The Prognostic Role of Pulmonary Arterial Elastance in Patients Undergoing Left Ventricular Assist Device Implantation: A Pilot Study
Abstract
1. Introduction
2. Methods
- (1)
- New York Heart Association class III (advanced) or IV;
- (2)
- Left ventricular ejection fraction (LVEF) ≤ 30%;
- (3)
- Persistence of a plasma level of NT-pro Brian Natriuretic Peptide > 2500 pg/mL;
- (4)
- Episodes of congestions (both pulmonary or systemic) requiring an IV dose of furosemide > 100 mg or the addition of metolazone or episodes of low output state requiring an infusion of inotropes or inodilators;
- (5)
- Distance covered at six-minutes walking distance test < 300 m or a peak VO2 < 12 mL/kg/min.
2.1. Echocardiography
2.2. Right Heart Catheterization
2.3. Evaluation of Pulmonary Elastance
- (1)
- The conventional method with the following formula: PASP/SV ratio (EaC);
- (2)
- The recent one presented by Brenner et al. with the following formula: (MPAP − PCWP)/SV ratio (EaB).
2.4. Left Ventricular Assist Device Implant
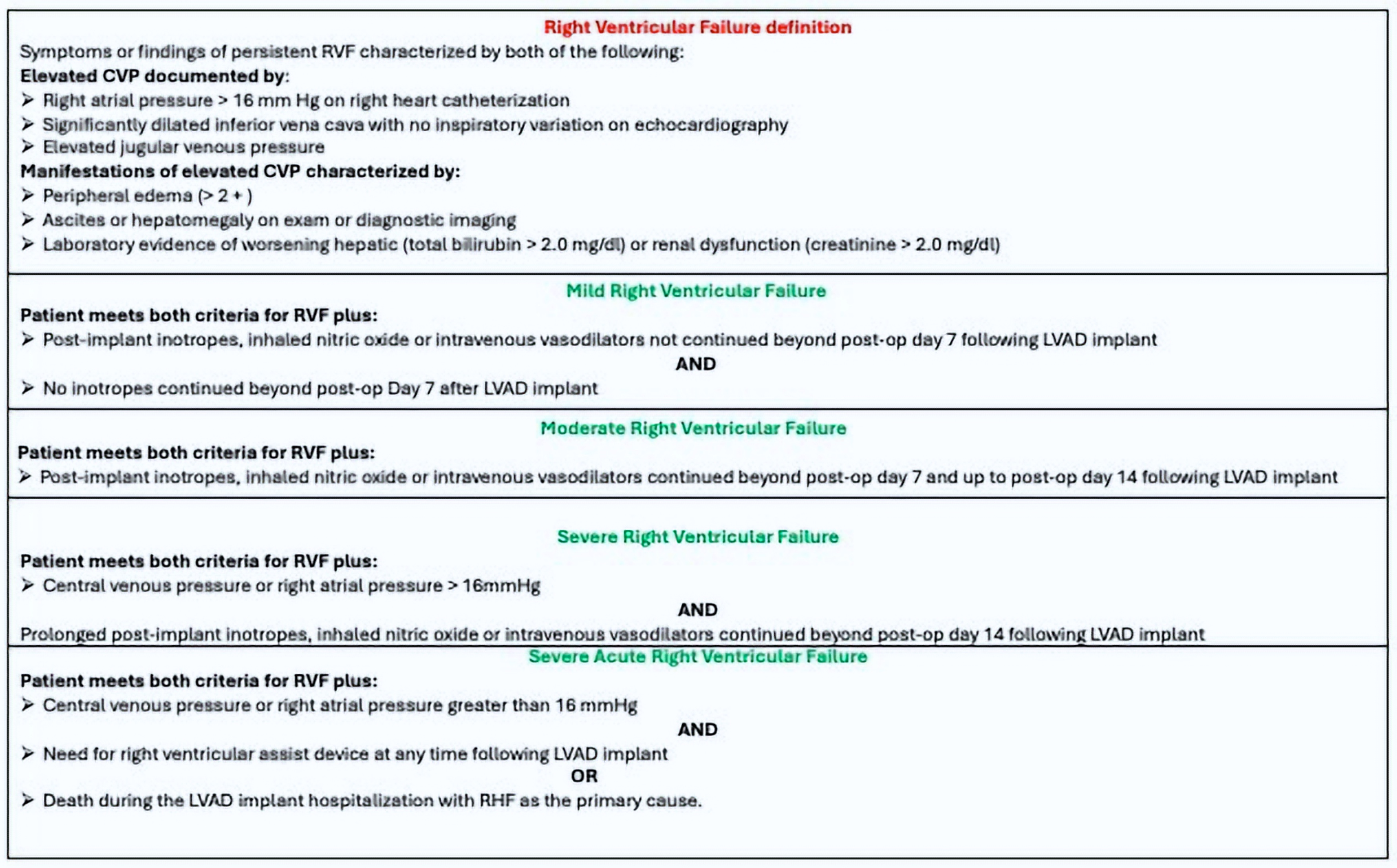
2.5. Definition of RVF
- (1)
- Symptoms or signs of RVF;
- (2)
- CVP > 16 mmHg at RHC;
- (3)
- Clinical evidence of congestion (peripheral edema printable with grade 2 or more), ascites, hepatomegaly (on clinical examination or liver echography), and biochemical parameters of hepatic or renal distress.
- (4)
- Occurrence < 30 days after LVAD implant.
2.6. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masarone, D.; Kittleson, M.; Petraio, A.; Pacileo, G. Advanced heart failure: State of the art and future directions. Rev. Cardiovasc. Med. 2022, 23, 48. [Google Scholar] [CrossRef] [PubMed]
- Masarone, D.; Houston, B.; Falco, L.; Martucci, M.L.; Catapano, D.; Valente, F.; Gravino, R.; Contaldi, C.; Petraio, A.; De Feo, M.; et al. How to Select Patients for Left Ventricular Assist Devices? A Guide for Clinical Practice. J. Clin. Med. 2023, 12, 5216. [Google Scholar] [CrossRef] [PubMed]
- Schmitto, J.D.; Shaw, S.; Garbade, J.; Gustafsson, F.; Morshuis, M.; Zimpfer, D.; Lavee, J.; Pya, Y.; Berchtold-Herz, M.; Wang, A.; et al. Fully magnetically centrifugal left ventricular assist device and long-term outcomes: The ELEVATE registry. Eur. Heart J. 2024, 45, 613–625. [Google Scholar] [CrossRef]
- Lampert, B.C.; Teuteberg, J.J. Right ventricular failure after left ventricular assist devices. J. Heart Lung Transplant. 2015, 34, 1123–1130. [Google Scholar] [CrossRef]
- Immohr, M.B.; Boeken, U.; Mueller, F.; Prashovikj, E.; Morshuis, M.; Böttger, C.; Aubin, H.; Gummert, J.; Akhyari, P.; Lichtenberg, A.; et al. Complications of left ventricular assist devices causing high urgency status on waiting list: Impact on outcome after heart transplantation. ESC Heart Fail. 2021, 8, 1253–1262. [Google Scholar] [CrossRef]
- Kilic, A.; Acker, M.A.; Atluri, P. Dealing with surgical left ventricular assist device complications. J. Thorac. Dis. 2015, 7, 2158–2164. [Google Scholar]
- Argiriou, M.; Kolokotron, S.-M.; Sakellaridis, T.; Argiriou, O.; Charitos, C.; Zarogoulidis, P.; Katsikogiannis, N.; Kougioumtzi, I.; Machairiotis, N.; Tsiouda, T.; et al. Right heart failure post left ventricular assist device implantation. J. Thorac. Dis. 2014, 6 (Suppl. S1), S52–S59. [Google Scholar] [PubMed]
- Meineri, M.; Van Rensburg, A.E.; Vegas, A. Right ventricular failure after LVAD implantation: Prevention and treatment. Best Pract. Res. Clin. Anaesthesiol. 2012, 26, 217–229. [Google Scholar] [CrossRef]
- Dang, N.; Topkara, V.; Mercando, M.; Kay, J.; Kruger, K.; Aboodi, M.; Oz, M.; Naka, Y. Right Heart Failure After Left Ventricular Assist Device Implantation in Patients with Chronic Congestive Heart Failure. J. Heart Lung Transplant. 2006, 25, 1–6. [Google Scholar] [CrossRef]
- Bellavia, D.; Iacovoni, A.; Scardulla, C.; Moja, L.; Pilato, M.; Kushwaha, S.S.; Senni, M.; Clemenza, F.; Agnese, V.; Falletta, C.; et al. Prediction of right ventricular failure after ventricular assist device implant: Systematic review and meta-analysis of observational studies. Eur. J. Heart Fail. 2017, 19, 926–946. [Google Scholar] [CrossRef]
- Kukucka, M.; Potapov, E.; Stepanenko, A.; Weller, K.; Mladenow, A.; Kuppe, H.; Habazettl, H. Acute impact of left ventricular unloading by left ventricular assist device on the right ventricle geometry and function: Effect of nitric oxide inhalation. J. Thorac. Cardiovasc. Surg. 2011, 141, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Bravo, C.A.; Navarro, A.G.; Dhaliwal, K.K.; Khorsandi, M.; Keenan, J.E.; Mudigonda, P.; O’Brien, K.D.; Mahr, C. Right heart failure after left ventricular assist device: From mechanisms to treatments. Front. Cardiovasc. Med. 2022, 9, 1023549. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.D.; Weiss, E.S.; Schaffer, J.; Ullrich, S.L.; Rivard, D.C.; Shah, A.S.; Russell, S.D.; Conte, J.V. Right heart dysfunction after left ventricular assist device implantation: A comparison of the pulsatile HeartMate I and axial-flow HeartMate II devices. Ann. Thorac. Surg. 2008, 86, 832–840; discussion 832–840. [Google Scholar] [CrossRef]
- Singh, S.K.; Takeda, K. Right heart failure after left ventricular assist device implantation—From prediction to action. J. Heart Lung Transplant. 2022, 41, 1727–1728. [Google Scholar] [CrossRef]
- Matthews, J.C.; Koelling, T.M.; Pagani, F.D.; Aaronson, K.D. The Right Ventricular Failure Risk Score. J. Am. Coll. Cardiol. 2008, 51, 2163–2172. [Google Scholar] [CrossRef]
- Jabagi, H.; Nantsios, A.; Ruel, M.; Mielniczuk, L.M.; Denault, A.Y.; Sun, L.Y. A standardized definition for right ventricular failure in cardiac surgery patients. ESC Heart Fail. 2022, 9, 1542–1552. [Google Scholar] [CrossRef]
- Imamura, T. What is Optimal Definition of Right Ventricular Dysfunction and Right Ventricular Failure? J. Card. Fail. 2019, 25, 698. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulos, A.P.; Kelkar, A.; Weinberger, J.F.; Morris, A.A.; Georgiopoulou, V.V.; Markham, D.W.; Butler, J.; Vega, J.D.; Smith, A.L. Validation of clinical scores for right ventricular failure prediction after implantation of continuous-flow left ventricular assist devices. J. Heart Lung Transplant. 2015, 34, 1595–1603. [Google Scholar] [CrossRef]
- Chriqui, L.-E.; Monney, P.; Kirsch, M.; Tozzi, P. Prediction of right ventricular failure after left ventricular assist device implantation in patients with heart failure: A meta-analysis comparing echocardiographic parameters. Interact. Cardiovasc. Thorac. Surg. 2021, 33, 784–792. [Google Scholar] [CrossRef]
- Bellavia, D.; Iacovoni, A.; Agnese, V.; Falletta, C.; Coronnello, C.; Pasta, S.; Novo, G.; di Gesaro, G.; Senni, M.; Maalouf, J.; et al. Usefulness of regional right ventricular and right atrial strain for prediction of early and late right ventricular failure following a left ventricular assist device implant: A machine learning approach. Int. J. Artif. Organs 2020, 43, 297–314. [Google Scholar] [CrossRef]
- Grant, A.D.M.; Smedira, N.G.; Starling, R.C.; Marwick, T.H. Independent and Incremental Role of Quantitative Right Ventricular Evaluation for the Prediction of Right Ventricular Failure After Left Ventricular Assist Device Implantation. J. Am. Coll. Cardiol. 2012, 60, 521–528. [Google Scholar] [CrossRef]
- Wagner, T.; Bernhardt, A.M.; Magnussen, C.; Reichenspurner, H.; Blankenberg, S.; Grahn, H. Right heart failure before LVAD implantation predicts right heart failure after LVAD implantation—Is it that easy? J. Cardiothorac. Surg. 2020, 15, 113. [Google Scholar] [CrossRef]
- Drakos, S.G.; Janicki, L.; Horne, B.D.; Kfoury, A.G.; Reid, B.B.; Clayson, S.; Horton, K.; Haddad, F.; Li, D.Y.; Renlund, D.G.; et al. Risk Factors Predictive of Right Ventricular Failure After Left Ventricular Assist Device Implantation. Am. J. Cardiol. 2010, 105, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.P.; Groves, L.; Vishram-Nielsen, J.K.K.; Karvasarski, E.; Valle, F.H.; Alba, A.C.; Mak, S. Elevated pulmonary arterial elastance and right ventricular uncoupling are associated with greater mortality in advanced heart failure. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2020, 39, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Adamopoulos, S.; Bonios, M.; Ben Gal, T.; Gustafsson, F.; Abdelhamid, M.; Adamo, M.; Bayes-Genis, A.; Böhm, M.; Chioncel, O.; Cohen-Solal, A.; et al. Right heart failure with left ventricular assist devices: Preoperative, perioperative and postoperative management strategies. A clinical consensus statement of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail 2004. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Morimont, P.; Lambermont, B.; Ghuysen, A.; Gerard, P.; Kolh, P.; Lancellotti, P.; Tchana-Sato, V.; Desaive, T.; D’Orio, V. Effective arterial elastance as an index of pulmonary vascular load. Am. J. Physiol.-Heart Circ. Physiol. 2008, 294, H2736–H2742. [Google Scholar] [CrossRef]
- Brener, M.I.; Burkhoff, D.; Sunagawa, K. Effective arterial elastance in the pulmonary arterial circulation: Derivation, assumptions, and clinical applications. Circ. Heart Fail. 2020, 13, e007117. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 36. [Google Scholar] [CrossRef]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 12. [Google Scholar]
- Rajagopalan, N.; Borlaug, B.A.; Bailey, A.L.; Eckman, P.M.; Guglin, M.; Hall, S.; Montgomery, M.; Ramani, G.; Khazanie, P. Practical Guidance for Hemodynamic Assessment by Right Heart Catheterization in Management of Heart Failure. JACC Heart Fail. 2024, 12, 1141–1156. [Google Scholar] [CrossRef]
- Kormos, R.L.; Antonides, C.F.; Goldstein, D.J.; Cowger, J.A.; Starling, R.C.; Kirklin, J.K.; Rame, J.E.; Rosenthal, D.; Mooney, M.L.; Caliskan, K.; et al. Updated definitions of adverse events for trials and registries of mechanical circulatory support: A consensus statement of the mechanical circulatory support academic research consortium. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2020, 39, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Roesel, M.J.; Nersesian, G.; Neuber, S.; Thau, H.; von Gudenberg, R.W.; Lanmueller, P.; Hennig, F.; Falk, V.; Potapov, E.; Knosalla, C.; et al. LVAD as a Bridge to Transplantation-Current Status and Future Perspectives. Rev. Cardiovasc. Med. 2024, 25, 176. [Google Scholar] [CrossRef] [PubMed]
- Kapelios, C.J.; Lund, L.H.; Wever-Pinzon, O.; Selzman, C.H.; Myers, S.L.; Cantor, R.S.; Stehlik, J.; Chamogeorgakis, T.; McKellar, S.H.; Koliopoulou, A.; et al. Right Heart Failure Following Left Ventricular Device Implantation: Natural History, Risk Factors, and Outcomes: An Analysis of the STS INTERMACS Database. Circ. Heart Fail. 2022, 15, e008706. [Google Scholar] [CrossRef] [PubMed]
- Lo Coco, V.; De Piero, M.E.; Massimi, G.; Chiarini, G.; Raffa, G.M.; Kowalewski, M.; Maessen, J.; Lorusso, R. Right ventricular failure after left ventricular assist device implantation: A review of the literature. J. Thorac. Dis. 2021, 13, 1256–1269. [Google Scholar] [CrossRef]
- Rodenas-Alesina, E.; Brahmbhatt, D.H.; Rao, V.; Salvatori, M.; Billia, F. Prediction, prevention, and management of right ventricular failure after left ventricular assist device implantation: A comprehensive review. Front. Cardiovasc. Med. 2022, 9, 1040251. [Google Scholar] [CrossRef]
- Turner, K.R. Right Ventricular Failure After Left Ventricular Assist Device Placement-The Beginning of the End or Just Another Challenge? J. Cardiothorac. Vasc. Anesth. 2019, 33, 1105–1121. [Google Scholar] [CrossRef]
- Sciaccaluga, C.; Procopio, M.C.; Potena, L.; Masetti, M.; Bernazzali, S.; Maccherini, M.; Landra, F.; Righini, F.M.; Cameli, M.; Valente, S. Right ventricular dysfunction in left ventricular assist device candidates: Is it time to change our prospective? Heart Fail. Rev. 2024, 29, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Tedford, R.J.; Mudd, J.O.; Girgis, R.E.; Mathai, S.C.; Zaiman, A.L.; Housten-Harris, T.; Boyce, D.; Kelemen, B.W.; Bacher, A.C.; Shah, A.A.; et al. Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circ. Heart Fail. 2013, 6, 953–963. [Google Scholar] [CrossRef]
- Abel, F.L.; Waldhausen, J.A. Effects of alterations in pulmonary vascular resistance on right ventricular function. J. Thorac. Cardiovasc. Surg. 1967, 54, 886–894. [Google Scholar] [CrossRef]
- Tampakakis, E.; Shah, S.J.; Borlaug, B.A.; Leary, P.J.; Patel, H.H.; Miller, W.L.; Kelemen, B.W.; Houston, B.A.; Kolb, T.M.; Damico, R.; et al. Pulmonary Effective Arterial Elastance as a Measure of Right Ventricular Afterload and Its Prognostic Value in Pulmonary Hypertension Due to Left Heart Disease. Circ. Heart Fail. 2018, 11, e004436. [Google Scholar] [CrossRef]
- Muslem, R.; Ong, C.S.; Tomashitis, B.; Schultz, J.; Ramu, B.; Craig, M.L.; Van Bakel, A.B.; Gilotra, N.A.; Sharma, K.; Hsu, S.; et al. Pulmonary Arterial Elastance and INTERMACS-Defined Right Heart Failure Following Left Ventricular Assist Device. Circ. Heart Fail. 2019, 12, e005923. [Google Scholar] [CrossRef]
- Sanz, J.; Sánchez-Quintana, D.; Bossone, E.; Bogaard, H.J.; Naeije, R. Anatomy, Function, and Dysfunction of the Right Ventricle: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1463–1482. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.; Fang, J.C.; Borlaug, B.A. Hemodynamics for the Heart Failure Clinician: A State-of-the-Art Review. J. Card. Fail. 2022, 28, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Aboumarie, H.S.; Pastore, M.C.; Caliskan, K.; Cikes, M.; Garbi, M.; Lim, H.S.; Muraru, D.; Mandoli, G.E.; Pergola, V.; et al. Multimodality imaging for the evaluation and management of patients with long-term (durable) left ventricular assist devices A Clinical Consensus Statement of the European Association of Cardiovascular Imaging (EACVI) of the ESC. Eur. Heart J. Cardiovasc. Imaging 2024, 25, e217–e240. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.S.; Cevasco, M.; Birati, E.Y.; Mazurek, J.A. Predicting, Recognizing, and Treating Right Heart Failure in Patients Undergoing Durable LVAD Therapy. J. Clin. Med. 2022, 11, 2984. [Google Scholar] [CrossRef]

| All | |
|---|---|
| Patients | 35 |
| Age | 59 ± 12 |
| Sex | 33 M, 2 F |
| BMI | 26 ± 3 kg/m2 |
| LVEF | 25 ± 6% |
| TAPSE | 17.4 ± 2.8 mm |
| Ischemic etiology | 23 (66%) |
| Idiopathic dilated cardiomyopathy | 10 (29%) |
| End-stage hypertrophic cardiomyopathy | 2 (5%) |
| HeartMate 3 | 35 (100%) |
| Parameter | Not-Severe RVF (n = 28) | Severe RVF (n = 6) | p-Value |
|---|---|---|---|
| TAPSE | 17.12 ± 3.9 | 17.8 ± 2.13 | 0.672 |
| TPG | 13.4 ± 9.1 | 13.8 ± 3.3 | 0.916 |
| DPG | 5.37 ± 8.34 | 2.66 ± 1.5 | 0.405 |
| PVR (WU) | 3.44 ± 2.57 | 4.36 ± 1.17 | 0.441 |
| RVSWi | 973.3 ± 714.99 | 934.3 ± 312.69 | 0.897 |
| CPI | 0.18 ± 0.053 | 0.177 ± 0.046 | 0.875 |
| Compliance | 3.055 ± 1.68 | 1.833 ± 0.401 | 0.09 |
| EaC | 0.947 ± 0.43 | 1.11 ± 0.2 | 0.361 |
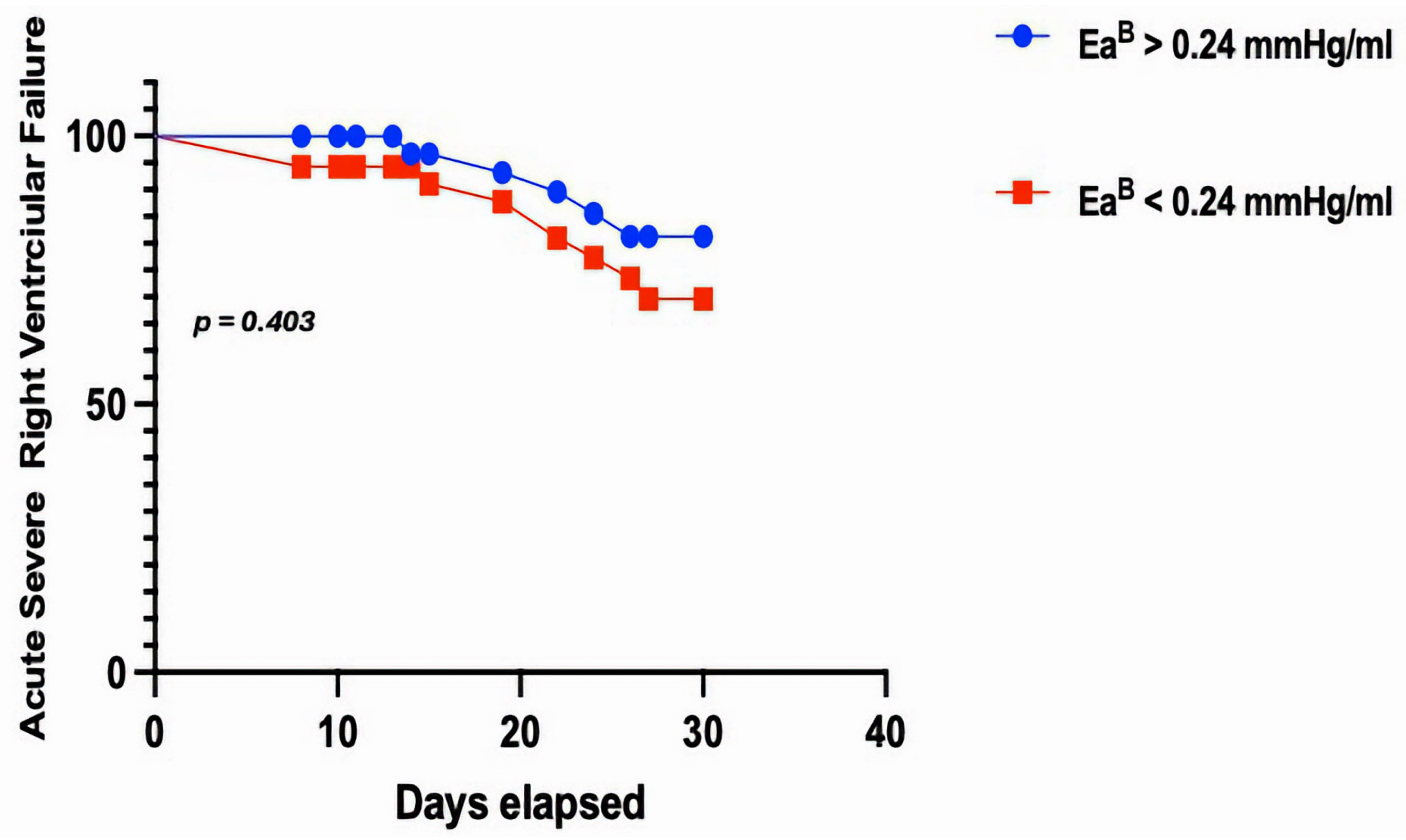
| EaB | 0.232 ± 0.13 | 0.278 ± 0.04 | 0.403 |
| Severe RVF | Not-Severe RVF | Severe RVF | Not-Severe RVF | ||
|---|---|---|---|---|---|
| CVP < 14 mmHg; EaC < 0.88 mmHg/ml | 0% | 46.4% | CVP < 14 mmHg; EaB < 0.24 mmHg/ml | 0% | 57.14% |
| CVP < 14 mmHg; EaC ≥ 0.88 mmHg/ml | 66.6% | 46.4% | CVP < 14 mmHg; EaB ≥ 0.24 mmHg/ml | 66.6% | 35.7% |
| CVP ≥ 14 mmHg; EaC < 0.88 mmHg/ml | 0% | 7.1% | CVP ≥ 14 mmHg; EaB < 0.24 mmHg/ml | 0% | 7.1% |
| CVP ≥ 14 mmHg; EaC ≥ 0.88 mmHg/ml | 33% | 0% | CVP ≥ 14 mmHg; EaB ≥ 0.24 mmHg/ml | 33% | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Mauro, M.; Kittleson, M.; Cacioli, G.; Piazza, V.; Putini, R.L.; Gravino, R.; Polizzi, V.; Montalto, A.; Comisso, M.; Sbaraglia, F.; et al. The Prognostic Role of Pulmonary Arterial Elastance in Patients Undergoing Left Ventricular Assist Device Implantation: A Pilot Study. J. Clin. Med. 2024, 13, 7102. https://doi.org/10.3390/jcm13237102
Di Mauro M, Kittleson M, Cacioli G, Piazza V, Putini RL, Gravino R, Polizzi V, Montalto A, Comisso M, Sbaraglia F, et al. The Prognostic Role of Pulmonary Arterial Elastance in Patients Undergoing Left Ventricular Assist Device Implantation: A Pilot Study. Journal of Clinical Medicine. 2024; 13(23):7102. https://doi.org/10.3390/jcm13237102
Chicago/Turabian StyleDi Mauro, Marco, Michelle Kittleson, Giulio Cacioli, Vito Piazza, Rita Lucia Putini, Rita Gravino, Vincenzo Polizzi, Andrea Montalto, Marina Comisso, Fabio Sbaraglia, and et al. 2024. "The Prognostic Role of Pulmonary Arterial Elastance in Patients Undergoing Left Ventricular Assist Device Implantation: A Pilot Study" Journal of Clinical Medicine 13, no. 23: 7102. https://doi.org/10.3390/jcm13237102
APA StyleDi Mauro, M., Kittleson, M., Cacioli, G., Piazza, V., Putini, R. L., Gravino, R., Polizzi, V., Montalto, A., Comisso, M., Sbaraglia, F., Monda, E., Petraio, A., De Feo, M., Amarelli, C., Marra, C., Musumeci, F., Di Lorenzo, E., & Masarone, D. (2024). The Prognostic Role of Pulmonary Arterial Elastance in Patients Undergoing Left Ventricular Assist Device Implantation: A Pilot Study. Journal of Clinical Medicine, 13(23), 7102. https://doi.org/10.3390/jcm13237102







