Artificial Intelligence-Driven Volumetric Analysis of Muscle Mass as a Predictor of Tumor Response to Neoadjuvant Chemoradiotherapy in Patients with Rectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Assessment of Sarcopenia
2.3. Assessment of Clinical Outcome
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Pre-nCRT Volumetric SMI and nCRT Response
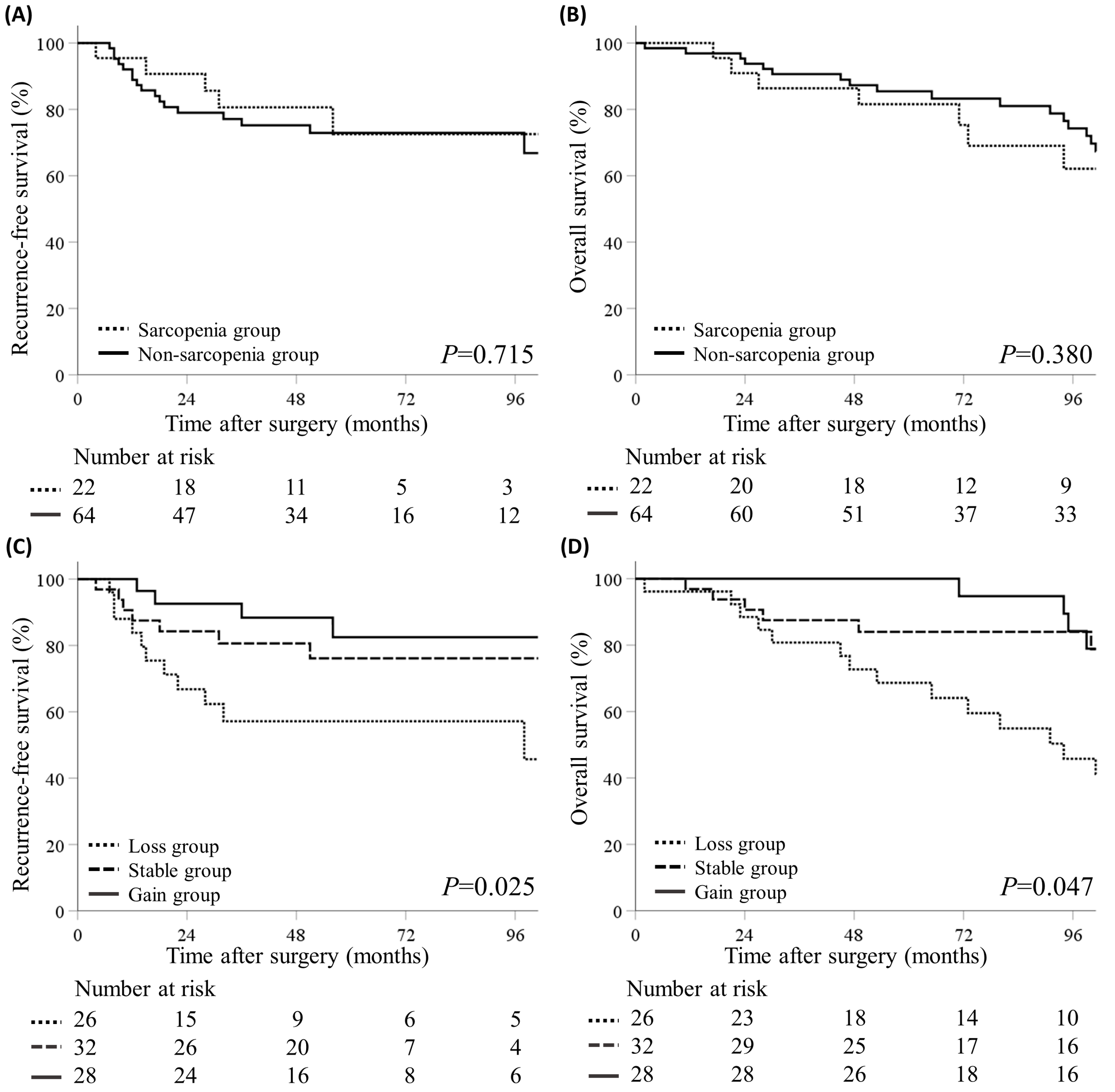
3.3. Volumetric SMI Change and Survival Outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.; Lee, H.S.; Cho, E.S.; Park, E.J.; Baik, S.H.; Lee, K.Y.; Kang, J. Prognostic significance of sarcopenia and skeletal muscle mass change during preoperative chemoradiotherapy in locally advanced rectal cancer. Clin. Nutr. 2020, 39, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. NCCN Guidelines Insights: Rectal Cancer, Version 6.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Kohl, V.K.B.; Weber, K.; Brunner, M.; Geppert, C.I.; Fietkau, R.; Grützmann, R.; Semrau, S.; Merkel, S. Factors influencing downstaging after neoadjuvant long-course chemoradiotherapy in rectal carcinoma. Int. J. Colorectal Dis. 2022, 37, 1355–1365. [Google Scholar] [CrossRef]
- Ryu, H.S.; Park, I.J.; Ahn, B.K.; Park, M.Y.; Kim, M.S.; Kim, Y.I.; Lim, S.B.; Kim, J.C. Prognostic significance of lymph node yield on oncologic outcomes according to tumor response after preoperative chemoradiotherapy in rectal cancer patients. Ann. Coloproctol. 2023, 39, 410–420. [Google Scholar] [CrossRef]
- Tan, Y.; Fu, D.; Li, D.; Kong, X.; Jiang, K.; Chen, L.; Yuan, Y.; Ding, K. Predictors and risk factors of pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancer: A population-based analysis. Front. Oncol. 2019, 9, 497. [Google Scholar] [CrossRef] [PubMed]
- Maas, M.; Nelemans, P.J.; Valentini, V.; Das, P.; Rödel, C.; Kuo, L.J.; Calvo, F.A.; García-Aguilar, J.; Glynne-Jones, R.; Haustermans, K.; et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol. 2010, 11, 835–844. [Google Scholar] [CrossRef]
- Park, I.J.; Yu, Y.S.; Mustafa, B.; Park, J.Y.; Seo, Y.B.; Kim, G.D.; Kim, J.; Kim, C.M.; Noh, H.D.; Hong, S.M.; et al. A nine-gene signature for predicting the response to preoperative chemoradiotherapy in patients with locally advanced rectal cancer. Cancers 2020, 12, 800. [Google Scholar] [CrossRef]
- Abe, S.; Nozawa, H.; Sasaki, K.; Murono, K.; Emoto, S.; Yokoyama, Y.; Matsuzaki, H.; Nagai, Y.; Yoshioka, Y.; Shinagawa, T.; et al. Sarcopenia is associated with oncological prognosis and the incidence of secondary cancer in patients with middle/lower rectal cancer. Clin. Colorectal Cancer 2023, 22, 143–152. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, X.; Zhang, Q.; Yang, Y. Prognostic value of sarcopenia in patients with rectal cancer: A meta-analysis. PLoS ONE 2022, 17, e0270332. [Google Scholar] [CrossRef]
- Bedrikovetski, S.; Traeger, L.; Vather, R.; Sammour, T.; Moore, J.W. Does sarcopenia predict local response rates after chemoradiotherapy for locally advanced rectal cancer? Dis. Colon Rectum 2023, 66, 965–972. [Google Scholar] [CrossRef]
- Bedrikovetski, S.; Traeger, L.; Price, T.J.; Carruthers, S.; Selva-Nayagam, S.; Moore, J.W.; Sammour, T. Can sarcopenia predict complete response after total neoadjuvant therapy in advanced rectal cancer? A multicentre observational cohort study. J. Surg. Oncol. 2023, 128, 75–84. [Google Scholar] [CrossRef]
- Thibault, R.; Pichard, C. The evaluation of body composition: A useful tool for clinical practice. Ann. Nutr. Metab. 2012, 60, 6–16. [Google Scholar] [CrossRef]
- Kim, M.; Lee, S.M.; Son, I.T.; Park, T.; Oh, B.Y. Prognostic value of artificial intelligence-driven, computed tomography-based, volumetric assessment of the volume and density of muscle in patients with colon cancer. Korean J. Radiol. 2023, 24, 849–859. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Han, Q.; Kim, S.I.; Yoon, S.H.; Kim, T.M.; Kang, H.C.; Kim, H.J.; Cho, J.Y.; Kim, J.W. Impact of computed tomography-based, artificial intelligence-driven volumetric sarcopenia on survival outcomes in early cervical cancer. Front. Oncol. 2021, 11, 741071. [Google Scholar] [CrossRef]
- Pickhardt, P.J.; Summers, R.M.; Garrett, J.W. Automated CT-based body composition analysis: A golden opportunity. Korean J. Radiol. 2021, 22, 1934–1937. [Google Scholar] [CrossRef]
- Lee, Y.S.; Hong, N.; Witanto, J.N.; Choi, Y.R.; Park, J.; Decazes, P.; Eude, F.; Kim, C.O.; Chang Kim, H.; Goo, J.M.; et al. Deep neural network for automatic volumetric segmentation of whole-body CT images for body composition assessment. Clin. Nutr. 2021, 40, 5038–5046. [Google Scholar] [CrossRef] [PubMed]
- Weston, A.D.; Korfiatis, P.; Kline, T.L.; Philbrick, K.A.; Kostandy, P.; Sakinis, T.; Sugimoto, M.; Takahashi, N.; Erickson, B.J. Automated abdominal segmentation of CT scans for body composition analysis using deep learning. Radiology 2019, 290, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Chung, J.Y.; Paik, H.; Seol, A.; Yoon, S.H.; Kim, T.M.; Kim, H.S.; Chung, H.H.; Cho, J.Y.; Kim, J.W.; et al. Prognostic role of computed tomography-based, artificial intelligence-driven waist skeletal muscle volume in uterine endometrial carcinoma. Insights Imaging 2021, 12, 192. [Google Scholar] [CrossRef] [PubMed]
- Çiçek, Ö.; Abdulkadir, A.; Lienkamp, S.S.; Brox, T.; Ronneberger, O. 3D U-Net: Learning dense volumetric segmentation from sparse annotation. In Medical Image Computing and Computer-Assisted Intervention–MICCAI 2016: Proceedings of the 19th International Conference, Athens, Greece, 17–21 October 2016; Proceedings, Part II 19; Springer: Cham, Switzerland, 2016; pp. 424–432. [Google Scholar]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional networks for biomedical image segmentation. In Medical Image Computing and Computer-Assisted Intervention–MICCAI 2015: Proceedings of the 18th International Conference, Munich, Germany, 5–9 October 2015; Proceedings, Part III 18; Springer: Cham, Switzerland, 2015; pp. 234–241. [Google Scholar]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist circumference as a vital sign in clinical practice: A consensus statement from the IAS and ICCR working group on visceral obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Baba, Y.; Sakamoto, Y.; Ohuchi, M.; Tokunaga, R.; Kurashige, J.; Hiyoshi, Y.; Iwagami, S.; Yoshida, N.; Yoshida, M.; et al. Sarcopenia is a negative prognostic factor after curative resection of colorectal cancer. Ann. Surg. Oncol. 2015, 22, 2663–2668. [Google Scholar] [CrossRef]
- Wang, S.; Xie, H.; Gong, Y.; Kuang, J.; Yan, L.; Ruan, G.; Gao, F.; Gan, J. The value of L3 skeletal muscle index in evaluating preoperative nutritional risk and long-term prognosis in colorectal cancer patients. Sci. Rep. 2020, 10, 8153. [Google Scholar] [CrossRef] [PubMed]
- Bimurzayeva, A.; Kim, M.J.; Ahn, J.S.; Ku, G.Y.; Moon, D.; Choi, J.; Kim, H.J.; Lim, H.K.; Shin, R.; Park, J.W.; et al. Three-dimensional body composition parameters using automatic volumetric segmentation allow accurate prediction of colorectal cancer outcomes. J. Cachexia Sarcopenia Muscle 2024, 15, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guan, J.; Ding, C.; Feng, M.; Gong, L.; Guan, W. Muscle loss 6 months after surgery predicts poor survival of patients with non-metastatic colorectal cancer. Front. Nutr. 2022, 9, 1047029. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Li, Y.; Peng, Y.; Lu, D.; Zhang, F.; Cui, X.; Zhang, Q.; Li, Z. Can sarcopenia be a predictor of prognosis for patients with non-metastatic colorectal cancer? A systematic review and meta-analysis. Int. J. Colorectal Dis. 2018, 33, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, J.J.; Reif, R.L.; Bigam, D.L.; Baracos, V.E.; Eurich, D.T.; Sawyer, M.B. The impact of muscle and adipose tissue on long-term survival in patients with stage I to III colorectal cancer. Dis. Colon Rectum 2019, 62, 549–560. [Google Scholar] [CrossRef]
- Malietzis, G.; Currie, A.C.; Athanasiou, T.; Johns, N.; Anyamene, N.; Glynne-Jones, R.; Kennedy, R.H.; Fearon, K.C.; Jenkins, J.T. Influence of body composition profile on outcomes following colorectal cancer surgery. Br. J. Surg. 2016, 103, 572–580. [Google Scholar] [CrossRef]
- Takeda, Y.; Akiyoshi, T.; Matsueda, K.; Fukuoka, H.; Ogura, A.; Miki, H.; Hiyoshi, Y.; Nagasaki, T.; Konishi, T.; Fujimoto, Y.; et al. Skeletal muscle loss is an independent negative prognostic factor in patients with advanced lower rectal cancer treated with neoadjuvant chemoradiotherapy. PLoS ONE 2018, 13, e0195406. [Google Scholar] [CrossRef]
- De Nardi, P.; Giani, A.; Maggi, G.; Braga, M. Relation between skeletal muscle volume and prognosis in rectal cancer patients undergoing neoadjuvant therapy. World J. Gastrointest. Oncol. 2022, 14, 423–433. [Google Scholar] [CrossRef]
- Luo, L.; Fan, Y.; Wang, Y.; Wang, Z.; Zhou, J. Prevalence and clinical outcomes of sarcopenia in patients with esophageal, gastric or colorectal cancers receiving preoperative neoadjuvant therapy: A meta-analysis. Asia Pac. J. Oncol. Nurs. 2024, 11, 100436. [Google Scholar] [CrossRef]
- De Nardi, P.; Salandini, M.; Chiari, D.; Pecorelli, N.; Cristel, G.; Damascelli, A.; Ronzoni, M.; Massimino, L.; De Cobelli, F.; Braga, M. Changes in body composition during neoadjuvant therapy can affect prognosis in rectal cancer patients: An exploratory study. Curr. Probl. Cancer 2020, 44, 100510. [Google Scholar] [CrossRef]
- Fukuoka, T.; Maeda, K.; Nagahara, H.; Shibutani, M.; Iseki, Y.; Matsutani, S.; Hirakawa, K.; Ohira, M. Change in PMI during neoadjuvant therapy is a predictive prognostic marker in rectal cancer. Anticancer Res. 2019, 39, 5157–5163. [Google Scholar] [CrossRef] [PubMed]
- Dossa, F.; Chesney, T.R.; Acuna, S.A.; Baxter, N.N. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef]
- Fleming, C.A.; O’Connell, E.P.; Kavanagh, R.G.; O’Leary, D.P.; Twomey, M.; Corrigan, M.A.; Wang, J.H.; Maher, M.M.; O’Connor, O.J.; Redmond, H.P. Body composition, inflammation, and 5-year outcomes in colon cancer. JAMA Netw. Open 2021, 4, e2115274. [Google Scholar] [CrossRef] [PubMed]

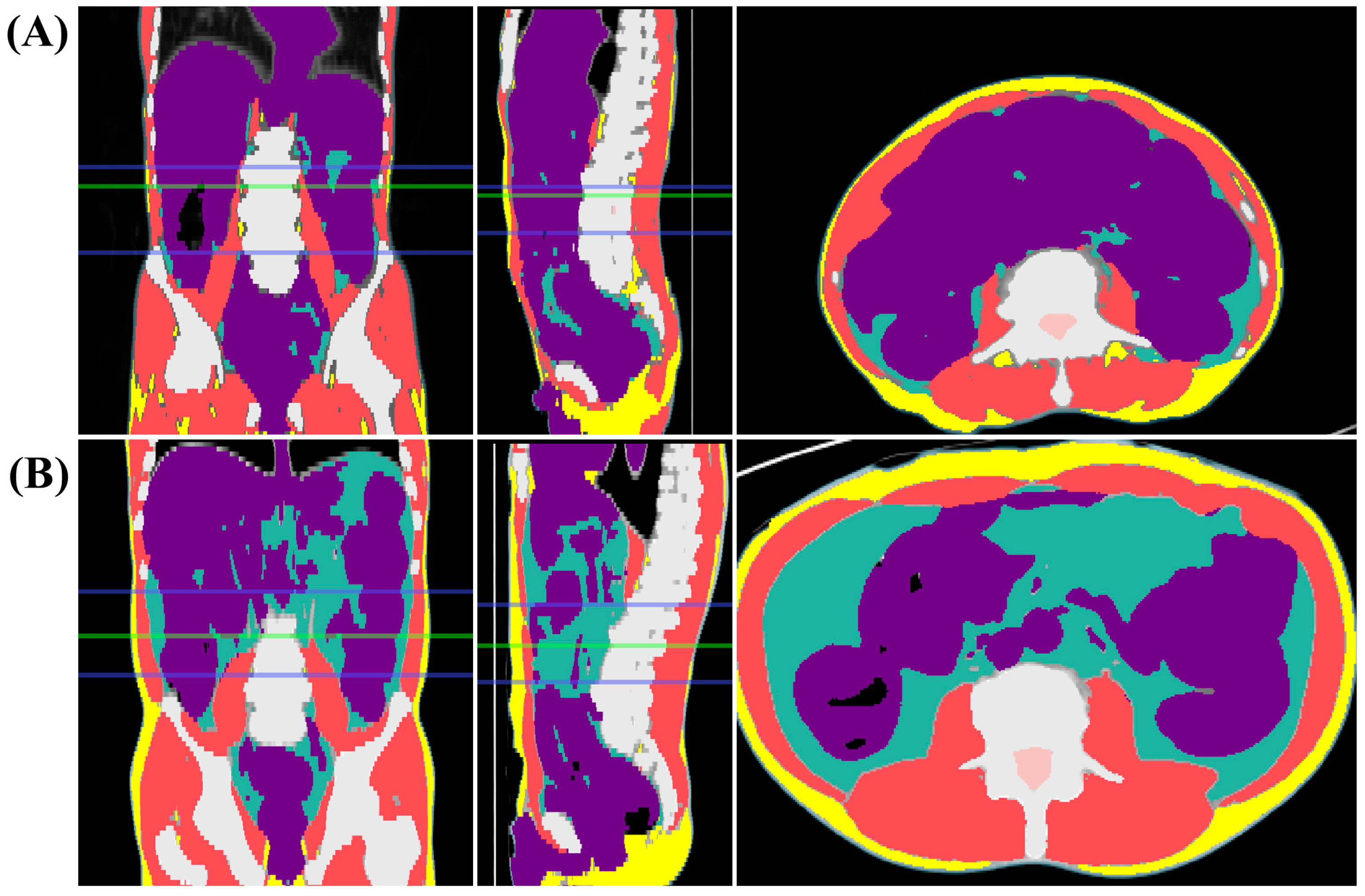
| Variables | Sarcopenia (n = 22) | Non-Sarcopenia (n = 64) | p-Value |
|---|---|---|---|
| Age | 69.9 ± 11.3 | 61.8 ± 10.7 | 0.004 |
| Sex | 1 | ||
| Male | 15 (68.2) | 44 (68.8) | |
| Female | 7 (31.8) | 20 (31.2) | |
| BMI (kg/m2) | 21.8 ± 2.3 | 24.2 ± 3.2 | 0.003 |
| cStage | 1 | ||
| I | 1 (4.5) | 4 (6.3) | |
| II | 6 (27.3) | 17 (26.6) | |
| III | 15 (68.2) | 43 (67.2) | |
| CEA (ng/mL) | 1 | ||
| ≥5.0 | 4 (18.2) | 13 (19.1) | |
| <5.0 | 18 (81.8) | 55 (80.9) | |
| ypStage | 0.863 | ||
| 0 or I | 8 (36.4) | 24 (37.5) | |
| II | 9 (40.9) | 22 (34.4) | |
| III | 5 (22.7) | 18 (28.1) | |
| Cell type | 1 | ||
| WD/MD | 21 (95.5) | 59 (92.2) | |
| PD/MUC/SRC | 1 (4.5) | 5 (7.8) | |
| LVI | 1 | ||
| Positive | 5 (22.7) | 16 (25.0) | |
| Negative | 17 (77.3) | 48 (75.0) | |
| PNI | 1 | ||
| Positive | 2 (9.1) | 6 (9.4) | |
| Negative | 20 (90.9) | 58 (90.6) | |
| nCRT response | 0.043 | ||
| Poor | 12 (54.5) | 19 (29.7) | |
| Good | 10 (45.5) | 45 (70.3) | |
| Parameter | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Pre-nCRT SMI | ||||
| Non-sarcopenia | 1 (Reference) | 1 (Reference) | ||
| Sarcopenia | 0.35 (0.13–0.95) | 0.04 | 0.34 (0.12–0.96) | 0.041 |
| Age | ||||
| <65 | 1 (Reference) | 1 (Reference) | ||
| ≥65 | 1.14 (0.46–2.80) | 0.778 | 2.10 (0.94–4.69) | 0.072 |
| Sex | ||||
| Male | 1 (Reference) | 1 (Reference) | ||
| Female | 1.52 (0.57–4.03) | 0.403 | 2.44 (0.99–6.00) | 0.053 |
| cStage | 0.066 | |||
| I | 1 (Reference) | |||
| II | 1.09 (0.48–2.47) | 0.835 | ||
| III | 1.90 (1.11–3.27) | 0.02 | ||
| CEA | ||||
| <5.0 | 1 (Reference) | |||
| ≥5.0 | 0.82 (0.26–2.55) | 0.726 | ||
| Parameter | Recurrence-Free Survival | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| cHR (95% CI) | p-Value | aHR (95% CI) | p-Value | cHR (95% CI) | p-Value | aHR (95% CI) | p-Value | |
| SMI change | 0.035 | 0.035 | 0.063 | 0.07 | ||||
| Loss group | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | ||||
| Stable group | 0.43 (0.17–1.11) | 0.081 | 0.38 (0.15–1.01) | 0.051 | 0.38 (0.15–0.94) | 0.037 | 0.41 (0.17–1.03) | 0.058 |
| Gain group | 0.25 (0.8–0.80) | 0.019 | 0.26 (0.08–0.83) | 0.023 | 0.44 (0.19–1.06) | 0.068 | 0.41 (0.17–0.99) | 0.049 |
| Pre-nCRT SMI | ||||||||
| Non-sarcopenia | 1 (Reference) | 1 (Reference) | ||||||
| Sarcopenia | 0.83 (0.31–2.25) | 0.716 | 1.42 (0.65–3.13) | 0.382 | ||||
| Age | ||||||||
| <65 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||||
| ≥65 | 1.53 (0.66–3.53) | 0.321 | 2.34 (1.11–4.94) | 0.026 | 2.75 (1.26–5.99) | 0.011 | ||
| Sex | ||||||||
| Male | 1 (Reference) | 1 (Reference) | ||||||
| Female | 0.61 (0.23–1.67) | 0.337 | 0.66 (0.28–1.54) | 0.334 | ||||
| cStage | 0.633 | 0.59 | ||||||
| I | 1 (Reference) | 1 (Reference) | ||||||
| II | 1.36 (0.16–11.72) | 0.779 | 2.72 (0.35–21.12) | 0.338 | ||||
| III | 2.01 (0.26–15.26) | 0.501 | 2.18 (0.29–16.49) | 0.451 | ||||
| CEA (ng/mL) | ||||||||
| <5.0 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||||
| ≥5.0 | 2.19 (0.89–5.39) | 0.087 | 2.39 (0.93–6.15) | 0.071 | 1.25 (0.47–3.31) | 0.653 | ||
| ypStage | 0.064 | 0.058 | 0.773 | |||||
| 0 or I | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||||
| II | 2.36 (0.73–7.70) | 0.153 | 3.17 (0.93–10.83) | 0.066 | 1.32 (0.56–3.14) | 0.529 | ||
| III | 3.76 (1.16–12.22) | 0.028 | 3.49 (1.07–11.40) | 0.038 | 1.36 (0.52–3.55) | 0.536 | ||
| Cell type | ||||||||
| WD/MD | 1 (Reference) | 1 (Reference) | ||||||
| PD/MUC/SRC | 1.50 (0.35–6.43) | 0.584 | 1.67 (0.50–5.55) | 0.404 | ||||
| LVI | ||||||||
| Negative | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||||
| Positive | 2.04 (0.85–4.86) | 0.109 | 2.11 (0.95–4.70) | 0.067 | 2.90 (1.24–6.80) | 0.014 | ||
| PNI | ||||||||
| Negative | 1 (Reference) | 1 (Reference) | ||||||
| Positive | 3.29 (1.10–9.82) | 0.033 | 2.63 (0.77–8.99) | 0.123 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Lee, S.M.; Son, I.T.; Kang, J.; Noh, G.T.; Oh, B.Y. Artificial Intelligence-Driven Volumetric Analysis of Muscle Mass as a Predictor of Tumor Response to Neoadjuvant Chemoradiotherapy in Patients with Rectal Cancer. J. Clin. Med. 2024, 13, 7018. https://doi.org/10.3390/jcm13237018
Kim M, Lee SM, Son IT, Kang J, Noh GT, Oh BY. Artificial Intelligence-Driven Volumetric Analysis of Muscle Mass as a Predictor of Tumor Response to Neoadjuvant Chemoradiotherapy in Patients with Rectal Cancer. Journal of Clinical Medicine. 2024; 13(23):7018. https://doi.org/10.3390/jcm13237018
Chicago/Turabian StyleKim, Minsung, Sang Min Lee, Il Tae Son, Jaewoong Kang, Gyoung Tae Noh, and Bo Young Oh. 2024. "Artificial Intelligence-Driven Volumetric Analysis of Muscle Mass as a Predictor of Tumor Response to Neoadjuvant Chemoradiotherapy in Patients with Rectal Cancer" Journal of Clinical Medicine 13, no. 23: 7018. https://doi.org/10.3390/jcm13237018
APA StyleKim, M., Lee, S. M., Son, I. T., Kang, J., Noh, G. T., & Oh, B. Y. (2024). Artificial Intelligence-Driven Volumetric Analysis of Muscle Mass as a Predictor of Tumor Response to Neoadjuvant Chemoradiotherapy in Patients with Rectal Cancer. Journal of Clinical Medicine, 13(23), 7018. https://doi.org/10.3390/jcm13237018






