Kidney Damage in Pediatric Obesity: Insights from an Emerging Perspective
Abstract
1. Introduction
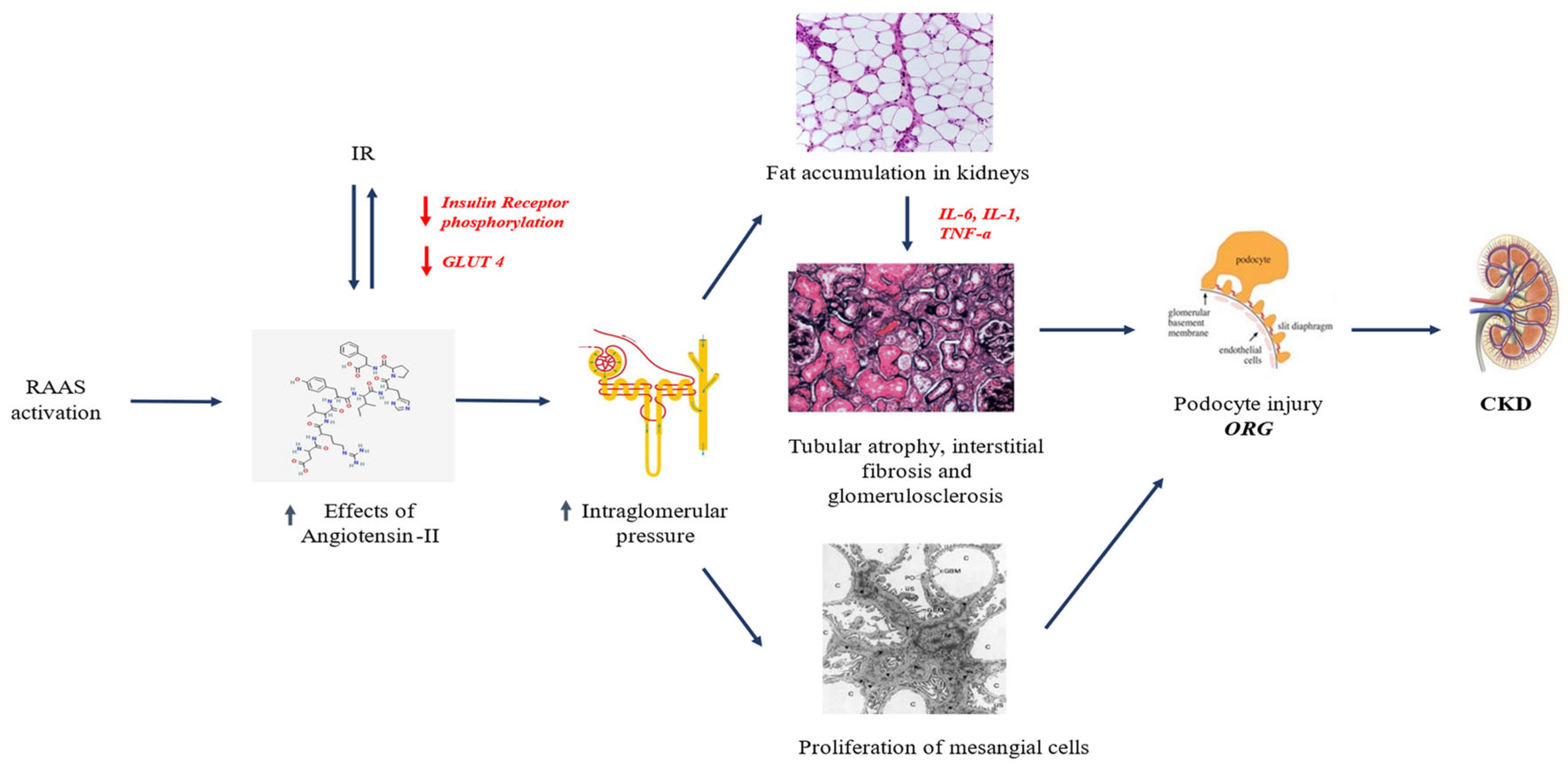
2. Pathophysiology of KD in Pediatric Obesity
2.1. RAAS Upregulation
2.2. Renal Lipotoxicity
2.3. IR
3. KD in Children with Obesity: State of the Art
| Reference | Study Design | Population and Methods | Main Findings |
|---|---|---|---|
| [9] | Case-control study | A total of 142 subjects was enrolled in the study group with the following inclusion criteria: (1) age of 10–16 years; (2) BMI z-score of >2; (3) no arterial hypertension; and (4) signed informed consent. Patients with obesity (BMI z-score > 2) were divided into “elevated GFR” (>130 mL/min/1.73 m2 [n = 42]), “normal GFR” (n = 85), and “decreased GFR” (<90 mL/min/1.73 m2 [n = 15]) groups according to GFR values estimated by Filler formula. A total of 62 subjects were enrolled as the control group. | Patients with obesity presented with albuminuria, lower serum adiponectin (p = 0.005), and higher urine Gal-3 concentrations (p = 0.004). Compared to controls, the “Decreased GFR” group showed higher serum uric acid (p = 0.004), triglycerides (p = 0.037), and cholesterol (p = 0.043) levels as well as significantly higher NGAL urine concentration and daily urine megalin excretion (p = 0.005). “Normal GFR” obese patients presented a strong correlation between urine Gal-3 concentration and urine NGAL concentration (p = 0.001, r = 0.706). |
| [11] | Cross-sectional study | 396 children and adolescents with obesity aged <18 years and a BMI that was >95th percentile according to reference values. Patients were classified according to obesity phenotypes such as MUO and MHO phenotypes. The study population was also stratified based on the presence or absence of KD (defined as the presence of reduced eGFR and/or albuminuria after a 3-month period of confirmation). | KD was found in 20.9% of the study population (25.8% of MUO group; 13.1% of MHO group). Children with KD showed higher BMI-SDS and higher HOMA-IR. MUO patients showed higher prevalence of hypertension, NAFLD and increased HOMA-IR values than those without KD (all p < 0.005). MHO patients showed significant differences for SBP-SDS, uric acid, fasting insulin, HOMA-IR, platelets, total cholesterol and low-density lipoprotein cholesterol values. MUO and MHO subjects had respectively an OR to show KD of 1.92 (95% CI: 1.22–3.01; p = 0.005) and 1.05 (95% CI: 1.00–1.09; p = 0.028) after adjustments. HOMA-IR was closely associated to KD in MUO group (OR = 2.07; 95% CI: 1.20–3.57; p = 0.007), while HOMA-IR (OR = 1.15; 95% CI: 1.02–1.29; p = 0.011) and uric acid (OR = 1.15; 95% CI: 1.02–1.30; p = 0.010) were the only significant risk factors for KD in MHO group. |
| [16] | Observational cross-sectional study | 117 children with CKD classified according to disease severity as stage I–II (eGFR > 60 mL/min/1.73 m2) or stage III–V (eGFR < 60 mL/min/1.73 m2). Overweight was defined as a BMI of >85th percentile for age and sex, and obesity was defined as a BMI of >95th percentile. Hypertension was defined as SBP of >95th percentile for age, sex, and height or receipt of one or more antihypertensive agents (excluding the use of angiotensin-converting enzyme inhibitors for proteinuria). eGFR was calculated using a modified Schwartz formula. | The prevalence of hyperuricemia in stage III–V was 70%. Children with stage III–V CKD were more likely to have hyperuricemia (OR, 4.6; 95% CI: 2.2–9.4; p < 0001). Children with hyperuricemia were more likely to be hypertensive (OR, 2.1; 95% CI: 1–4.1; p = 0.03). Hyperuricemia was significantly associated with increased BMI, albuminuria, and renal dysfunction with reduced eGFR and hypertension (all p < 0.05). Significant linear relationships between eGFR and urate and between BMI and urate were detected (both p = 0.0001). |
| [27] | Retrospective cohort study | A total of 801,019 youths aged 3 to 17 years (mean [SD] age, 9.4 [4.6] years; 409,167 [51.1%] female]; 391,852 [48.9%] male) were recruited. Baseline sex-specific BMI for age and change in the distance to the median BMI for age during the 5-year follow-up were compared. Cox proportional hazards regression models with age as a time scale were used to assess hypertension risk, adjusted for sex, race, and ethnicity, socioeconomic status, baseline year, and birth year. | Compared with youths with a baseline BMI for age in the 40th to 59th percentiles, the aHR for hypertension within a maximum of 5 years was 1.26 (95% CI: 1.20–1.33) for youths between the 60th and 84th percentiles. With every 1-unit annual increase in the distance to the median BMI for age, the aHR increased by 1.04 (95% CI: 1.04–1.05). The aHR was 4.94 (95% CI: 4.72–5.18) in youths with a baseline BMI for age in the 97th percentile or higher who maintained their body weight. Weight gain increased the risk associated with baseline BMI for age in the 97th percentile or higher with an aHR of 1.04 (95% CI: 1.04–1.05) per 1-unit annual increase in the distance to the median BMI. |
| [34] | Cohort study | The study population included 598,702 Israeli adolescents (age 16–20 years) evaluated for eligibility for military service. 505,816 (84.5%) were lean (BMI < 85th percentile), and 92,886 (15.5%) had a high BMI (BMI ≥ 85th percentile). According to BP levels, patients were clustered into group A (<120/<80 mm Hg; reference group), group B (120/<80–129/<80 mm Hg), group C (130/80–139/89 mm Hg), and group D (≥140/90 mm Hg). Early KD in young adulthood was defined as albuminuria of ≥30 mg/g with an estimated glomerular filtration rate of ≥60 mL/(min·1.73 m2). | Of 598 702 adolescents (54% men), 2004 (0.3%) developed early KD during a mean follow-up of 15.1 (7.2) years. The aHRs for early KD in BP group C were 1.17 (1.03–1.32) and 1.51 (1.22–1.86) among adolescents with lean and high BMIs, respectively. Corresponding HRs for KD in group D were 1.49 (1.15–1.93) and 1.79 (1.35–2.38) among adolescents with lean and high BMIs, respectively. |
| [38] | Cross-sectional study | A cohort of 1078 youths of both sexes in the range of 11–18 years of age was recruited. Patients were classified based on sex and age BMI percentiles (LMS method) into five BMI groups (as severely thin, thin, healthy, overweight, and obese), and measurements of urinary biomarkers of kidney injury (KIM-1, NGAL, and ACR) were obtained. | The median urinary levels of NGAL, ACR, and particularly KIM-1 (as a more sensitive indicator of kidney injury), showed no significant differences across the BMI groups. Notably, moderate correlations between BMI, KIM-1 and NGAL were identified in severely thin girls. |
| [46] | Review | 7 observational studies (4 prospective cohorts and 3 retrospective cohorts) evaluating the effect of obesity in childhood and adolescence on the occurrence of kidney diseases later in life were included. | Out of 7 cohort studies reported statistically significant positive links between obesity in early life and kidney disease later in life. 5 of the included studies adjusted for hypertension and only 2 adjusted for diabetes as major risk factors for CKD. |
| [47] | Cross-sectional cohort study | 600 children with overweight and obesity were enrolled (mean age was 12.20 ± 3.28 years; mean BMI z-score was 3.31 ± 0.75), of which 53.5% were female. 21.3% of the children were OW, 44.7% OB, and 34.0% with severe OB. eGFR equations and rSCr/Q-age or -/Q-height were assessed. | 96.5% of the children had SCr/Q-height and SCr/Q-age within the reference interval (0.67–1.33). SCr/Q-height was weakly inversely associated with BMI z-score (r = −0.109, p = 0.007). SCr and SCr/Q-age did not correlate with BMI z-score. SCr/Q-age and nearly all eGFR equations correlated with HOMA-IR and HDL cholesterol, triacylglyceride, serum uric acid, and ALT concentrations. All examined creatinine-based eGFR equations were positively correlated with fat mass and waist-to-hip ratio. |
| [49] | Review | 16 papers examining the prevalence of various biomarkers in adolescents with obesity and CKD were included. | Microalbuminuria was found in 75% of the studies analyzed as the most prevalent biomarker in adolescents. Hypertension was found to be as low as 13.6% and as high as 70.6%. Low HDL-C values were reported with a prevalence range of 1.39% to 20%. |
| [53] | Cross-sectional study | 3118 youth with OW/OB (aged 5–14 years) and 286 healthy normal weight youth. MRGFR was defined as eGFR > 60 and <90 mL/min/1.73 m2. eGFR was calculated through eGFRBSE and eGFRFAS. | MRGFR was found in 3.8% in NW vs. 7.8% in OW/OB (p = 0.016) by eGFRBSE, and 8.7% in NW versus 19.4% in OW/OB (p < 0.0001) by eGFRFAS. MRGFR was associated with an altered CMR profile in youths with OW/OB. The eGFRFAS equation identified a higher prevalence of youth with MRGFR compared to eGFRBSE equation. |
| [56] | Prospective cohort study | 614 children with overweight and obesity (mean age of 12.17 ± 3.28 years, 53.6% female, mean BMI z-score of 3.32 ± 0.75). Of these, 557 patients completed follow-up interventions. SCr was rescaled using Q-age and Q-height polynomials. At baseline, 95–97% of the children had a SCr/Q-height and SCr/Q-age in the normal reference range [0.67–1.33]. | SCr/Q significantly increased in every follow-up visit, and linear mixed regression analyses demonstrated slopes between 0.01 and 0.04 (corresponding to eGFR FAS reduction of 1.1–4.1 mL/min/1.73 m2) per visit. BMI z-score reduced in both sexes, and this reduction was significantly higher in males (slope = −0.07556, p < 0.0001). No correlation between change in rescaled SCr and BMI z-score reduction was demonstrated. |
4. Clinical Implications
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sawyer, A.; Zeitler, E.; Trachtman, H.; Bjornstad, P. Kidney considerations in pediatric obesity. Curr. Obes. Rep. 2023, 12, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Barlabà, A.; Grella, C.; Tammaro, M.; Petrone, D.; Guarino, S.; Miraglia Del Giudice, E.; Marzuillo, P.; Di Sessa, A. Kidney function evaluation in children and adolescents with obesity: A not-negligible need. Eur. J. Pediatr. 2024, 183, 3655–3664. [Google Scholar] [CrossRef] [PubMed]
- Carullo, N.; Zicarelli, M.; Michael, A.; Faga, T.; Battaglia, Y.; Pisani, A.; Perticone, M.; Costa, D.; Ielapi, N.; Coppolino, G.; et al. Childhood Obesity: Insight into kidney involvement. Int. J. Mol. Sci. 2023, 24, 17400. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018; NCHS Data Brief; CDC National Center for Health Statistics: Hyattsville, MD, USA, 2020; pp. 1–8. [Google Scholar]
- Kopple, J.D. Obesity and chronic kidney disease. J. Ren. Nutr. 2010, 20 (Suppl. S5), S29–S30. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.R.; Kim, H.; Park, J.T.; Chang, T.I.; Yoo, T.H.; Kang, S.W.; Choi, K.H.; Sung, S.; Kim, S.W.; Lee, J.; et al. Obesity, metabolic abnormality, and progression of CKD. Am. J. Kidney Dis. 2018, 72, 400–410. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Song, Y.; Caballero, B.; Cheskin, L.J. Association between obesity and kidney disease: A systematic review and meta-analysis. Kidney Int. 2008, 73, 19–33. [Google Scholar] [CrossRef]
- Vivante, A.; Golan, E.; Tzur, D.; Leiba, A.; Tirosh, A.; Skorecki, K.; Calderon-Margalit, R. Body mass index in 1.2 million adolescents and risk for end-stage renal disease. Arch. Intern. Med. 2012, 172, 1644–1650. [Google Scholar] [CrossRef]
- Mackowiak-Lewandowicz, K.; Ostalska-Nowicka, D.; Zaorska, K.; Kaczmarek, E.; Zachwieja, J.; Witt, M.; Nowicki, M. Chronic kidney disease predictors in obese adolescents. Pediatr. Nephrol. 2022, 37, 2479–2488. [Google Scholar] [CrossRef]
- Valerio, G.; Di Bonito, P.; Calcaterra, V.; Cherubini, V.; Corica, D.; De Sanctis, L.; Di Sessa, A.; Faienza, M.F.; Fornari, E.; Iughetti, L.; et al. Cardiometabolic risk in children and adolescents with obesity: A position paper of the Italian Society for Pediatric Endocrinology and Diabetology. Ital. J. Pediatr. 2024, 50, 205. [Google Scholar] [CrossRef]
- Di Sessa, A.; Passaro, A.P.; Colasante, A.M.; Cioffi, S.; Guarino, S.; Umano, G.R.; Papparella, A.; Miraglia Del Giudice, E.; Marzuillo, P. Kidney damage predictors in children with metabolically healthy and metabolically unhealthy obesity phenotype. Int. J. Obes. 2023, 47, 1247–1255. [Google Scholar] [CrossRef]
- Marzuillo, P.; Grandone, A.; Di Sessa, A.; Guarino, S.; Diplomatico, M.; Umano, G.R.; Polito, C.; La Manna, A.; Perrone, L.; Miraglia Del Giudice, E. Anthropometric and biochemical determinants of estimated glomerular filtration rate in a large cohort of obese children. J. Ren. Nutr. 2018, 28, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Di Sessa, A.; Guarino, S.; Umano, G.R.; Miraglia Del Giudice, E.; Marzuillo, P. MASLD vs. NAFLD: A better definition for children with obesity at higher risk of kidney damage. J. Hepatol. 2024, 80, e87–e89. [Google Scholar] [CrossRef] [PubMed]
- Hannon, T.S.; Arslanian, S.A. Obesity in adolescents. N. Engl. J. Med. 2023, 389, 251–261. [Google Scholar] [CrossRef] [PubMed]
- The Lancet Diabetes Endocrinology. Childhood obesity: A growing pandemic. Lancet Diabetes Endocrinol. 2022, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Noone, D.G.; Marks, S.D. Hyperuricemia is associated with hypertension, obesity, and albuminuria in children with chronic kidney disease. J. Pediatr. 2013, 162, 128–132. [Google Scholar] [CrossRef]
- Gicchino, M.F.; Di Sessa, A.; Guarino, S.; Miraglia Del Giudice, E.; Olivieri, A.N.; Marzuillo, P. Prevalence of and factors associated to chronic kidney disease and hypertension in a cohort of children with juvenile idiopathic arthritis. Eur. J. Pediatr. 2021, 180, 655–661. [Google Scholar] [CrossRef]
- Andrassy, K.M. Comments on ‘KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease’. Kidney Int. 2013, 84, 622–623. [Google Scholar] [CrossRef]
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; de Ferranti, S.D.; Dionne, J.M.; Falkner, B.; Flinn, S.K.; et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 2017, 140, e20171904. [Google Scholar] [CrossRef]
- Kounatidis, D.; Vallianou, N.G.; Stratigou, T.; Voukali, M.; Karampela, I.; Dalamaga, M. The kidney in obesity: Current evidence, perspectives and controversies. Curr. Obes. Rep. 2024, 13, 680–702. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Harada, R.; Hamasaki, Y.; Okuda, Y.; Hamada, R.; Ishikura, K. Epidemiology of pediatric chronic kidney disease/kidney failure: Learning from registries and cohort studies. Pediatr. Nephrol. 2022, 37, 1215–1229. [Google Scholar] [CrossRef] [PubMed]
- Câmara, N.O.; Iseki, K.; Kramer, H.; Liu, Z.H.; Sharma, K. Kidney disease and obesity: Epidemiology, mechanisms and treatment. Nat. Rev. Nephrol. 2017, 13, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Masalskienė, J.; Rudaitis, Š.; Vitkevič, R.; Čerkauskienė, R.; Dobilienė, D.; Jankauskienė, A. Epidemiology of Chronic Kidney Disease in Children: A Report from Lithuania. Medicina 2021, 57, 112. [Google Scholar] [CrossRef] [PubMed]
- Colasante, A.M.; Bartiromo, M.; Nardolillo, M.; Guarino, S.; Marzuillo, P.; Mangoni di S Stefano, G.S.R.C.; Miraglia Del Giudice, E.; Di Sessa, A. Tangled relationship between insulin resistance and microalbuminuria in children with obesity. World J. Clin. Pediatr. 2022, 11, 455–462. [Google Scholar] [CrossRef]
- Adebayo, O.C.; Nkoy, A.B.; van den Heuvel, L.P.; Labarque, V.; Levtchenko, E.; Delanaye, P.; Pottel, H. Glomerular hyperfiltration: Part 2-clinical significance in children. Pediatr. Nephrol. 2023, 38, 2529–2547. [Google Scholar] [CrossRef]
- Koebnick, C.; Sidell, M.A.; Li, X.; Woolford, S.J.; Kuizon, B.D.; Kunani, P. Association of high normal body weight in youths with risk of hypertension. JAMA Netw. Open 2023, 6, e231987. [Google Scholar] [CrossRef]
- Yau, K.; Kuah, R.; Cherney, D.Z.I.; Lam, T.K.T. Obesity and the kidney: Mechanistic links and therapeutic advances. Nat. Rev. Endocrinol. 2024, 20, 321–335. [Google Scholar] [CrossRef]
- Mangat, G.; Nair, N.; Barat, O.; Abboud, B.; Pais, P.; Bagga, S.; Raina, R. Obesity-related glomerulopathy in children: Connecting pathophysiology to clinical care. Clin. Kidney J. 2022, 16, 611–618. [Google Scholar] [CrossRef]
- Nawaz, S.; Chinnadurai, R.; Al-Chalabi, S.; Evans, P.; Kalra, P.A.; Syed, A.A.; Sinha, S. Obesity and chronic kidney disease: A current review. Obes. Sci. Pract. 2022, 9, 61–74. [Google Scholar] [CrossRef]
- Rüster, C.; Wolf, G. The role of the renin-angiotensin-aldosterone system in obesity-related renal diseases. Semin. Nephrol. 2013, 33, 44–53. [Google Scholar] [CrossRef]
- Luo, Z.; Chen, Z.; Hu, J.; Ding, G. Interplay of lipid metabolism and inflammation in podocyte injury. Metabolism 2024, 150, 155718. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, M.F. Safety in glomerular numbers. Pediatr. Nephrol. 2012, 27, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Tsur, A.M.; Akavian, I.; Derazne, E.; Tzur, D.; Vivante, A.; Grossman, E.; Rotem, R.S.; Fishman, B.; Afek, A.; Coresh, J.; et al. Adolescent blood pressure and the risk for early kidney damage in young adulthood. Hypertension 2022, 79, 974–983. [Google Scholar] [CrossRef]
- Gómez-Hernández, A.; Beneit, N.; Díaz-Castroverde, S.; Escribano, Ó. Differential role of adipose tissues in obesity and related metabolic and vascular complications. Int. J. Endocrinol. 2016, 2016, 1216783. [Google Scholar] [CrossRef]
- Kaneko, K.; Kimata, T.; Tsuji, S.; Shiraishi, K.; Yamauchi, K.; Murakami, M.; Kitagawa, T. Impact of obesity on childhood kidney. Pediatr. Rep. 2011, 3, e27. [Google Scholar] [CrossRef] [PubMed]
- Gunta, S.S.; Mak, R.H. Is obesity a risk factor for chronic kidney disease in children? Pediatr. Nephrol. 2013, 28, 1949–1956. [Google Scholar] [CrossRef]
- Gunasekara, T.D.K.S.C.; De Silva, P.M.C.S.; Chandana, E.P.S.; Jayasinghe, S.; Herath, C.; Siribaddana, S.; Jayasundara, N. Body mass index and implications for pediatric kidney health: A cross-sectional study with urinary biomarkers. Pediatr. Nephrol. 2024, 39, 167–175. [Google Scholar] [CrossRef]
- Sawada, K.; Chung, H.; Softic, S.; Moreno-Fernandez, M.E.; Divanovic, S. The bidirectional immune crosstalk in metabolic dysfunction-associated steatotic liver disease. Cell Metab. 2023, 35, 1852–1871. [Google Scholar] [CrossRef]
- Yi, J.; Qu, C.; Li, X.; Gao, H. Insulin resistance assessed by estimated glucose disposal rate and risk of atherosclerotic cardiovascular diseases incidence: The multi-ethnic study of atherosclerosis. Cardiovasc. Diabetol. 2024, 23, 349. [Google Scholar] [CrossRef]
- Sotiropoulos, C.; Giormezis, N.; Pertsas, V.; Tsirkas, T. Biomarkers and data visualization of insulin resistance and metabolic syndrome: An applicable approach. Life 2024, 14, 1197. [Google Scholar] [CrossRef]
- Coward, R.; Fornoni, A. Insulin signaling: Implications for podocyte biology in diabetic kidney disease. Curr. Opin. Nephrol. Hypertens. 2015, 24, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.C.; Yu, P.H.; Niu, S.W.; Kuo, I.C.; Lee, J.J.; Shen, F.C.; Chang, J.M.; Hwang, S.J. Association between Body Mass Index and renal outcomes modified by chronic kidney disease and anemia: The obesity paradox for renal outcomes. J. Clin. Med. 2022, 11, 2787. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Sietsma, S.J.; Navis, G.; Janssen, W.M.; de Zeeuw, D.; Gans, R.O.; de Jong, P.E.; PREVEND Study Group. A central body fat distribution is related to renal function impairment, even in lean subjects. Am. J. Kidney Dis. 2003, 41, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Martin-Del-Campo, F.; Batis-Ruvalcaba, C.; Ordaz-Medina, S.M.; Martínez-Ramírez, H.R.; Vizmanos-Lamotte, B.; Romero-Velarde, E.; Cortes-Sanabria, L.; Cueto-Manzano, A.M. Frequency and risk factors of kidney alterations in children and adolescents who are overweight and obese in a primary health-care setting. J. Ren. Nutr. 2019, 29, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Pourghazi, F.; Mohammadi, S.; Eslami, M.; Zoshk, M.Y.; Asadi, S.; Ejtahed, H.S.; Qorbani, M. Association between childhood obesity and later life kidney disorders: A systematic review. J. Ren. Nutr. 2023, 33, 520–528. [Google Scholar] [CrossRef]
- van Dam, M.J.C.M.; Pottel, H.; Vreugdenhil, A.C.E. Relation between obesity-related comorbidities and kidney function estimation in children. Pediatr. Nephrol. 2023, 38, 1867–1876. [Google Scholar] [CrossRef]
- Friedman, A.N.; Ogden, C.L.; Hales, C.M. Prevalence of obesity and CKD among adults in the United States, 2017–2020. Kidney Med. 2022, 5, 100568. [Google Scholar] [CrossRef]
- Nair, N.; Kalra, R.; Chandra Bhatt, G.; Narang, A.; Kumar, G.; Raina, R. The effect and prevalence of comorbidities in adolescents with CKD and obesity. Adv. Chronic Kidney Dis. 2022, 29, 251–262. [Google Scholar] [CrossRef]
- ESCAPE Trial Group; Wühl, E.; Trivelli, A.; Picca, S.; Litwin, M.; Peco-Antic, A.; Zurowska, A.; Testa, S.; Jankauskiene, A.; Emre, S.; et al. Strict blood-pressure control and progression of renal failure in children. N. Engl. J. Med. 2009, 361, 1639–1650. [Google Scholar] [CrossRef]
- Myette, R.L.; Flynn, J.T. The ongoing impact of obesity on childhood hypertension. Pediatr. Nephrol. 2024, 39, 2337–2346. [Google Scholar] [CrossRef]
- García-Carro, C.; Vergara, A.; Bermejo, S.; Azancot, M.A.; Sellarés, J.; Soler, M.J. A Nephrologist Perspective on Obesity: From Kidney Injury to Clinical Management. Front. Med. 2021, 8, 655871. [Google Scholar] [CrossRef] [PubMed]
- Di Bonito, P.; Licenziati, M.R.; Campana, G.; Chiesa, C.; Pacifico, L.; Manco, M.; Miraglia Del Giudice, E.; Di Sessa, A.; Baroni, M.G.; Marzuillo, P.; et al. Prevalence of Mildly Reduced Estimated GFR by height- or age-related equations in young people with obesity and its association with cardiometabolic risk factors. J. Ren. Nutr. 2021, 31, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Mallamaci, F.; Ruggenenti, P.; Perna, A.; Leonardis, D.; Tripepi, R.; Tripepi, G.; Remuzzi, G.; Zoccali, C.; REIN Study Group. ACE inhibition is renoprotective among obese patients with proteinuria. J. Am. Soc. Nephrol. 2011, 22, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Smeets, N.J.L.; Schreuder, M.F.; Dalinghaus, M.; Male, C.; Lagler, F.B.; Walsh, J.; Laer, S.; de Wildt, S.N. Pharmacology of enalapril in children: A review. Drug Discov. Today 2020, 25, 1957–1970. [Google Scholar] [CrossRef]
- van Dam, M.J.C.M.; Pottel, H.; Delanaye, P.; Vreugdenhil, A.C.E. The evaluation of kidney function estimation during lifestyle intervention in children with overweight and obesity. Pediatr. Nephrol. 2024, 39, 3271–3278. [Google Scholar] [CrossRef]
- Stefan, N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020, 8, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Khokhar, A.; Chin, V.; Perez-Colon, S.; Farook, T.; Bansal, S.; Kochummen, E.; Umpaichitra, V. Differences between metabolically healthy vs unhealthy obese children and adolescents. J. Natl. Med. Assoc. 2017, 109, 203–210. [Google Scholar] [CrossRef]
- Wan Mohd Zin, R.M.; Jalaludin, M.Y.; Yahya, A.; Nur Zati Iwani, A.K.; Md Zain, F.; Hong, J.Y.H.; Mokhtar, A.H.; Wan Mohamud, W.N. Prevalence and clinical characteristics of metabolically healthy obese versus metabolically unhealthy obese school children. Front. Endocrinol. 2022, 13, 971202. [Google Scholar] [CrossRef]
- Vukovic, R.; Dos Santos, T.J.; Ybarra, M.; Atar, M. Children with metabolically healthy obesity: A review. Front. Endocrinol. 2019, 10, 865. [Google Scholar] [CrossRef]
- Blüher, S.; Schwarz, P. Metabolically healthy obesity from childhood to adulthood—Does weight status alone matter? Metabolism 2014, 63, 1084–1092. [Google Scholar] [CrossRef]
- Di Bonito, P.; Miraglia Del Giudice, E.; Chiesa, C.; Licenziati, M.R.; Manco, M.; Franco, F.; Tornese, G.; Baroni, M.G.; Morandi, A.; Maffeis, C.; et al. Preclinical signs of liver and cardiac damage in youth with metabolically healthy obese phenotype. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Correia-Costa, L.; Azevedo, A.; Caldas Afonso, A. Childhood Obesity and Impact on the Kidney. Nephron 2019, 143, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Medyńska, A.; Chrzanowska, J.; Zubkiewicz-Kucharska, A.; Zwolińska, D. New Markers of Early Kidney Damage in Children and Adolescents with Simple Obesity. Int. J. Mol. Sci. 2024, 25, 10769. [Google Scholar] [CrossRef] [PubMed]
- Salinas, M.P.; Sepúlveda, J.; Hidalgo, L.; Peirano, D.; Morel, M.; Uribe, P.; Rotemberg, V.; Briones, J.; Mery, D.; Navarrete-Dechent, C. A systematic review and meta-analysis of artificial intelligence versus clinicians for skin cancer diagnosis. npj Digit. Med. 2024, 7, 125. [Google Scholar] [CrossRef]
- Mondillo, G.; Frattolillo, V.; Colosimo, S.; Perrotta, A.; Di Sessa, A.; Guarino, S.; Miraglia Del Giudice, E.; Marzuillo, P. Basal Knowledge in the Field of Pediatric Nephrology and Its Enhancement Following Specific Training of ChatGPT-4 “Omni” and Gemini 1.5 Flash. Pediatr. Nephrol. 2024. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forcina, G.; Luciano, M.; Frattolillo, V.; Mori, S.; Monaco, N.; Guarino, S.; Marzuillo, P.; Miraglia del Giudice, E.; Di Sessa, A. Kidney Damage in Pediatric Obesity: Insights from an Emerging Perspective. J. Clin. Med. 2024, 13, 7025. https://doi.org/10.3390/jcm13237025
Forcina G, Luciano M, Frattolillo V, Mori S, Monaco N, Guarino S, Marzuillo P, Miraglia del Giudice E, Di Sessa A. Kidney Damage in Pediatric Obesity: Insights from an Emerging Perspective. Journal of Clinical Medicine. 2024; 13(23):7025. https://doi.org/10.3390/jcm13237025
Chicago/Turabian StyleForcina, Gianmario, Margherita Luciano, Vittoria Frattolillo, Simona Mori, Noemi Monaco, Stefano Guarino, Pierluigi Marzuillo, Emanuele Miraglia del Giudice, and Anna Di Sessa. 2024. "Kidney Damage in Pediatric Obesity: Insights from an Emerging Perspective" Journal of Clinical Medicine 13, no. 23: 7025. https://doi.org/10.3390/jcm13237025
APA StyleForcina, G., Luciano, M., Frattolillo, V., Mori, S., Monaco, N., Guarino, S., Marzuillo, P., Miraglia del Giudice, E., & Di Sessa, A. (2024). Kidney Damage in Pediatric Obesity: Insights from an Emerging Perspective. Journal of Clinical Medicine, 13(23), 7025. https://doi.org/10.3390/jcm13237025








