 16% [47]. However, the results of urine cytology are affected by the presence of other disorders related to bladder function, such as inflammation, urinary tract infection, or urolithiasis [48]. To monitor BC, optical biopsy techniques such as optical coherence tomography (OCT) or confocal laser endomicroscopy (CLE) are also used. Combinations of imaging with other diagnostic methods give high sensitivity and specificity values, such as 89.7% and 100%, respectively, when PDD is combined with OCT [45,49]. Ultrasound examinations are also used to diagnose BC. An example of a relatively effective technique is high-resolution 29 mhz micro-ultrasound (mUS), which shows high sensitivity and specificity for BC with low malignancy (85% and 89%, respectively) [45,50]. Despite continuous improvements in BC diagnostic techniques, there are still many cases of this disease detected at a late stage. Diagnosis of BC is expensive and, among the diagnostic methods, invasive cystoscopy is still the most commonly used, which carries a high burden for patients involving the possibility of complications such as painful urination, bleeding, urinary tract infections, fever, or urinary retention [51]. Therefore, a search is in progress for new diagnostic methods based on biomarkers that could significantly reduce the costs of both initial diagnosis and health monitoring of patients and could enable non-invasive detection of BC even at low stages of advancement.
3.2. Epigenetic Biomarkers in the Diagnosis of BC
The use of epigenetic alterations as biomarkers for the early detection of BC has been the subject of intense research in recent times. Currently, many potential biomarkers based on the modifications in question are already known, but still, few of them have sufficient sensitivity and specificity, especially when it comes to the detection of NMIBC, or have high costs or complicated procedures for their analysis. Therefore, many issues regarding the published findings are still in question, and there is a great need to search for new high-specificity and high-sensitivity epigenetic alterations that could, in the future, provide a basis for the diagnosis of the disease entity in question and help reduce the need for invasive cystoscopy, which is still the gold standard for BC diagnosis. In this review, the authors summarise selected epigenetic biomarkers, including changes in the methylation levels of selected genes and expression of selected ncRNAs undergoing validation studies, and compare the potential for their use, including analysis of parameters such as sensitivity (SN) and specificity (SP). In addition, discussed are selected epigenetic changes showing high potential as BC diagnostic biomarkers that are in the phase of research using BC cell lines or have been selected on the basis of available bioinformatic analyses.
3.3. Available Tests Based on Analysis of Epigenetic Changes for BC Diagnosis
Several tests based on the methylation of selected DNA fragments have already been developed so far and investigated for use in BC diagnosis. A number of diagnostic panels based solely on DNA methylation have now been developed. The first of these is Bladder EpiCheck, which is a commercially available test. The diagnostic panel consists of the DNA methylation in 15 genes, which uses qPCR to assess their methylation levels (SN = 68.2%, SP = 88%) [48,52]. The next test is UroMark, which shows very high sensitivity and specificity (98% and 97%, respectively). It is based on the analysis of 150 methylation sites using next-generation DNA sequencing (NGS) in which the genetic material has undergone bisulfide conversion [48,53]. Another test is BladMetrix, which evaluates the methylation levels of eight selected genes using droplet digital PCR (ddPCR). In a study involving patients with haematuria, the sensitivity and specificity of the test were 92.1% and 93.3%, respectively [48,54]. A 2021 clinical evaluation of the Bladder CARE test by Piatti et al. showed its overall sensitivity and specificity to be 93.5% and 92.6%, respectively. For the determination, it uses urine samples in which the analysis of three methylated BC-specific genes is performed: TRNA-Cys, SIM2, and NKX1. The analysis is performed using methylation-sensitive restriction enzymes [55]. Also published in 2024 was a paper by Bang et al. presenting the results of a diagnostic evaluation of the EarlyTest BCD test that analysed the methylation level of the PENK gene in samples of excreted urine. This study revealed the high potential of the test, given that the overall sensitivity and specificity were 81% and 91.5%, respectively, in detecting all stages of BC in patients with haematuria. Sensitivity was significantly related to the stage of BC [56]. A surprisingly positive result was shown by the use of the GynTect test, originally designed for use in the diagnosis of cervical cancer. It is based on the use of six DNA methylation markers (ASTN1, DLX1, ITGA4, RXFP3, SOX17, and ZNF671), and the sensitivity and specificity of the test were 60% and 96.7%, respectively, noting that the material was collected only from patients with NMIBC [57]. A summary of the aforementioned tests can be found in Table 1.

Table 1.
Available tests based on DNA methylation for BC diagnosis.
In most of these studies, the level of methylation was assessed using biological material such as exfoliated cells found in urine. The discussed tests showed promising results in terms of their implementation in routine diagnostic testing in BC. However, the problems included often too low sensitivity, especially for BC with low malignancy, complicated testing procedures, or high costs associated with the use of these assays in practice. These results, however, have still not translated into the practical use of test molecules or modifications in routine diagnostic procedures. Several diagnostic tests approved by the Food and Drug Administration (FDA) based on urine samples are currently available, but none of them are based on the epigenetic changes discussed in the articles. Among them are four tests based on protein biomarkers and a test based on the examination of exfoliated cells. Protein biomarkers include BTA (bladder tumour antigen) on the basis of which two diagnostic tests have been constructed. Qualitative BTA-Stat is a rapid immunochromatographic test (sensitivity and specificity of 64% and 77%, respectively) and BTA-TRAK is an ELISA immunoenzymatic test (sensitivity and specificity of 65% and 74%, respectively). Both tests detect and measure the level of human complement factor H-related protein (hCFHrp) in urine samples. Another FDA-approved BC protein biomarker is NMP22 (nuclear matrix protein 22) on the basis of which two diagnostic tests have also been developed: the quantitative NMP22 ELISA (sensitivity and specificity of 69% and 77%, respectively) and the qualitative NMP22 BladderChek test (sensitivity and specificity of 58% and 88%, respectively), which have been validated for initial diagnosis. Also commercially available is the UroVysion test, which uses a multi-probe FISH (fluorescence in situ hybridisation) technique to detect four chromosomal abnormalities that have been linked to the development of BC: aneuploidy of chromosomes 3, 7, and 7, and loss of the 9p21 locus. UroVysion achieved a specificity of 87.7% and a sensitivity of 63%. Although the aforementioned tests have been approved by the FDA, due to their limitations, they are not widely used in clinical practice [48,58].
3.4. Epigenetic Biomarkers in BC—Cohort Studies
Recently, there have also been the results of studies evaluating the usefulness of analysing the methylation levels of single genes or panels of a few selected genes using material collected from BC patients such as blood, urine, or tumour tissues. Most of them, despite surprisingly positive sensitivity and specificity values, require further studies and proper clinical validation. The 2022 methylation panel of three genes developed by Fang et al., including PCDH17, POU4F2, and PENK hypermethylation, showed a sensitivity of 87% and a specificity of 97%. The biomarkers were tested using a sediment of cells present in urine. However, in the present study, it was not possible to determine the sensitivity and specificity in early and late stages of the disease due to the study involving too few patients and missing data. In addition, in the conducted study, the methylation panel did not show the potential to distinguish between different subtypes of BC [58,59]. In 2022, Hentschel et al. showed high potential for the combined use of methylated GHSR and MAL genes with a diagnostic potential that reached a sensitivity of 80% and a specificity of 93% [60]. Jiang et al. in 2024 also published a paper evaluating the utility of using a panel based on assessing the hypermethylation of three genes, ZNF671, OTX1, and IRF8, which were tested in patients’ excreted urine samples. The panel achieved a specificity of 90.9% and a sensitivity of 75% [61]. Another panel studied by Wu et al. consisting of four hypermethylated genes, ONECUT2+, HOXA9, PCDH17, and POU4F2, showed a specificity of 73.2% and a sensitivity of 90.5% in urine samples from patients with haematuria [58,62]. In 2020, Chen et al. evaluated the diagnostic utility of a methylation panel of two BC-specific genes (OTX1 and SOX1-OT) in the DNA of exfoliated cells found in urine. The sensitivity and specificity values of this panel were 91.7% and 77.3%, respectively [48,63]. Another publication in 2024 by Zhang et al. showed sensitivity and specificity values of 78% and 83%, respectively, for distinguishing patients with BC of urothelial origin from healthy volunteers using methylation analysis of TWIST1 and VIM gene promoters in the deposits of cells contained in urine [64]. It is worth noting that the following review considers only selected markers based on DNA methylation that could be useful in the diagnosis of BC, while most of them still show many limitations due to, among other things, too few patients studied and require further approaches for their clinical validation.
A summary of the discussed DNA methylation-based biomarkers and proposed biomarkers not mentioned in the text is provided in Table 2 and Figure 1.

Table 2.
DNA methylation-based biomarkers for BC diagnosis validated in cohort studies.
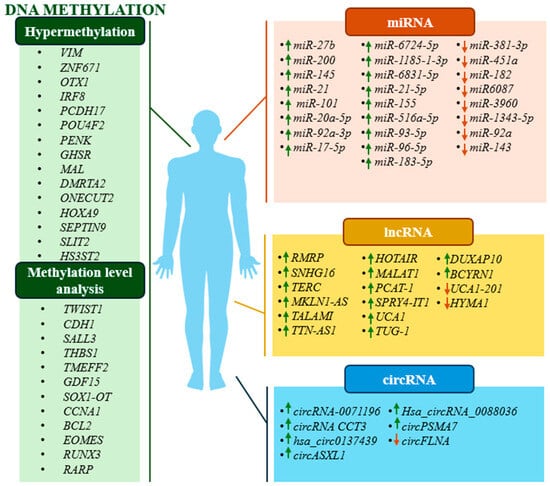
Figure 1.
Schematic representation of studied changes in methylation status and expression levels of potential diagnostic biomarkers. Green arrows indicate an increase in the expression level and red arrows indicate a decrease in the expression level of a particular ncRNA.
Changes in the expression levels of non-coding RNA (ncRNA) molecules are another epigenetic mechanism within which BC diagnostic biomarkers are sought. One of the types of ncRNAs considered in this review are miRNA molecules. An enormous amount of data has been published in recent years suggesting that miRNAs have great diagnostic potential in BC. A 2021 paper by Wang et al. analysed the diagnostic potential of miRNAs. It showed that three molecules detected in blood, miR-20a-5p, miR-92a-3P, and miR-17-5p, the expression levels of which were increased in BC, had a sensitivity of 90.4% and a specificity of 94.4% [71,72]. On the other hand, the results of the analysis by Yu et al. in 2023 of the expression levels of three miRNA molecules (miR-27b, miR-381-3p, and miR-451a) showed high values of sensitivity and specificity (86.9% and 77.38%, respectively) in detecting BC. These molecules are detected in blood serum, are associated with the regulation of the expression of genes such as SMAD4 and FOXO1, and appear to show great potential as non-invasive biomarkers for the early detection of BC [73]. A broad spectrum of miRNA molecules is detected in urine and they often have high sensitivity and specificity. For example, the analysis of the expression levels of two molecules, downregulated miR-145 and upregulated miR-182, by Suarez-Cabrera et al. was characterised by sensitivity and specificity values of 93% and 86%, respectively [74]. In 2021, El-Shal et al. reported high sensitivity and specificity of a panel comprising two molecules: miR-96-5p and miR-183-5p (88.2% and 87.8%, respectively). The researchers suggested that these miRNA molecules could serve as potential novel diagnostic biomarkers for BC [75]. Other scientific studies have indicated that these molecules are involved in the regulation of transcription factors from the FOXO family, which function as tumour suppressors. Additionally, miR-183-5p directly promotes PDCD4, a pro-apoptotic molecule, whereas miR-96 has been shown to act as a tumour suppressor in pancreatic cancer by regulating the oncogene KRAS [76,77,78]. Relatively high diagnostic performance parameters were observed by Lin et al. for two molecules, miR-93-5p and miR-516-5p, with enhanced expression in BC. The molecular target of these miRNAs is the regulation of BTG2. In this case, miRNAs located in extracellular vesicles found in urine were studied and, combined together, they achieved sensitivity and specificity values of 85.2% and 82.4%, respectively [72,79]. In a 2017 study, Matsuzaki et al. reported the high diagnostic potential of miR-21-5p (SN = 75%, SP= 98%) for early detection of BC [80]. In 2019, Chen assessed the diagnostic potential of miR-101 as a biomarker to distinguish patients with BC from healthy volunteers. This potential biomarker showed a relatively satisfactory sensitivity and specificity of 82% and 80.9%, respectively [81]. In 2024, Lu et al. evaluated the diagnostic potential of six miRNAs (miR-221-5P, miR-181a-5p, miR98-5p, miR-15a-5p, miR-222-3p, and miR-197-3p). Among them, a panel of four molecules (miR-221-5P, miR-181a-5p, miR-15a-5p, and miR-222-3p) showed sensitivity and specificity values of 82.14% and 85.71%, respectively [82]. Currently, a huge number of studies exploring the potential of miRNA molecules as diagnostic biomarkers of BC are emerging. However, most of them, despite promising sensitivity and specificity results, require further study due to various limitations of the experiments performed, as the results are often not very reproducible [83]. Despite this, the currently available literature reports clearly indicate the definite diagnostic potential of miRNA molecules.
Another type of ncRNAs that is promising for BC detection is lncRNAs. In a comparative analysis of seven publications providing specificity and sensitivity results of the UCA1 molecule, it was found that it achieved a sensitivity of 84% and specificity of 87% [48,84]. On the other hand, a diagnostic panel consisting of three different lncRNAs (MALAT1, PCAT-1, and SPRY4-IT1) showed a specificity of 85.6% and a sensitivity of 62.5% [85]. Another panel, combining four lncRNAs (UCA1-201, HOTAIR, HYMA1, and MALAT1) showed a sensitivity of 93.3% and specificity of 96.7% and was able to effectively distinguish NMIBC patients from BC patients [86]. In 2024, Gao et al. investigated the diagnostic potential of the hypermethylated RMRP gene for BC diagnostics. Their study demonstrated that it exhibited high diagnostic potential when using the RT-qPCR method, with a sensitivity and specificity of 83% and 70%, respectively. However, the most intriguing results were obtained using the modern RT-RAA-CRISPR/Cas12a method, which showed a surprisingly high diagnostic potential for this epigenetic modification, with a sensitivity and specificity of 95% and 92.5%, respectively [87]. Additionally, Liu et al. demonstrated that hypermethylation of the TERC gene also shows promising diagnostic potential, with reported sensitivity and specificity levels of 78.7% and 77.8%, respectively [88].
Other ncRNAs studied for their potential use as diagnostic biomarkers for BC include circRNAs. In 2024, Yang et al. demonstrated the high diagnostic potential of circRNA0071196, with a sensitivity and specificity of 87.5% and 85%, respectively. This circRNA is likely involved in regulating miR-19b-3p and CIT expression [89]. Another interesting example is hsa-circ0137439, which exhibited a diagnostic potential to distinguish BC patients from controls, with a sensitivity of 87.9% and specificity of 80.1%. For distinguishing NMIBC patients from MIBC patients, the sensitivity was 88.6% and the specificity was 73.5% [90].
A summary of the ncRNA-based biomarkers discussed and proposals for biomarkers not mentioned in the text are provided in Table 3 and Figure 1.

Table 3.
NcRNA-based biomarkers for BC diagnosis validated in cohort studies.
Despite the promising results and the high potential offered by epigenetic alterations, most of the proposed biomarkers still require further research. None of the available biomarkers have found application in clinical practice yet due to the lack of adequate validation, the high cost of developed tests and diagnostic panels, or complicated and expensive analytical procedures. Some of the proposed biomarkers show too low sensitivity; additionally, they do not allow, at this point, the detection of cancer at an early stage, which is key to increasing the chances of patient survival and accelerating the selection of appropriate treatment. Therefore, there is still a need to search for new highly sensitive and highly specific biomarkers for early detection of BC.
3.5. Potential Epigenetic Biomarkers in BC (Bioinformatic Analyses and Cell Line Studies)
The following section presents examples of proposed diagnostic biomarkers for BC. It includes both selected results of recent bioinformatic analyses and results of studies conducted on cell lines, suggesting possible development paths and potential new subjects for future research using biological material such as readily available urine and blood.
In the year 2023, a bioinformatic analysis of potential biomarkers related to BC diagnosis based mainly on data from the Gene Expression Omnibus (GEO) database was published. The analysis included selected genes hypermethylated in BC for which their expression was downregulated following this epigenetic modification [105]. One of them was SPARCL1, which belongs to the acidic and cysteine-rich protein family secreted in the cellular matrix and is responsible for encoding an extracellular matrix glycoprotein. It is involved in the development and progression of various cancers. Significant downregulation of gene expression is observed in BC, breast, cervical, rectal, lung, and ovarian cancers, among others, and it is considered a tumour suppressor gene [106]. In addition, the product of the SPARCL1 gene affects the viability of cancer cells and exhibits anti-adhesion effects [107,108,109]. Therefore, SPARCL1 methylation shows great potential as a biomarker for BC detection and a prognostic biomarker [105,106]. The above-mentioned bioinformatic analysis also indicated a significant difference in the methylation level of the MFAP4 gene, which encodes an extracellular matrix protein. In comparison between healthy and BC tissues, the expression of MFAP4 gene was reduced in BC. COX7A1 was also mentioned as a potential diagnostic biomarker in BC. This gene encodes the 7A1 subunit of cytochrome c oxidase, which plays a key role in the mitochondrial electron transport chain. Its role as a useful biomarker in cancer prognosis, such as in gastric cancer, is now suggested [110]. The bioinformatic analysis mentioned earlier also suggested the potential of using the methylation level of the EFEMP1 gene as a potential BC diagnostic biomarker [105]. It encodes the epidermal growth factor-containing fibulin-like extracellular matrix protein 1, but its function in tumorigenesis is not yet fully understood. Additionally, it shows high sensitivity and specificity (77.8% and 97.3%, respectively) for prostate cancer diagnosis [111]. The analysis also suggested the potential of using ABCA8 and MAMDC2 gene methylation as potential BC diagnostic biomarkers, but as with the methylation patterns mentioned earlier, they require further analysis [105].
Among potential BC diagnostic biomarkers, there are also several emerging studies using BC cell lines to analyse expression levels and the role that miRNAs play in BC pathogenesis. An interesting biomarker candidate is the miR-3622 molecule, which is a negative regulator of LASS2, known as a tumour metastasis suppressor gene. Overexpression of miR-3622 was observed to lead to increased proliferation and invasion of BC cells [112]. In 2022, Xu et al. conducted an analysis of miR-494-3p expression using BC cell lines and human bladder epithelial cells in which they showed that the expression level of this molecule was increased in BC cells. In addition, it was determined that it promoted the growth of BC cells by regulating the KLF9/RGS axis, suggesting that it may be an interesting object of study using patient-derived biological material to analyse its potential use as a diagnostic biomarker or therapeutic target in BC [113]. Another miRNA molecule is miR-616-5p, the expression level of which in BC cell lines was studied in 2021 by Ren et al. It was proven to promote the proliferation of BC cells in which its expression level was increased [114].
Currently, numerous studies are focused on the role that lncRNAs play in both cancer diagnosis and therapy. A recent study conducted on cell lines also suggested the exploratory potential of the lncRNA DUXAP10 [115]. This experiment showed that the expression of DUXAP10 in human BC cell lines was significantly higher than in non-BC cell lines. After knockdown of the DUXAP10 gene, it was found that BC cell proliferation was inhibited, whereas in normal cells, the process was not significantly affected. In addition, a significant reduction in Akt and mTOR phosphorylation involved in the PI3K/Akt/mTOR signalling pathway, which is critical for cancer, was observed [116]. Such results provide hope not only for the use of assessing lncRNA DUXAP10 expression levels as a diagnostic or prognostic biomarker in BC but also for the development of novel therapeutic approaches for this disease. In 2024, a paper by Arim et al. was published showing the results of a study of the diagnostic potential of lncRNA BCYRN1. A knockdown was performed, which resulted in a decrease in BC cell viability. In addition, the serum levels of BCYRN1 in BC patients and healthy subjects were also examined, showing that its levels were significantly higher in BC patients. Such results suggest the possibility of the use of this lncRNA both as an epigenetic biomarker for the diagnosis of BC as well as a potential molecular target for therapy of this disease [117]. Another potential lncRNA-based biomarker is upregulated XIST, the molecular targets of which include TET1 and the p53 protein, where it negatively regulates the p53 protein by binding to TET1. XIST plays an important role in regulating proliferation, migration, and apoptosis in BC cells [118,119]. In 2024, Zhou et al. reported the possible potential of GAS6-AS1 as a novel biomarker useful in the diagnosis, prognosis, and treatment of BC. The researchers investigated the mechanism of action of this lncRNA and indicated that it was involved in an interaction with miR-367-3p and PRC1. GAS6-AS1 was found to increase MMP7 expression through interaction with miR-367-3p and decreased APC expression through PRC1 binding, thereby promoting BC progression [120].
Nowadays, many scientific reports attribute an important role in BC pathogenesis to circRNA molecules. Given this, many researchers are looking for potential diagnostic biomarkers among them. In 2022, Yang et al. investigated the expression level of Hsa_circRNA_0088036 using BC cell lines and tissues from BC patients and proved that the expression level in BC cells was significantly higher compared to that in healthy cells. Knockdown of Hsa_circRNA_0088036 led to the inhibition of growth, migration, and invasion of BC cells. The increased expression of this molecule suggested the possibility of using it as a diagnostic biomarker of BC [121]. In 2021, the molecular mechanism of action and expression levels of circFLNA were analysed. It was found that this molecule played an important role in inhibiting BC cellular grounding by regulating miR-216a-3p and BTG2, and its level in BC cells and tissues was downregulated [122]. In 2024, Yi et al. identified a novel circPSMA7 molecule that showed increased expression levels in both BC cell lines and tissues. Additionally, it was found to promote cell proliferation and metastasis formation, playing a key role in tumour progression. Therefore, the authors suggested that circPSMA7 may be considered a potential diagnostic biomarker and additionally may represent a novel therapeutic target in BC patients [123].
4. Epigenetic Biomarkers in Clinical Prognosis of BC
4.1. DNA Methylation Biomarkers—Cohort Studies
There are many reports indicating the potential of DNA methylation biomarkers in predicting the course of cancers as well as the response to the treatment used [124]. An example is the study by Yoon HY. et al. in 2016, which looked at RSPH9 methylation in BC. They indicated that hypermethylation of this gene was an independent predictor of relapse and disease progression, as evidenced by hazard ratios (HR) of 3.02 (p = 0.001) and 8.25 (p = 0.028), respectively [125]. A similar analysis was performed by Zhan L. et al. in 2017, who showed that hypermethylation of the RASSF1A gene was significantly more common in patients with BC and was associated with a shortened OS (HR = 2.24, 95% CI: 1.45; 3.48; p = 0.0003) [126]. On the other hand, Shivakumar M. et al. in 2017 observed a correlation between hypomethylation of the NACC2 gene and OS (HR not reported; p = 0.0321). NACC2 contributes to the inhibition of MDM2 expression, which is responsible for normal p53 expression, leading to BC progression and shorter patient survival [127]. Analogous results were reported by Zhang Y. et al. in 2018, who showed that hypomethylation of the CDH1 gene was associated with the occurrence of reduced OS in BC patients (HR not reported; p > 0.0001). The protein encoded by CDH1 is closely associated with the regulation of cell division, and hypomethylation of this gene results in excessive cellular proliferation and BC progression [128].
Recently, there have been other new biomarkers based on DNA methylation crucial in predicting the course of the disease as well as the survival of BC patients [129]. An analysis by Takagi K. et al. in 2022 indicated that CALN1 hypomethylation was correlated with advanced tumour stage (rho = −0.563, p = 0.0007) and was an independent risk factor for recurrence, as suggested by an HR of 3.83 (95% CI; 1.14–13.0, p = 0.031) [130]. Analogous results in peripheral blood samples were obtained by Chen JQ. et al. in 2022, as they observed a statistically significant relationship between reduced methylation levels of the BLCAP gene and cancer recurrence or death within 10 years (p = 0.03; false discovery rate (FDR) = 2.63 × 10−14) [131]. A similar study was conducted by Zhang C. et al. in 2020 to assess the predictive value of methylation of certain genes, including KRT8. Their results showed that a hypomethylated KRT8 gene was correlated closely with the prognosis of survival of patients with bladder cancer (p = 0.003), which was also confirmed by an HR of 6.74 [132]. Kim C. et al. also focused on the relationship between a certain level of DNA methylation and the prognosis of patients with BC and observed that hypermethylation of the PTK2 gene was associated with a statistically significant increased mortality (HR not reported, p = 0.022). In addition, increased PTK2 expression also contributed to poor survival outcomes in this group of patients [133]. However, DNA methylation-based biomarkers, despite their potential use in bladder cancer prognosis, still require further study [134].
A summary of the discussed DNA methylation-based biomarkers and proposed biomarkers not mentioned in the text are provided in Table 4 and Figure 2.

Table 4.
DNA methylation-based biomarkers for BC prognosis validated in cohort studies.
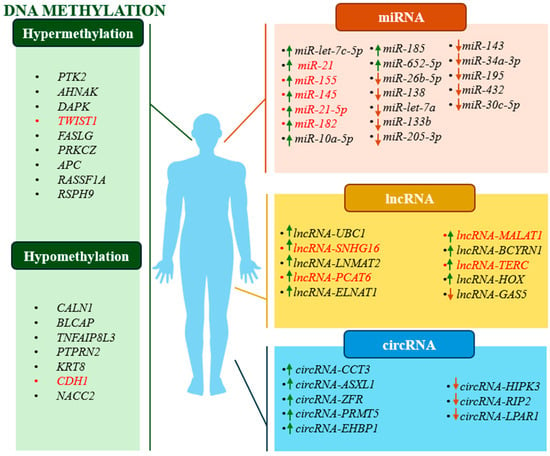
Figure 2.
Schematic representation of studied changes in methylation status and type of regulation of potential prognostic biomarkers. Green arrows indicate upregulation and red arrows indicate downregulation of a given ncRNA. Red text indicates examples of biomarkers that show both diagnostic and prognostic potential.
4.2. ncRNA Biomarkers—Cohort Studies
Many have suggested that ncRNAs may also act as markers in predicting the development and recurrence of various cancers and patient response to therapy [119]. There has been a lot of work on the existence of these correlations, and one example is the analysis performed by Mitash et al. in 2017. It showed that there was a correlation between high miR-21 expression in tumour tissues and shortened time to recurrence-free survival (RFS) (HR = 0.64, 95% CI; log rank = 0.03). MiR-21 negatively affects the expression of PTEN, a tumour suppressor gene, contributing to the uncontrolled development of BC [139]. A 2016 paper by Zhang X. et al. showed that high miR-155 expression in urine samples of BC patients correlated with shortened RFS (HR = 3.497; 95% CI: 1.722–7.099; p = 0.001) and progression-free survival (PFS) (HR = 10.146; 95% CI: 389–74.091; p = 0.022). MiR-155 causes inhibition of APC gene expression, and this in turn affects Wnt/β-catenin pathway activation and BC progression [98]. Another analysis, which in turn observed the effect of low miR-133b expression in tissues from BC patients on shorter PFS (RR = 2.97; 95% CI: 1.78–6.44; p = 0.009) and OS (RR = 4.23; 95% CI: 1.51–11.8; p = 0.011), is the study by Chen X. et al. in 2016. MiR-133b activates the EGFR gene, which induces the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) and phosphoinositide-3-kinase/protein kinase B/Akt (PI3K/AKT) signalling pathways. The result is excessive proliferation of cancer cells [140,141]. A 2018 paper by Avgeris M. et al. reported that patients with reduced lncRNA-GAS5 expression in tumour-lesioned tissues showed significantly shorter PFS (HR = 6.628; 95% CI: 1.494–29.40; p = 0.013). Silencing of lncRNA-GAS5 contributed to an increased proportion of tumour cells in the S/G2 phase of the cell cycle, which was associated with overexpression of the CDK6 gene, responsible for cycle regulation [142]. Jiao R. et al. in 2020 indicated that increased expression of lncRNA-SNHG16 in tested tumour tissues was significantly associated with decreased OS in patients (HR = 2.523, 95% CI: 1.540–4.133, p < 0.001). By interacting with CCL5, LncRNA-SNHG16 disrupts signalling pathways, affecting chemokine expression and creating a microenvironment that promotes BC growth [143]. Chen C. et al., in 2020, addressed a similar issue, observing a correlation between overexpression of lncRNA-LNMAT2 in tumour-transformed tissues and shorter OS (HR = 0.62; 95% CI: 0.42–0.91; p < 0.05). Overexpression of lncRNA-LNMAT2 activates the PROX1 gene, which promotes BC progression and lymph node metastasis [144]. By contrast, Lin G. et al. in 2019 observed an association between low expression of circRNA-LPAR1 in tissues of BC patients and shortened disease-specific survival (DSS, HR = 0.364; 95% CI: 0.197–0.673; p = 0.001). Circ-LPAR1 interacting with the WNT5A gene activates the Wnt/β-catenin pathway and leads to BC cell proliferation [145,146]. A similar study was conducted by Tang G. et al. in 2017, where they evaluated the relationship between high expression of circRNA-ASXL1 in tumour tissue and reduced OS (HR = 4.831; 95% CI: 1.718–13.514; p = 0.007). CircRNA-ASXL1 affects the TP53 gene by deregulating the mechanism of action of the p53 protein, resulting in disruption of the body’s defence against BC development [104].
Noteworthy is the fact that the topic of ncRNA research and BC prediction is constantly being expanded with new studies that provide a lot of valuable information. A paper by Andrew AS. et al. in 2019 showed that low expression of miR-26b-5p in tumour tissue was associated with reduced PFS in BC patients (HR = 0.043, 95% CI; 0.0079–0.24, p = 0.0008). MiR-26b-5p affects the level of the PLOD2 gene, which in turn is involved in collagen maturation. In case of aberrant expression of PLOD2, stiffness and dysregulation of the extracellular matrix occur, and further migration and invasion of BC cells follows [147,148]. Borkowska et al. in 2019 also focused on demonstrating an association between high miR-21-5p expression in bladder cancer tissues and shortened overall survival (OS, HR = 1.00009, 95% CI: 1.000025–1.00015, p = 0.0069). MiR-21-5p may interact with the TP53 gene to inhibit the mechanisms controlled by p53, reducing the effectiveness of the cell’s natural defences against tumorigenesis [149]. On the other hand, an analysis by Juracek J. et al. in 2019 showed a correlation between reduced miR-34a-3p expression in tissues and shortened OS in patients with BC (HR = 0.3184, 95% CI: 0.003–0.681, p = 0.0258). MiR-34a-3p interacts with the tumour suppressor gene PTEN, contributing to BC progression [150,151]. As another example, a study by Yang L. et al. in 2021 observed a correlation between high miR-10a-5p expression in the plasma of bladder cancer patients and reduced OS (HR = 1.74, 95% CI: 1.31–2.01, p = 0.002). MiR-10a-5p regulates the expression of the FGFR3 gene, which, when activated, causes dimerisation of fibroblast growth factor receptor 3, which promotes BC cell proliferation and survival [149,152,153]. Yin et al. 2019 noted that elevated miR-185 expression in tumour tissues of BC patients was correlated with shorter OS (HR = 0.820; 95% CI: 0.688–0.978; p = 0.027). MiR-185, through interaction with the ITGB5 gene, may lead to excessive proliferation and invasion of tumour-lesioned cells [154]. In 2022, Yerukala S. et al. also analysed the correlation between high miR-652-5p expression in tumour tissues and shortened OS (HR = 0.54, 95% CI: 0.40–0.72, p = 0.00004). The effect of miR-652-5p on the KCNN3 gene, which regulates the flow of potassium ions in biological membranes, can lead to abnormal membrane potential, resulting in abnormal cell proliferation [155,156]. By contrast, Chen C. et al. in 2021 showed that overexpression of lncRNA-ELNAT1 in urine was correlated with reduced OS in patients with BC (HR = 1.76; 95% CI: 1.21–2.55; p < 0.01). LncRNA-ELNAT1, like lncRNA-LNMAT2, also promotes lymphatic metastasis by affecting the UBC9 gene in bladder cancer [157]. Liang T. et al. in 2021 noted that there was a correlation between high expression of lncRNA-MALAT1a and shortened OS (HR = 3.278; 95% CI: 2.159–3.430; p = 0.004). LncRNA-MALAT1, through its activating effect on the MDM2 gene, can lead to decreased p53 activity, which is associated with BC progression [158]. In a study by Zheng H. et al. in 2021, an association between lncRNA-BCYRN1 overexpression in tissues of BC patients and shorter OS was observed (HR = 1.58; 95% CI: 1.07–2.33; p < 0.05). This was because increased lncRNA-BCYRN1 expression targeted the WNT5A gene, contributed to activation of the Wnt/β-catenin signalling pathway, and consequently to the growth and migration of BC cells [159]. Another study, also related to proving a correlation between high lncRNA- TERC expression in BC patient tissues and reduced OS, is the analysis by Chen C. et al. in 2022 (HR = 1.51; 95% CI: 0.95–2.39). Overexpressed lncRNA-TERC interacts with the TERT gene, promoting increased telomerase activation in cancer cells and leading to uncontrolled BC progression [88,160]. A study by Xie F. et al. in 2020 reported that low expression of circRNA-HIPK3 in BC tissues was associated with shorter OS in these patients (HR = 0.4155; 95% CI: 0.2148–0.8036; p = 0.0091). Silencing of circRNA-HIPK3 contributed to the inhibition of SOX4 gene expression, which regulates mesenchymal features and may result in an increased ability to migrate and metastasise in BC patients [161,162]. Analogous results were obtained by Su Y. et al. in 2020, as they showed that low circRNA-RIP2 expression in tumour-lesioned tissues was associated with shortened OS in BC patients (HR = 0.339; 95% CI: 0.128–0.898; p = 0.029). Reduced circRNA-RIP2 expression deregulates normal SMAD3 gene expression, leading to uncontrolled activation of the TGF-β/SMAD signalling pathway, and this promotes BC cell invasion [163]. A similar relationship was studied by Zhu J. et al. in 2021 in the context of circRNA-EHBP1 and OS. As a result of the analyses, they showed that high expression of this molecule in tissues from BC patients was associated with shorter OS (HR = 0.48; 95% CI: 0.31–0.74; p < 0.01). CircEHBP1 increases TGFβR1 gene expression, thereby activating the TGF-β/SMAD3 signalling pathway, which promotes lymph angiogenesis in BC [164].
A summary of the ncRNA-based biomarkers discussed and proposals for biomarkers not mentioned in the text are provided in Table 5 and Figure 2.

Table 5.
The ncRNA-based biomarkers for BC prognosis validated in cohort studies.
5. Strength and Limitations
One of the main strengths of this review is the wide range of research papers included in the analysis, as evidenced by the extensive bibliography, which focuses mainly on recent work, thus providing a solid update of the current state of knowledge. The selection of papers analysed was based on several different epigenetic mechanisms with high potential for use as diagnostic and prognostic biomarkers of BC, allowing a wide range of data to be presented. This review article summarises and analyses data on the diagnostic potential of selected biomarkers, which are presented in a clear and lucid manner and can be a valuable resource for those wishing to learn about the current state of knowledge and trends discussed in this field. On the other hand, we are aware of the limitations of this narrative review. Selection of the literature was based mainly on English language articles, which may exclude important studies available in other languages. In addition, the focus was on specific indicators for assessing the diagnostic and prognostic utility of epigenetic biomarker data, while others were discarded. Attention was focused primarily on recent articles, which may have excluded older studies that are relevant to the field. Due to these limitations, the findings of the review should be interpreted with caution.
6. Conclusions
Early diagnosis and accurate assessment of prognosis are key to successful treatment of BC. The research findings included in this review demonstrate the high potential of epigenetic alterations as diagnostic and prognostic biomarkers in BC. For early-stage cancers, DNA methylation-based biomarkers are of particular importance Recent scientific work indicates the high potential of hypermethylated genes TWIST1, VIM, ZNF671, OTX1, and IRF8 for early diagnosis and PTK2, AHNAK, and DAPK for BC prognosis. On the other hand, among ncRNAs, a panel of three miRNA molecules, miR-20a-5p, miR-921-3p, and miR-17-5p, or lncRNA RMRP, among others, had the highest sensitivity and specificity values for detecting BC. The ncRNA molecules also appear to show potential in the prognosis of BC. Recently, the utility of circRNA CCT3, lncRNA-HOX, and miR-205-3P as promising prognostic biomarkers of BC has been proposed. In addition, some epigenetic changes, such as miR-155 and circRNA CCT3, are useful in both early detection and prognosis of BC. An important advantage of the epigenetic changes discussed is that they can be studied in readily available biological materials such as blood or urine. However, despite the promising results of these studies, epigenetic alterations are still not part of the standard methods for diagnosing and predicting BC, e.g., due to the study groups being too small, lack of reproducibility of results, lack of adequate validation of the results obtained, and the need for appropriate facilities, equipment, and highly qualified personnel, which can generate high costs. On the other hand, finding an epigenetic biomarker with high sensitivity and specificity could, in practice, be more cost efficient than a standardised expensive cystoscopy. In addition, it is worth noting that finding an effective biomarker to detect BC at an early stage of development could dramatically reduce the costs associated with both diagnosis and treatment of the patient itself. Given the above, further research, especially conducted on sufficiently large groups of patients, taking into account the validation of the results obtained, as well as aiming to optimise diagnostic methods and obtain biomarkers that combine all of the characteristics of a desirable BC biomarker, seems fully justified.
Author Contributions
Conceptualization, N.J. and R.M.; Methodology, N.J. and R.M.; Resources, N.J. and A.B.; Data Curation, N.J. and A.B.; Writing—Original Draft Preparation, N.J., A.B., J.S., R.S., W.K. and R.M.; Writing—Review & Editing, N.J. and R.M.; Visualization, N.J.; Supervision, R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Silverman, D.T.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Association between smoking and risk of bladder cancer among men and women. JAMA 2011, 306, 737–745. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. List of Classifications by Cancer Sites with Sufficient or Limited Evidence in Humans. IARC Monographs Volumes 1–136a. Available online: https://monographs.iarc.who.int/wp-content/uploads/2019/07/Classifications_by_cancer_site.pdf (accessed on 5 July 2024).
- Dobruch, J.; Oszczudłowski, M. Bladder cancer: Current challenges and future directions. Medicina 2021, 57, 749. [Google Scholar] [CrossRef] [PubMed]
- Henning, G.M.; Barashi, N.S.; Smith, Z.L. Advances in biomarkers for detection, surveillance, and prognosis of bladder cancer. Clin. Genitourin. Cancer 2021, 19, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Kluth, L.A.; Black, P.C.; Bochner, B.H.; Catto, J.; Lerner, S.P.; Stenzl, A.; Sylvester, R.; Vickers, A.J.; Xylinas, E.; Shariat, S.F. Prognostic and prediction tools in bladder cancer: A comprehensive review of the literature. Eur. Urol. 2015, 68, 238–253. [Google Scholar] [CrossRef]
- Gilyazova, I.; Enikeeva, K.; Rafikova, G.; Kagirova, E.; Sharifyanova, Y.; Asadullina, D.; Pavlov, V. Epigenetic and Immunological Features of Bladder Cancer. Int. J. Mol. Sci. 2023, 24, 9854. [Google Scholar] [CrossRef]
- Lobo, N.; Afferi, L.; Moschini, M.; Mostafid, H.; Porten, S.; Psutka, S.P.; Gupta, S.; Smith, A.B.; Williams, S.B.; Lotan, Y. Epidemiology, screening, and prevention of bladder cancer. Eur. Urol. Oncol. 2022, 5, 628–639. [Google Scholar] [CrossRef]
- Dyrskjøt, L.; Hansel, D.E.; Efstathiou, J.A.; Knowles, M.A.; Galsky, M.D.; Teoh, J.; Theodorescu, D. Bladder cancer. Nat. Rev. Dis. Primers 2023, 9, 58. [Google Scholar] [CrossRef]
- Hurst, C.D.; Alder, O.; Platt, F.M.; Droop, A.; Stead, L.F.; Burns, J.E.; Burghel, G.J.; Jain, S.; Klimczak, L.J.; Lindsay, H.; et al. Genomic subtypes of non-invasive bladder cancer with distinct metabolic profile and female gender bias in KDM6A mutation frequency. Cancer Cell 2017, 32, 701–715.e7. [Google Scholar] [CrossRef]
- Ségal-Bendirdjian, E.; Geli, V. Non-canonical roles of telomerase: Unraveling the imbroglio. Front. Cell Dev. Biol. 2019, 7, 332. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Rinaldetti, S.; Cheikh, B.B.; Zhou, Q.; Hass, E.P.; Jones, R.T.; Joshi, M.; LaBarbera, D.V.; Knott, S.R.V.; Cech, T.R.; et al. TRIM28 is a transcriptional activator of the mutant TERT promoter in human bladder cancer. Proc. Natl. Acad. Sci. USA 2021, 118, e2102423118. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, E.; L’hôte, C.G.; Kennedy, W.; Tomlinson, D.C.; Knowles, M.A. Mutant fibroblast growth factor receptor 3 induces intracellular signaling and cellular transformation in a cell type-and mutation-specific manner. Oncogene 2009, 28, 4306–4316. [Google Scholar] [CrossRef] [PubMed]
- Hurst, C.D.; Cheng, G.; Platt, F.M.; Castro, M.A.; Marzouka, N.-A.S.; Eriksson, P.; Black, E.V.; Alder, O.; Lawson, A.R.; Lindskrog, S.V.; et al. Stage-stratified molecular profiling of non-muscle-invasive bladder cancer enhances biological, clinical, and therapeutic insight. Cell Rep. Med. 2021, 2, 100472. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Borresen-Dale, A.L.; et al. Erratum: Signatures of mutational processes in human cancer (Nature (2013) 500 (415–421). Nature 2013, 502, 258. [Google Scholar] [CrossRef]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 2017, 171, 540–556.e25. [Google Scholar] [CrossRef]
- Li, Q.; Damish, A.W.; Frazier, Z.; Liu, D.; Reznichenko, E.; Kamburov, A.; Bell, A.; Zhao, H.; Jordan, E.J.; Gao, S.P.; et al. ERCC2 helicase domain mutations confer nucleotide excision repair deficiency and drive cisplatin sensitivity in muscle-invasive bladder cancer. Clin. Cancer Res. 2019, 25, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.V.; Hurst, C.D.; Knowles, M.A. Oncogenic FGFR3 gene fusions in bladder cancer. Hum. Mol. Genet. 2013, 22, 795–803. [Google Scholar] [CrossRef]
- Huan, J.; Grivas, P.; Birch, J.; Hansel, D.E. Emerging roles for mammalian target of rapamycin (mTOR) complexes in bladder cancer progression and therapy. Cancers 2022, 14, 1555. [Google Scholar] [CrossRef] [PubMed]
- Goriki, A.; Seiler, R.; Wyatt, A.W.; Contreras-Sanz, A.; Bhat, A.; Matsubara, A.; Hayashi, T.; Black, P.C. Unravelling disparate roles of NOTCH in bladder cancer. Nat. Rev. Urol. 2018, 15, 345–357. [Google Scholar] [CrossRef]
- Li, S.; Xin, K.; Pan, S.; Wang, Y.; Zheng, J.; Li, Z.; Liu, X.; Liu, B.; Xu, Z.; Chen, X. Blood-based liquid biopsy: Insights into early detection, prediction, and treatment monitoring of bladder cancer. Cell. Mol. Biol. Lett. 2023, 28, 28. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.K.; Covarrubias, S.; Carpenter, S. The how and why of lncRNA function: An innate immune perspective. Biochim. Biophys. Acta (BBA)—Gene Regul. Mech. 2020, 1863, 194419. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Yang, L. Regulation of circRNA biogenesis. RNA Biol. 2015, 12, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Tsukahara, T. A view of pre-mRNA splicing from RNase R resistant RNAs. Int. J. Mol. Sci. 2014, 15, 9331–9342. [Google Scholar] [CrossRef]
- Geng, Y.; Jiang, J.; Wu, C. Function and clinical significance of circRNAs in solid tumors. J. Hematol. Oncol. 2018, 11, 98. [Google Scholar] [CrossRef]
- Poletajew, S.; Krajewski, W.; Kaczmarek, K.; Kopczyński, B.; Stamirowski, R.; Tukiendorf, A.; Zdrojowy, R.; Słojewski, M.; Radziszewski, P. The learning curve for transurethral resection of bladder tumour: How many is enough to be independent, safe and effective surgeon? J. Surg. Educ. 2020, 77, 978–985. [Google Scholar] [CrossRef]
- Morales, A.; Eidinger, D.; Bruce, A.W. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J. Urol. 1976, 116, 180–182. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology guidelines on non–muscle-invasive bladder cancer (Ta, T1, and carcinoma in situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef]
- Huncharek, M.; Geschwind, J.F.; Witherspoon, B.; McGarry, R.; Adcock, D. Intravesical chemotherapy prophylaxis in primary superficial bladder cancer: A meta-analysis of 3703 patients from 11 randomized trials. J. Clin. Epidemiol. 2000, 53, 676–680. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Espinós, E.L.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology guidelines on muscle-invasive and metastatic bladder cancer: Summary of the 2020 guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef]
- Compérat, E.; Amin, M.B.; Cathomas, R.; Choudhury, A.; De Santis, M.; Kamat, A.; Stenzl, A.; Thoeny, H.C.; Witjes, J.A. Current best practice for bladder cancer: A narrative review of diagnostics and treatments. Lancet 2022, 400, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
- De Santis, M.; Bellmunt, J.; Mead, G.; Kerst, J.M.; Leahy, M.; Maroto, P.; Gil, T.; Marreaud, S.; Daugaard, G.; Skoneczna, I.; et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J. Clin Oncol. 2012, 30, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazadeh, A.; Mellado, B.; Varlamov, S.; et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 2019, 381, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Hoimes, C.J.; Flaig, T.W.; Milowsky, M.I.; Friedlander, T.W.; Bilen, M.A.; Gupta, S.; Srinivas, S.; Merchan, J.R.; McKay, R.R.; Petrylak, D.P.; et al. Enfortumab vedotin plus pembrolizumab in previously untreated advanced urothelial cancer. J. Clin. Oncol. 2023, 41, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, S.T.; Balar, A.V.; Petrylak, D.P.; Kalebasty, A.R.; Loriot, Y.; Fléchon, A.; Jain, R.K.; Agarwal, N.; Bupathi, M.; Barthelemy, P.; et al. TROPHY-U-01: A phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J. Clin. Oncol. 2021, 39, 2474–2485. [Google Scholar] [CrossRef]
- Pan, S.; Zhan, Y.; Chen, X.; Wu, B.; Liu, B. Bladder cancer exhibiting high immune infiltration shows the lowest response rate to immune checkpoint inhibitors. Front. Oncol. 2019, 9, 1101. [Google Scholar] [CrossRef]
- Zhou, B.; Guo, R. Integrative analysis of genomic and clinical data reveals intrinsic characteristics of bladder urothelial carcinoma progression. Genes 2019, 10, 464. [Google Scholar] [CrossRef]
- Matuszczak, M.; Kiljańczyk, A.; Salagierski, M. A liquid biopsy in bladder Cancer—The current Landscape in urinary biomarkers. Int. J. Mol. Sci. 2022, 23, 8597. [Google Scholar] [CrossRef]
- Chatterjee, D.; Mou, S.I.; Sultana, T.; Hosen, M.I.; Faruk, M.O. Identification and validation of prognostic signature genes of bladder cancer by integrating methylation and transcriptomic analysis. Sci. Rep. 2024, 14, 368. [Google Scholar] [CrossRef]
- Cancer.gov. Available online: https://www.cancer.gov/types/bladder/survival (accessed on 27 April 2023).
- Lucca, I.; de Martino, M.; Klatte, T.; Shariat, S.F. Novel biomarkers to predict response and prognosis in localized bladder cancer. Urol. Clin. 2015, 42, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.G.; Munera-Maravilla, E.; Bernardini, A.; Rubio, C.; Suarez-Cabrera, C.; Segovia, C.; Lodewijk, I.; Dueñas, M.; Martínez-Fernández, M.; Paramio, J.M. Epigenetics of bladder cancer: Where biomarkers and therapeutic targets meet. Front. Genet. 2019, 10, 1125. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, G.; Wu, S. Advances in diagnosis and therapy for bladder cancer. Cancers 2022, 14, 3181. [Google Scholar] [CrossRef] [PubMed]
- Veeratterapillay, R.; Gravestock, P.; Nambiar, A.; Gupta, A.; Aboumarzouk, O.; Rai, B.; Vale, L.; Heer, R. Time to turn on the blue lights: A systematic review and meta-analysis of photodynamic diagnosis for bladder cancer. Eur. Urol. Open Sci. 2021, 31, 17–27. [Google Scholar] [CrossRef]
- Yafi, F.A.; Brimo, F.; Steinberg, J.; Aprikian, A.G.; Tanguay, S.; Kassouf, W. Prospective analysis of sensitivity and specificity of urinary cytology and other urinary biomarkers for bladder cancer. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2015; pp. 66.e25–66.e31. [Google Scholar]
- Tomiyama, E.; Fujita, K.; Hashimoto, M.; Uemura, H.; Nonomura, N. Urinary markers for bladder cancer diagnosis: A review of current status and future challenges. Int. J. Urol. 2024, 31, 208–219. [Google Scholar] [CrossRef]
- Schmidbauer, J.; Remzi, M.; Klatte, T.; Waldert, M.; Mauermann, J.; Susani, M.; Marberger, M. Fluorescence cystoscopy with high-resolution optical coherence tomography imaging as an adjunct reduces false-positive findings in the diagnosis of urothelial carcinoma of the bladder. Eur. Urol. 2009, 56, 914–919. [Google Scholar] [CrossRef]
- Li, Q.; Tang, J.; He, E.; Li, Y.; Zhou, Y.; Wang, B. Differentiation between high-and low-grade urothelial carcinomas using contrast enhanced ultrasound. Oncotarget 2017, 8, 70883. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stav, K.; Leibovici, D.; Goren, E.; Livshitz, A.; Siegel, Y.I.; Lindner, A.; Zisman, A. Adverse effects of cystoscopy and its impact on patients’ quality of life and sexual performance. Isr. Med. Assoc. J. 2004, 6, 474–478. [Google Scholar]
- Witjes, J.A.; Morote, J.; Cornel, E.B.; Gakis, G.; van Valenberg, F.J.P.; Lozano, F.; Sternberg, I.A.; Willemsen, E.; Hegemann, M.L.; Paitan, Y.; et al. Performance of the bladder EpiCheck™ methylation test for patients under surveillance for non–muscle-invasive bladder cancer: Results of a multicenter, prospective, blinded clinical trial. Eur. Urol. Oncol. 2018, 1, 307–313. [Google Scholar] [CrossRef]
- Feber, A.; Dhami, P.; Dong, L.; de Winter, P.; Tan, W.S.; Martínez-Fernández, M.; Paul, S.S.; Hynes-Allen, A.; Rezaee, S.; Gurung, P.; et al. UroMark—A urinary biomarker assay for the detection of bladder cancer. Clin. Epigenetics 2017, 9, 8. [Google Scholar] [CrossRef]
- Pharo, H.D.; Jeanmougin, M.; Ager-Wick, E.; Vedeld, H.M.; Sørbø, A.K.; Dahl, C.; Larsen, L.K.; Honne, H.; Brandt-Winge, S.; Five, M.; et al. BladMetrix: A novel urine DNA methylation test with high accuracy for detection of bladder cancer in hematuria patients. Clin. Epigenetics 2022, 14, 115. [Google Scholar] [CrossRef] [PubMed]
- Piatti, P.; Chew, Y.C.; Suwoto, M.; Yamada, T.; Jara, B.; Jia, X.Y.; Guo, W.; Ghodoussipour, S.; Daneshmand, S.; Ahmadi, H.; et al. Clinical evaluation of Bladder CARE, a new epigenetic test for bladder cancer detection in urine samples. Clin. Epigenetics 2021, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Bang, B.R.; Zhong, J.; Oh, T.J.; Lee, J.Y.; Seo, Y.; Woo, M.A.; Lim, J.S.; Na, Y.G.; Song, K.H.; Shin, J.H.; et al. EarlyTect BCD, a Streamlined PENK Methylation Test in Urine DNA, Effectively Detects Bladder Cancer in Patients with Hematuria. J. Mol. Diagn. 2024, 26, 613–623. [Google Scholar] [CrossRef]
- Steinbach, D.; Kaufmann, M.; Hippe, J.; Gajda, M.; Grimm, M.O. High Detection Rate for Non–Muscle-Invasive Bladder Cancer Using an Approved DNA Methylation Signature Test. Clin. Genitourin. Cancer 2020, 18, 210–221. [Google Scholar] [CrossRef]
- Harsanyi, S.; Novakova, Z.V.; Bevizova, K.; Danisovic, L.; Ziaran, S. Biomarkers of bladder cancer: Cell-free DNA, epigenetic modifications and non-coding RNAs. Int. J. Mol. Sci. 2022, 23, 13206. [Google Scholar] [CrossRef]
- Fang, Q.; Zhang, X.; Nie, Q.; Hu, J.; Zhou, S.; Wang, C. Improved urine DNA methylation panel for early bladder cancer detection. BMC Cancer 2022, 22, 237. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, A.E.; Beijert, I.J.; Bosschieter, J.; Kauer, P.C.; Vis, A.N.; Lissenberg-Witte, B.I.; Moorselaar, R.J.A.; Steenbergen, R.D.M.; Nieuwenhuijzen, J.A. Bladder cancer detection in urine using DNA methylation markers: A technical and prospective preclinical validation. Clin. Epigenetics 2022, 14, 19. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Liu, Y.S.; Wei, Y.C.; Jhang, J.F.; Kuo, H.C.; Huang, H.H.; Chan, M.W.Y.; Lin, G.L.; Cheng, W.C.; Lin, S.C.; et al. Hypermethylation Loci of ZNF671, IRF8, and OTX1 as Potential Urine-Based Predictive Biomarkers for Bladder Cancer. Diagnostics 2024, 14, 468. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jiang, G.; Zhang, N.; Liu, S.; Lin, X.; Perschon, C.; Zheng, S.L.; Ding, Q.; Wang, X.; Na, R.; et al. HOXA9, PCDH17, POU4F2, and ONECUT2 as a urinary biomarker combination for the detection of bladder cancer in Chinese patients with hematuria. Eur. Urol. Focus 2020, 6, 284–291. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Ruan, W.; Huang, M.; Wang, C.; Wang, H.; Jiang, Z.; Wang, S.; Liu, Z.; Liu, C.; et al. Urine DNA methylation assay enables early detection and recurrence monitoring for bladder cancer. J. Clin. Investig. 2020, 130, 6278–6289. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, X.; Wang, T.; Lu, Y.; Lu, Z.; Wang, T.; Pan, Z. Clinical performance and utility of a noninvasive urine-based methylation biomarker: TWIST1/Vimentin to detect urothelial carcinoma of the bladder. Sci. Rep. 2024, 14, 7941. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Chao, H.; Deng, H.; Yu, Z.; Zhao, R.; Huang, L.; Gong, Y.; Zhu, Y.; Wang, Q.; Li, F.; et al. A novel and sensitive DNA methylation marker for the urine-based liquid biopsies to detect bladder cancer. BMC Cancer 2022, 22, 510. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Chen, X.; Huang, M.; Wang, H.; Chen, J.; Liang, Z.; Zhang, J.; Yu, Y.; Chen, S.; Xu, S.; et al. A urine-based DNA methylation assay to facilitate early detection and risk stratification of bladder cancer. Clin. Epigenetics 2021, 13, 91. [Google Scholar] [CrossRef]
- Guo, R.Q.; Xiong, G.Y.; Yang, K.W.; Zhang, L.; He, S.M.; Gong, Y.Q.; He, Q.; Li, X.Y.; Wang, Z.C.; Bao, Z.Q.; et al. Detection of urothelial carcinoma, upper tract urothelial carcinoma, bladder carcinoma, and urothelial carcinoma with gross hematuria using selected urine-DNA methylation biomarkers: A prospective, single-center study. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2018; pp. 342.e15–342.e23. [Google Scholar]
- Roperch, J.P.; Grandchamp, B.; Desgrandchamps, F.; Mongiat-Artus, P.; Ravery, V.; Ouzaid, I.; Roupret, M.; Phe, V.; Ciofu, C.; Tubach, F.; et al. Promoter hypermethylation of HS3ST2, SEPTIN9 and SLIT2 combined with FGFR3 mutations as a sensitive/specific urinary assay for diagnosis and surveillance in patients with low or high-risk non-muscle-invasive bladder cancer. BMC Cancer 2016, 16, 704. [Google Scholar] [CrossRef]
- Dahmcke, C.M.; Steven, K.E.; Larsen, L.K.; Poulsen, A.L.; Abdul-Al, A.; Dahl, C.; Guldberg, P. A prospective blinded evaluation of urine-DNA testing for detection of urothelial bladder carcinoma in patients with gross hematuria. Eur. Urol. 2016, 70, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Tian, Y.; Xu, H. Improved Noninvasive Bladder Cancer Diagnosis using Urine Sediments and Novel DNA Methylation Biomarker Panels. Clin. Lab. 2016, 62, 327–336. [Google Scholar] [CrossRef]
- Wang, J.; Peng, X.; Li, R.; Liu, K.; Zhang, C.; Chen, X.; Huang, G.; Zhao, L.; Chen, Z.; Lai, Y. Evaluation of serum miR-17-92 cluster as noninvasive biomarkers for bladder cancer diagnosis. Front. Oncol. 2021, 11, 795837. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wei, X.; Qu, X.; Zhu, Y. Potential clinical application of microRNAs in bladder cancer. J. Biomed. Res. 2024, 38, 289–306. [Google Scholar] [CrossRef]
- Yu, Z.; Lu, C.; Lai, Y. A serum miRNAs signature for early diagnosis of bladder cancer. Ann. Med. 2023, 55, 736–745. [Google Scholar] [CrossRef]
- Suarez-Cabrera, C.; Estudillo, L.; Ramón-Gil, E.; Martínez-Fernández, M.; Peral, J.; Rubio, C.; Lodewijk, I.; de Bernardo, A.M.; Garcia-Escudero, R.; Villacampa, F.; et al. BlaDimiR: A urine-based miRNA score for accurate bladder cancer diagnosis and follow-up. Eur. Urol. 2022, 82, 663–667. [Google Scholar] [CrossRef]
- El-Shal, A.S.; Shalaby, S.M.; Abouhashem, S.E.; Elbary, E.H.A.; Azazy, S.; Rashad, N.M.; Sarhan, W. Urinary exosomal microRNA-96-5p and microRNA-183-5p expression as potential biomarkers of bladder cancer. Mol. Biol. Rep. 2021, 48, 4361–4371. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Lu, Z.; Liu, C.; Meng, Y.; Ma, Y.; Zhao, W.; Liu, J.; Yu, J.; Chen, J. miRNA-96 suppresses KRAS and functions as a tumor suppressor gene in pancreatic cancer. Cancer Res. 2010, 70, 6015–6025. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fu, H.; Xu, C.; Tie, Y.; Xing, R.; Zhu, J.; Qin, Y.; Sun, Z.; Zheng, X. miR-183 inhibits TGF-β1-induced apoptosis by downregulation of PDCD4 expression in human hepatocellular carcinoma cells. BMC Cancer 2010, 10, 354. [Google Scholar] [CrossRef]
- Myatt, S.S.; Wang, J.; Monteiro, L.J.; Christian, M.; Ho, K.K.; Fusi, L.; Dina, R.E.; Brosens, J.J.; Ghaem-Maghami, S.; Lam, E.W. Definition of microRNAs that repress expression of the tumor suppressor gene FOXO1 in endometrial cancer. Cancer Res. 2010, 70, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Shi, X.; Li, H.; Hui, J.; Liu, R.; Chen, Z.; Lu, Y.; Tan, W. Urinary Exosomal miRNAs as biomarkers of bladder Cancer and experimental verification of mechanism of miR-93-5p in bladder Cancer. BMC Cancer 2021, 21, 1293. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Fujita, K.; Jingushi, K.; Kawashima, A.; Ujike, T.; Nagahara, A.; Ueda, Y.; Tanigawa, G.; Yoshioka, I.; Ueda, K.; et al. MiR-21-5p in urinary extracellular vesicles is a novel biomarker of urothelial carcinoma. Oncotarget 2017, 8, 24668. [Google Scholar] [CrossRef]
- Chen, X. MiR-101 acts as a novel bio-marker in the diagnosis of bladder carcinoma. Medicine 2019, 98, e16051. [Google Scholar] [CrossRef]
- Lu, C.; Lin, S.; Wen, Z.; Sun, C.; Ge, Z.; Chen, W.; Li, Y.; Zhang, P.; Wu, Y.; Wang, W.; et al. Testing the accuracy of a four serum microRNA panel for the detection of primary bladder cancer: A discovery and validation study. Biomarkers 2024, 29, 276–284. [Google Scholar] [CrossRef]
- Grimaldi, A.M.; Lapucci, C.; Salvatore, M.; Incoronato, M.; Ferrari, M. Urinary miRNAs as a diagnostic tool for bladder cancer: A systematic review. Biomedicines 2022, 10, 2766. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Zhang, D.; Yu, Y.; Cai, L.; Zhang, C. Long non-coding RNA urothelial carcinoma–associated 1 as a tumor biomarker for the diagnosis of urinary bladder cancer. Tumor Biol. 2017, 39, 1010428317709990. [Google Scholar] [CrossRef]
- Zhan, Y.; Du, L.; Wang, L.; Jiang, X.; Zhang, S.; Li, J.; Yan, K.; Duan, W.; Zhao, Y.; Wang, L.; et al. Expression signatures of exosomal long non-coding RNAs in urine serve as novel non-invasive biomarkers for diagnosis and recurrence prediction of bladder cancer. Mol. Cancer 2018, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, R.; Han, C.; Wang, Z.; Jin, X. A panel of urinary long non-coding RNAs differentiate bladder cancer from urocystitis. J. Cancer 2020, 11, 781. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, X.; Wang, X.; Sun, R.; Li, Y.; Li, J.; Quan, W.; Yao, Y.; Hou, Y.; Li, D.; et al. The clinical value of rapidly detecting urinary exosomal lncRNA RMRP in bladder cancer with an RT-RAA-CRISPR/Cas12a method. Clin. Chim. Acta 2024, 562, 119855. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Shang, A.; Sun, Z.; Gao, Y.; Huang, J.; Ping, Y.; Chang, W.; Gu, C.; Sun, J.; Ji, P.; et al. Urinary exosomal long noncoding RNA TERC as a noninvasive diagnostic and prognostic biomarker for bladder urothelial carcinoma. J. Immunol. Res. 2022, 2022, 9038808. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Yao, W.; Zou, G.; Ye, X.; Mo, Q. Diagnostic value of urine cyclic RNA-0071196 for bladder urothelial carcinoma. BMC Urol. 2024, 24, 88. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, Q.; Zhu, J.; Yin, G.; Lin, L.; Liang, C. Identification of urinary hsa_circ_0137439 as a potential biomarker and tumor regulator of bladder cancer. Neoplasma 2020, 67, 137–146. [Google Scholar] [CrossRef]
- Mamdouh, S.; Sherif, H.; Romeih, M.; Elesaily, K. Urine micro-RNA signature as a potential non-invasive diagnostic biomarker in bladder cancer. Asian Pac. J. Cancer Prev. APJCP 2023, 24, 121. [Google Scholar]
- Zhang, X.F.; Zhang, X.Q.; Chang, Z.X.; Wu, C.C.; Guo, H. microRNA-145 modulates migration and invasion of bladder cancer cells by targeting N-cadherin. Mol. Med. Rep. 2018, 17, 8450–8456. [Google Scholar] [CrossRef]
- Zhang, H.H.; Qi, F.; Cao, Y.H.; Zu, X.B.; Chen, M.F. Expression and clinical significance of microRNA-21, maspin and vascular endothelial growth factor-C in bladder cancer. Oncol. Lett. 2015, 10, 2610–2616. [Google Scholar] [CrossRef]
- Piao, X.M.; Jeong, P.; Kim, Y.H.; Byun, Y.J.; Xu, Y.; Kang, H.W.; Ha, Y.S.; Kim, W.T.; Lee, J.Y.; Woo, S.H.; et al. Urinary cell-free microRNA biomarker could discriminate bladder cancer from benign hematuria. Int. J. Cancer 2019, 144, 380–388. [Google Scholar] [CrossRef]
- Usuba, W.; Urabe, F.; Yamamoto, Y.; Matsuzaki, J.; Sasaki, H.; Ichikawa, M.; Takizawa, S.; Aoki, Y.; Niida, S.; Kato, K.; et al. Circulating miRNA panels for specific and early detection in bladder cancer. Cancer Sci. 2019, 110, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhao, H.; Qian, X.; Qiu, J. MiR-20a in cell-free urine as a potential diagnostic biomarker for non-muscle invasive bladder cancer: A Chinese population-based study. Int. J. Clin. Exp. Med. 2018, 11, 209–216. [Google Scholar]
- Urquidi, V.; Netherton, M.; Gomes-Giacoia, E.; Serie, D.J.; Eckel-Passow, J.; Rosser, C.J.; Goodison, S. A microRNA biomarker panel for the non-invasive detection of bladder cancer. Oncotarget 2016, 7, 86290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Liu, X.; Fang, A.; Wang, J.; Yang, Y.; Wang, L.; Du, L.; Wang, C. Direct quantitative detection for cell-free miR-155 in urine: A potential role in diagnosis and prognosis for non-muscle invasive bladder cancer. Oncotarget 2016, 7, 3255. [Google Scholar] [CrossRef] [PubMed]
- Motawi, T.K.; Rizk, S.M.; Ibrahim, T.M.; Ibrahim, I.A.R. Circulating microRNAs, miR-92a, miR-100 and miR-143, as non-invasive biomarkers for bladder cancer diagnosis. Cell Biochem. Funct. 2016, 34, 142–148. [Google Scholar] [CrossRef]
- Liu, C.; Xu, P.; Shao, S.; Wang, F.; Zheng, Z.; Li, S.; Liu, W.; Li, G. The value of urinary exosomal lncRNA SNHG16 as a diagnostic biomarker for bladder cancer. Mol. Biol. Rep. 2023, 50, 8297–8304. [Google Scholar] [CrossRef]
- Bian, B.; Li, L.; Ke, X.; Chen, H.; Liu, Y.; Zheng, N.; Zheng, Y.; Ma, Y.; Zhou, Y.; Yang, J.; et al. Urinary exosomal long non-coding RNAs as noninvasive biomarkers for diagnosis of bladder cancer by RNA sequencing. Front. Oncol. 2022, 12, 976329. [Google Scholar] [CrossRef]
- Sarfi, M.; Abbastabar, M.; Khalili, E. Increased expression of urinary exosomal LnCRNA TUG-1 in early bladder cancer. Gene Rep. 2021, 22, 101010. [Google Scholar] [CrossRef]
- Luo, L.; Xie, Q.; Wu, Y.; Li, P.; Qin, F.; Liao, D.; Wang, K. Circular RNA CCT3 is a unique molecular marker in bladder cancer. BMC Cancer 2023, 23, 977. [Google Scholar] [CrossRef]
- Tang, G.; Xie, W.; Qin, C.; Zhen, Y.; Wang, Y.; Chen, F.; Du, Z.; Wu, Z.; Zhang, B.; Shen, Z.; et al. Expression of circular RNA circASXL1 correlates with TNM classification and predicts overall survival in bladder cancer. Int. J. Clin. Exp. Pathol. 2017, 10, 8495. [Google Scholar]
- Cheng, H.; Liu, Y.; Chen, G. Identification of potential DNA methylation biomarkers related to diagnosis in patients with bladder cancer through integrated bioinformatic analysis. BMC Urol. 2023, 23, 135. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, X.; Yang, A.; Fu, W.; Yin, F.; Zeng, X. Associations of tumor suppressor SPARCL1 with cancer progression and prognosis. Oncol. Lett. 2017, 14, 2603–2610. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, H.; Ge, W.; Liu, X.; Loera, S.; Chu, P.; Chen, H.; Peng, J.; Zhou, L.; Yu, S.; et al. Secreted protein acidic and rich in cysteines-like 1 suppresses aggressiveness and predicts better survival in colorectal cancers. Clin. Cancer Res. 2012, 18, 5438–5448. [Google Scholar] [CrossRef]
- Esposito, I.; Kayed, H.; Keleg, S.; Giese, T.; Sage, E.H.; Schirmacher, P.; Friess, H.; Kleeff, J. Tumor-suppressor function of SPARC-like protein 1/Hevin in pancreatic cancer. Neoplasia 2007, 9, 8–17. [Google Scholar] [CrossRef]
- Turtoi, A.; Musmeci, D.; Naccarato, A.G.; Scatena, C.; Ortenzi, V.; Kiss, R.; Murtas, D.; Patsos, G.; Mazzucchelli, G.; Pauw, E.D.; et al. Sparc-like protein 1 is a new marker of human glioma progression. J. Proteome Res. 2012, 11, 5011–5021. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Wang, Y.X.; Shen, A.; Yang, X.Q.; Liang, C.C.; Huang, R.J.; Jian, R.; An, N.; Xiao, Y.L.; Wang, L.S.; et al. Construction of a gene model related to the prognosis of patients with gastric cancer receiving immunotherapy and exploration of COX7A1 gene function. Eur. J. Med. Res. 2024, 29, 180. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, L.; Zhou, J.; Chen, Z.; Yang, G.; Liao, Y.; Zhu, M. Epidermal growth factor-containing fibulin-like extracellular matrix protein 1 (EFEMP1) acts as a potential diagnostic biomarker for prostate cancer. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 216. [Google Scholar] [CrossRef]
- Fu, S.; Luan, T.; Jiang, C.; Huang, Y.; Li, N.; Wang, H.; Wang, J. miR-3622a promotes proliferation and invasion of bladder cancer cells by downregulating LASS2. Gene 2019, 701, 23–31. [Google Scholar] [CrossRef]
- Xu, X.H.; Sun, J.M.; Chen, X.F.; Zeng, X.Y.; Zhou, H.Z. MicroRNA-494-3p facilitates the progression of bladder cancer by mediating the KLF9/RGS2 axis. Kaohsiung J. Med. Sci. 2022, 38, 1070–1079. [Google Scholar] [CrossRef]
- Ren, W.; Hu, J.; Li, H.; Chen, J.; Ding, J.; Zu, X.; Fan, B. miR-616-5p promotes invasion and migration of bladder cancer via downregulating NR2C2 expression. Front. Oncol. 2021, 11, 762946. [Google Scholar] [CrossRef]
- Lv, X.Y.; Ma, L.; Chen, J.F.; Yu, R.; Li, Y.; Yan, Z.J.; Cheng, Y.; Ma, Q. Knockdown of DUXAP10 inhibits proliferation and promotes apoptosis in bladder cancer cells via PI3K/Akt/mTOR signaling pathway. Int. J. Oncol. 2017, 52, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; German, P.; Bai, S.; Barnes, S.; Guo, W.; Qi, X.; Lou, H.; Liang, J.; Jonasch, E.; Mills, G.B.; et al. The PI3K/AKT pathway and renal cell carcinoma. J. Genet. Genom. 2015, 42, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Arima, J.; Yoshino, H.; Fukumoto, W.; Kawahara, I.; Saito, S.; Li, G.; Fukuda, I.; Lizasa, S.; Mitsuke, A.; Sakaguchi, T.; et al. LncRNA BCYRN1 as a Potential Therapeutic Target and Diagnostic Marker in Serum Exosomes in Bladder Cancer. Int. J. Mol. Sci. 2024, 25, 5955. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Shi, G.; Li, Q.; Li, W.; Zhou, H. Long noncoding RNA XIST participates in bladder cancer by downregulating p53 via binding to TET1. J. Cell. Biochem. 2019, 120, 6330–6338. [Google Scholar] [CrossRef]
- Li, H.J.; Gong, X.; Li, Z.K.; Qin, W.; He, C.X.; Xing, L.; Zhou, X.; Zhao, D.; Cao, H.L. Role of long non-coding RNAs on bladder cancer. Front. Cell Dev. Biol. 2021, 9, 672679. [Google Scholar] [CrossRef]
- Zhou, X.; Xiao, L.; Meng, F.; Zuo, F.; Wu, W.; Li, G.; Han, F.; Peng, G.; Shen, H. GAS6-AS1 drives bladder cancer progression by increasing MMP7 expression in a ceRNA-and RBP-dependent manner. Transl. Oncol. 2024, 48, 102065. [Google Scholar] [CrossRef]
- Yang, J.; Qi, M.; Fei, X.; Wang, X.; Wang, K. Hsa_circRNA_0088036 acts as a ceRNA to promote bladder cancer progression by sponging miR-140-3p. Cell Death Dis. 2022, 13, 322. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wang, L.; Shi, Z.; Zhu, A.; Zhang, G.; Hong, Z.; Cheng, C. Circular RNA circFLNA inhibits the development of bladder carcinoma through microRNA miR-216a-3p/BTG2 axis. Bioengineered 2021, 12, 11376–11389. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Ma, X.; Ying, Y.; Liu, Z.; Tang, Y.; Shu, X.; Sun, J.; Wu, Y.; Lu, D.; Wang, X.; et al. N6-methyladenosine-modified CircPSMA7 enhances bladder cancer malignancy through the miR-128–3p/MAPK1 axis. Cancer Lett. 2024, 585, 216613. [Google Scholar] [CrossRef]
- Oliver, J.; Garcia-Aranda, M.; Chaves, P.; Alba, E.; Cobo-Dols, M.; Onieva, J.L.; Barragan, I. Emerging noninvasive methylation biomarkers of cancer prognosis and drug response prediction. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2022; pp. 584–595. [Google Scholar]
- Yoon, H.Y.; Kim, Y.J.; Kim, J.S.; Kim, Y.W.; Kang, H.W.; Kim, W.T.; Yun, S.J.; Ryu, K.H.; Lee, S.C.; Kim, W.J. RSPH9 methylation pattern as a prognostic indicator in patients with non-muscle invasive bladder cancer. Oncol. Rep. 2016, 35, 1195–1203. [Google Scholar] [CrossRef][Green Version]
- Zhan, L.; Zhang, B.; Tan, Y.; Yang, C.; Huang, C.; Wu, Q.; Zhang, Y.; Chen, X.; Zhou, M.; Shu, A. Quantitative assessment of the relationship between RASSF1A gene promoter methylation and bladder cancer (PRISMA). Medicine 2017, 96, e6097. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, M.; Lee, Y.; Bang, L.; Garg, T.; Sohn, K.A.; Kim, D. Identification of epigenetic interactions between miRNA and DNA methylation associated with gene expression as potential prognostic markers in bladder cancer. BMC Med. Genom. 2017, 10, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, L.; Zang, Y.; Xu, Z. Identification of core genes and key pathways via integrated analysis of gene expression and DNA methylation profiles in bladder cancer. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 3024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, J.; Xu, M.; Zhu, T. Exploring prognostic DNA methylation genes in bladder cancer: A comprehensive analysis. Discov. Oncol. 2024, 15, 331. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Naruse, A.; Akita, K.; Muramatsu-Maekawa, Y.; Kawase, K.; Koie, T.; Horie, M.; Kikuchi, A. CALN1 hypomethylation as a biomarker for high-risk bladder cancer. BMC Urol. 2022, 22, 176. [Google Scholar] [CrossRef]
- Chen, J.Q.; Salas, L.A.; Wiencke, J.K.; Koestler, D.C.; Molinaro, A.M.; Andrew, A.S.; Seigne, J.D.; Karagas, M.R.; Kelsey, K.T.; Christensen, B.C. Immune profiles and DNA methylation alterations related with non-muscle-invasive bladder cancer outcomes. Clin. Epigenetics 2022, 14, 14. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, K.; Zheng, Y.; Qi, F.; Luo, J. Genome-wide screening of abberant methylated drivers combined with relative risk loci in bladder cancer. Cancer Med. 2020, 9, 768–782. [Google Scholar] [CrossRef]
- Kim, C.; Oh, S.; Im, H.; Gim, J. Unveiling Bladder Cancer Prognostic Insights by Integrating Patient-Matched Sample and CpG Methylation Analysis. Medicina 2024, 60, 1175. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, J.; Zhang, Q.; Liang, Y.; Du, Y.; Wang, G. Identification of prognostic biomarkers for bladder cancer based on DNA methylation profile. Front. Cell Dev. Biol. 2022, 9, 817086. [Google Scholar] [CrossRef]
- Koukourikis, P.; Papaioannou, M.; Georgopoulos, P.; Apostolidis, I.; Pervana, S.; Apostolidis, A. A Study of DNA Methylation of Bladder Cancer Biomarkers in the Urine of Patients with Neurogenic Lower Urinary Tract Dysfunction. Biology 2023, 12, 1126. [Google Scholar] [CrossRef]
- Azzouzi, M.; El Ahanidi, H.; Alaoui, C.H.; Chaoui, I.; Benbacer, L.; Tetou, M.; Hassan, I.; Bensaid, M.; Oukabli, M.; Ameur, A.; et al. Evaluation of DNA methylation in promoter regions of hTERT, TWIST1, VIM and NID2 genes in Moroccan bladder cancer patients. Cancer Genet. 2022, 260, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yin, J.; Dai, Y.; Guan, Y.; Chen, P.; Chen, Y.; Huang, C.; Lu, Y.J.; Zhang, L.; Song, D. A novel CpG methylation risk indicator for predicting prognosis in bladder cancer. Front. Cell Dev. Biol. 2021, 9, 642650. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, Q.; Chen, X.; Hao, L. Bioinformatics analysis to screen DNA methylation-driven genes for prognosis of patients with bladder cancer. Transl. Androl. Urol. 2021, 10, 3604. [Google Scholar] [CrossRef]
- Mitash, N.; Agnihotri, S.; Tiwari, S.; Agrawal, V.; Mandhani, A. MicroRNA-21 could be a molecular marker to predict the recurrence of nonmuscle invasive bladder cancer. Indian J. Urol. 2017, 33, 283–290. [Google Scholar] [PubMed]
- Chen, X.; Wu, B.; Xu, Z.; Li, S.; Tan, S.; Liu, X.; Wang, K. Downregulation of miR-133b predict progression and poor prognosis in patients with urothelial carcinoma of bladder. Cancer Med. 2016, 5, 1856–1862. [Google Scholar] [CrossRef]
- Zeng, W.; Zhu, J.F.; Liu, J.Y.; Li, Y.L.; Dong, X.; Huang, H.; Shan, L. miR-133b inhibits cell proliferation, migration and invasion of esophageal squamous cell carcinoma by targeting EGFR. Biomed. Pharmacother. 2019, 111, 476–484. [Google Scholar] [CrossRef]
- Avgeris, M.; Tsilimantou, A.; Levis, P.K.; Tokas, T.; Sideris, D.C.; Stravodimos, K.; Ardavanis, A.; Scorilas, A. Loss of GAS5 tumour suppressor lncRNA: An independent molecular cancer biomarker for short-term relapse and progression in bladder cancer patients. Br. J. Cancer 2018, 119, 1477–1486. [Google Scholar] [CrossRef]
- Jiao, R.; Jiang, W.; Wei, X.; Zhang, M.; Zhao, S.; Huang, G. Clinicopathological significance and prognosis of long noncoding RNA SNHG16 expression in human cancers: A meta-analysis. BMC Cancer 2020, 20, 662. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Luo, Y.; He, W.; Zhao, Y.; Kong, Y.; Liu, H.; Zhong, G.; Li, Y.; Li, J.; Huang, J.; et al. Exosomal long noncoding RNA LNMAT2 promotes lymphatic metastasis in bladder cancer. J. Clin. Investig. 2020, 130, 404–421. [Google Scholar] [CrossRef]
- Lin, G.; Sheng, H.; Xie, H.; Zheng, Q.; Shen, Y.; Shi, G.; Ye, D. circLPAR1 is a novel biomarker of prognosis for muscle-invasive bladder cancer with invasion and metastasis by miR-762. Oncol. Lett. 2019, 17, 3537–3547. [Google Scholar] [CrossRef]
- Liu, J.; Liu, T.; Wang, X.; He, A. Circles reshaping the RNA world: From waste to treasure. Mol. Cancer 2017, 16, 58. [Google Scholar] [CrossRef] [PubMed]
- Andrew, A.S.; Karagas, M.R.; Schroeck, F.R.; Marsit, C.J.; Schned, A.R.; Pettus, J.R.; Armstrong, D.A.; Seigne, J.D. MicroRNA Dysregulation and Non-Muscle–Invasive Bladder Cancer Prognosis. Cancer Epidemiol. Biomark. Prev. 2019, 28, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Seki, N.; Matsushita, R.; Yonemori, M.; Yoshino, H.; Nakagawa, M.; Enokida, H. Tumour-suppressive miRNA-26a-5p and miR-26b-5p inhibit cell aggressiveness by regulating PLOD2 in bladder cancer. Br. J. Cancer 2016, 115, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Borkowska, E.M.; Konecki, T.; Pietrusiński, M.; Borowiec, M.; Jabłonowski, Z. MicroRNAs which can prognosticate aggressiveness of bladder cancer. Cancers 2019, 11, 1551. [Google Scholar] [CrossRef]
- Juracek, J.; Stanik, M.; Vesela, P.; Radova, L.; Dolezel, J.; Svoboda, M.; Slaby, O. Tumor expression of miR-34a-3p is an independent predictor of recurrence in non–muscle-invasive bladder cancer and promising additional factor to improve predictive value of EORTC nomogram. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2019; pp. 184.e1–184.e7. [Google Scholar]
- Ding, Z.S.; He, Y.H.; Deng, Y.S.; Peng, P.X.; Wang, J.F.; Chen, X.; Zhao, P.Y.; Zhou, X.F. MicroRNA-34a inhibits bladder cancer cell migration and invasion and upregulates PTEN expression. Oncol. Lett. 2019, 18, 5549–5554. [Google Scholar] [CrossRef]
- Yang, L.; Sun, H.F.; Guo, L.Q.; Cao, H.B. MiR-10a-5p: A promising biomarker for early diagnosis and prognosis evaluation of bladder cancer. Cancer Manag. Res. 2021, 13, 7841–7850. [Google Scholar] [CrossRef] [PubMed]
- Bogale, D.E. The roles of FGFR3 and c-MYC in urothelial bladder cancer. Discov. Oncol. 2024, 15, 295. [Google Scholar] [CrossRef]
- Yin, X.H.; Jin, Y.H.; Cao, Y.; Wong, Y.; Weng, H.; Sun, C.; Deng, J.H.; Zeng, X.T. Development of a 21-miRNA signature associated with the prognosis of patients with bladder cancer. Front. Oncol. 2019, 9, 729. [Google Scholar] [CrossRef]
- Yerukala Sathipati, S.; Tsai, M.J.; Shukla, S.K.; Ho, S.Y.; Liu, Y.; Beheshti, A. MicroRNA signature for estimating the survival time in patients with bladder urothelial carcinoma. Sci. Rep. 2022, 12, 4141. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Zhan, D.M.; Chong, Y.K.; Ding, L.; Yang, Y.G. MiR-652-3p promotes bladder cancer migration and invasion by targeting KCNN3. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8806–8812. [Google Scholar]
- Chen, C.; Zheng, H.; Luo, Y.; Kong, Y.; An, M.; Li, Y.; He, W.; Gao, B.; Zhao, Y.; Huang, J.; et al. UMOylation promotes extracellular vesicle–mediated transmission of lncRNA ELNAT1 and lymph node metastasis in bladder cancer. J. Clin. Investig. 2021, 131, e146431. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Xu, F.; Wan, P.; Zhang, L.; Huang, S.; Yang, N.; Wang, Y. Malat-1 expression in bladder carcinoma tissues and its clinical significance. Am. J. Transl. Res. 2021, 13, 3555. [Google Scholar] [PubMed]
- Zheng, H.; Chen, C.; Luo, Y.; Yu, M.; He, W.; An, M.; Gao, B.; Kong, Y.; Ya, Y.; Lin, Y.; et al. Tumor-derived exosomal BCYRN1 activates WNT5A/VEGF-C/VEGFR3 feedforward loop to drive lymphatic metastasis of bladder cancer. Clin. Transl. Med. 2021, 11, e497. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Fujita, K.; Netto, G.J.; Nonomura, N. Clinical application of TERT promoter mutations in urothelial carcinoma. Front. Oncol. 2021, 11, 705440. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhao, N.; Zhang, H.; Xie, D. Circular RNA CircHIPK3 promotes gemcitabine sensitivity in bladder cancer. J. Cancer 2020, 11, 1907. [Google Scholar] [CrossRef]
- Zhang, J.X.; Lu, J.; Xie, H.; Wang, D.P.; Ni, H.E.; Zhu, Y.; Ren, L.H.; Meng, X.X.; Wang, R.L. circHIPK3 regulates lung fibroblast-to-myofibroblast transition by functioning as a competing endogenous RNA. Cell Death Dis. 2019, 10, 182. [Google Scholar] [CrossRef]
- Su, Y.; Feng, W.; Shi, J.; Chen, L.; Huang, J.; Lin, T. circRIP2 accelerates bladder cancer progression via miR-1305/Tgf-β2/smad3 pathway. Mol. Cancer 2020, 19, 23. [Google Scholar] [CrossRef]
- Zhu, J.; Luo, Y.; Zhao, Y.; Kong, Y.; Zheng, H.; Li, Y.; Gao, B.; Ai, L.; Huang, H.; Huang, J.; et al. circEHBP1 promotes lymphangiogenesis and lymphatic metastasis of bladder cancer via miR-130a-3p/TGFβR1/VEGF-D signaling. Mol. Ther. 2021, 29, 1838–1852. [Google Scholar] [CrossRef]
- Zhenhai, Z.; Qi, C.; Shuchao, Z.; Zhongqi, W.; Xue, S.; Zhijun, G.; Zhijie, M.; Jianmin, L.; Beibei, L.; Yuanyuan, G. MiR-205-3p suppresses bladder cancer progression via GLO1 mediated P38/ERK activation. BMC Cancer 2023, 23, 956. [Google Scholar] [CrossRef]
- Hao, Y.; Zhu, Y.; Sun, F.; Xu, D.; Wang, C. MicroRNA-30c-5p arrests bladder cancer G2/M phase and suppresses its progression by targeting PRC1-mediated blocking of CDK1/Cyclin B1 axis. Cell. Signal. 2023, 110, 110836. [Google Scholar] [CrossRef]
- Awadalla, A.; Abol-Enein, H.; Hamam, E.T.; Ahmed, A.E.; Khirallah, S.M.; El-Assmy, A.; Mostafa, S.A.; Babalghith, A.O.; Ali, M.; Abdel-Rahim, M.; et al. Identification of epigenetic interactions between miRNA and gene expression as potential prognostic markers in bladder cancer. Genes 2022, 13, 1629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sang, Y.; Liu, Z.; Shao, J. Negative correlation between circular RNA SMARC5 and MicroRNA 432, and their clinical implications in bladder cancer patients. Technol. Cancer Res. Treat. 2021, 20, 15330338211039110. [Google Scholar] [CrossRef]
- Spagnuolo, M.; Costantini, M.; Ferriero, M.; Varmi, M.; Sperduti, I.; Regazzo, G.; Cicchillitti, L.; Mendez, A.B.D.; Cigliana, G.; Pompeo, V.; et al. Urinary expression of let-7c cluster as non-invasive tool to assess the risk of disease progression in patients with high grade non-muscle invasive bladder Cancer: A pilot study. J. Exp. Clin. Cancer Res. 2020, 39, 68. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Buiga, R.; Cojocneanu, R.; Buse, M.; Raduly, L.; Pop, L.A.; Chira, S.; Budisan, L.; Jurj, A.; Ciocan, C.; et al. Connecting the dots between different networks: miRNAs associated with bladder cancer risk and progression. J. Exp. Clin. Cancer Res. 2019, 38, 433. [Google Scholar] [CrossRef]
- Chen, H.; Xu, L.; Wang, L. Expression of miR-182 and Foxo3a in patients with bladder cancer correlate with prognosis. Int. J. Clin. Exp. Pathol. 2019, 12, 4193. [Google Scholar] [PubMed]
- Yang, K.; Tang, H.; Ding, M.; Guo, Y.; Kai, K.; Xiao, J.; Shen, Y.; Miao, S.; Zhou, R. Expression of miR-195 and MEK1 in patients with bladder cancer and their relationship to prognosis. Int. J. Clin. Exp. Pathol. 2019, 12, 843. [Google Scholar]
- Li, D.; Hao, X.; Song, Y. An integrated analysis of key microRNAs, regulatory pathways and clinical relevance in bladder cancer. OncoTargets Ther. 2018, 11, 3075–3085. [Google Scholar] [CrossRef]
- Martins, E.P.; Vieira de Castro, J.; Fontes, R.; Monteiro-Reis, S.; Henrique, R.; Jerónimo, C.; Costa, B.M. Relevance of HOTAIR rs920778 and rs12826786 Genetic Variants in Bladder Cancer Risk and Survival. Cancers 2024, 16, 434. [Google Scholar] [CrossRef]
- Novikova, E.L.; Kulakova, M.A. There and back again: Hox clusters use both DNA strands. J. Dev. Biol. 2021, 9, 28. [Google Scholar] [CrossRef]
- Xia, W.; Chen, C.; Zhang, M.R.; Zhu, L.N. LncRNA PCAT6 aggravates the progression of bladder cancer cells by targeting miR-513a-5p. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9908–9914. [Google Scholar]
- Zhang, S.; Du, L.; Wang, L.; Jiang, X.; Zhan, Y.; Li, J.; Yan, K.; Duan, W.; Zhao, Y.; Wang, L.; et al. Evaluation of serum exosomal Lnc RNA-based biomarker panel for diagnosis and recurrence prediction of bladder cancer. J. Cell. Mol. Med. 2019, 23, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Miao, P.; Ming, Y.; Tao, J.; Shen, H. Circ-ZFR promotes progression of bladder cancer by upregulating WNT5A via sponging miR-545 and miR-1270. Front. Oncol. 2021, 10, 596623. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, R.X.; Wei, W.S.; Li, Y.H.; Feng, Z.H.; Tan, L.; Chen, J.W.; Yuan, G.J.; Chen, S.L.; Guo, S.J.; et al. PRMT5 Circular RNA Promotes Metastasis of Urothelial Carcinoma of the Bladder through Sponging miR-30c to Induce Epithelial–Mesenchymal Transition. Clin. Cancer Res. 2021, 27, 2664. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).