Efficacy and Blood Levels of Lacosamide in Patients with Focal Epilepsy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Considerations
2.2. Patient Selection and Treatment
2.3. Sample Collection and Evaluation
- B: paroxysmal frequency for 28 days before evaluation.
- T: paroxysmal frequency for 28 days after evaluation.
2.4. Statistical Analysis
3. Results
3.1. Details of Patients’ Age Ranges, Doses, and Blood Levels by Timing of Sampling and Seizure Type, and the Time from the Most Recent Administration to Blood Sampling
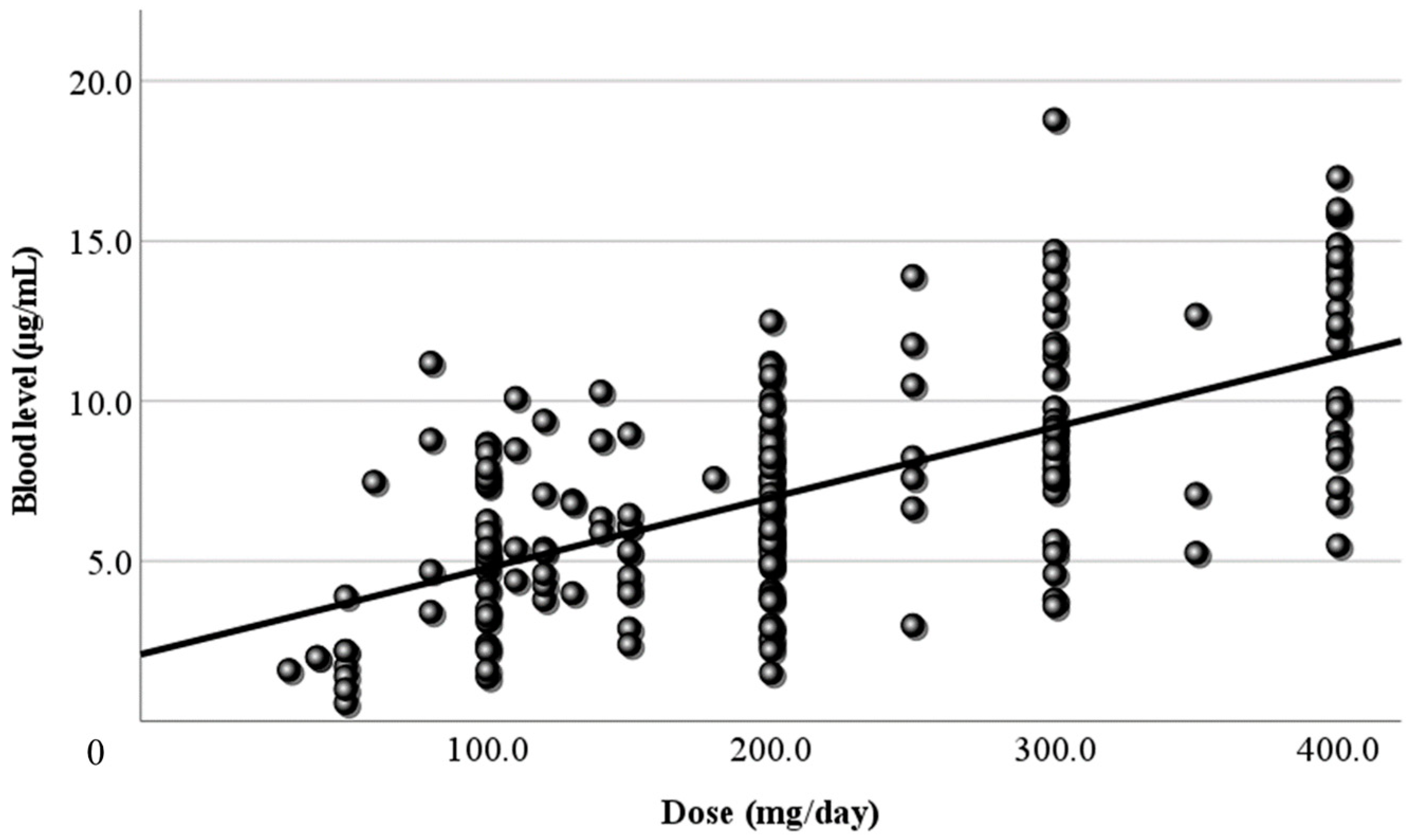
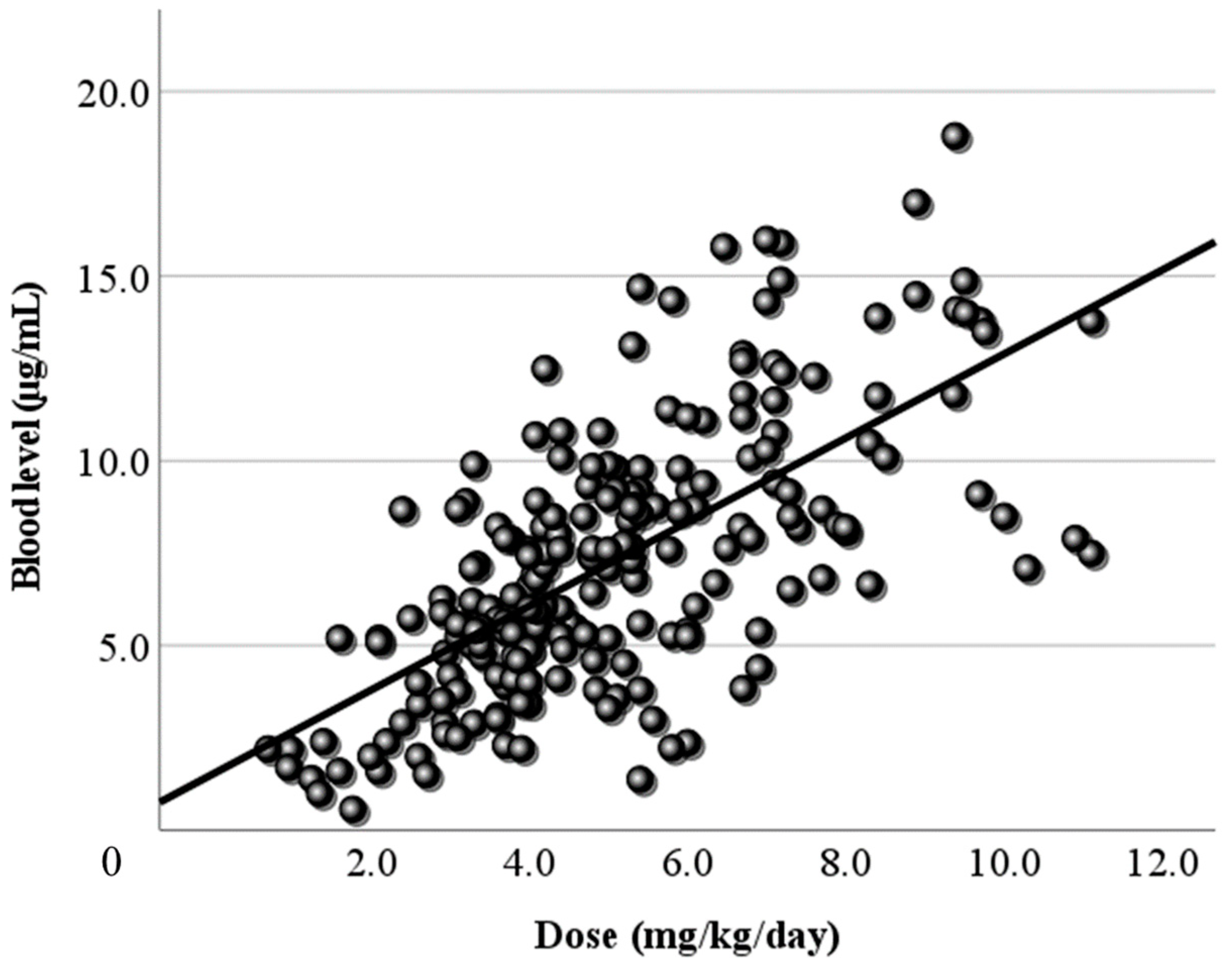
3.2. Relationship Between Dose and Blood Level
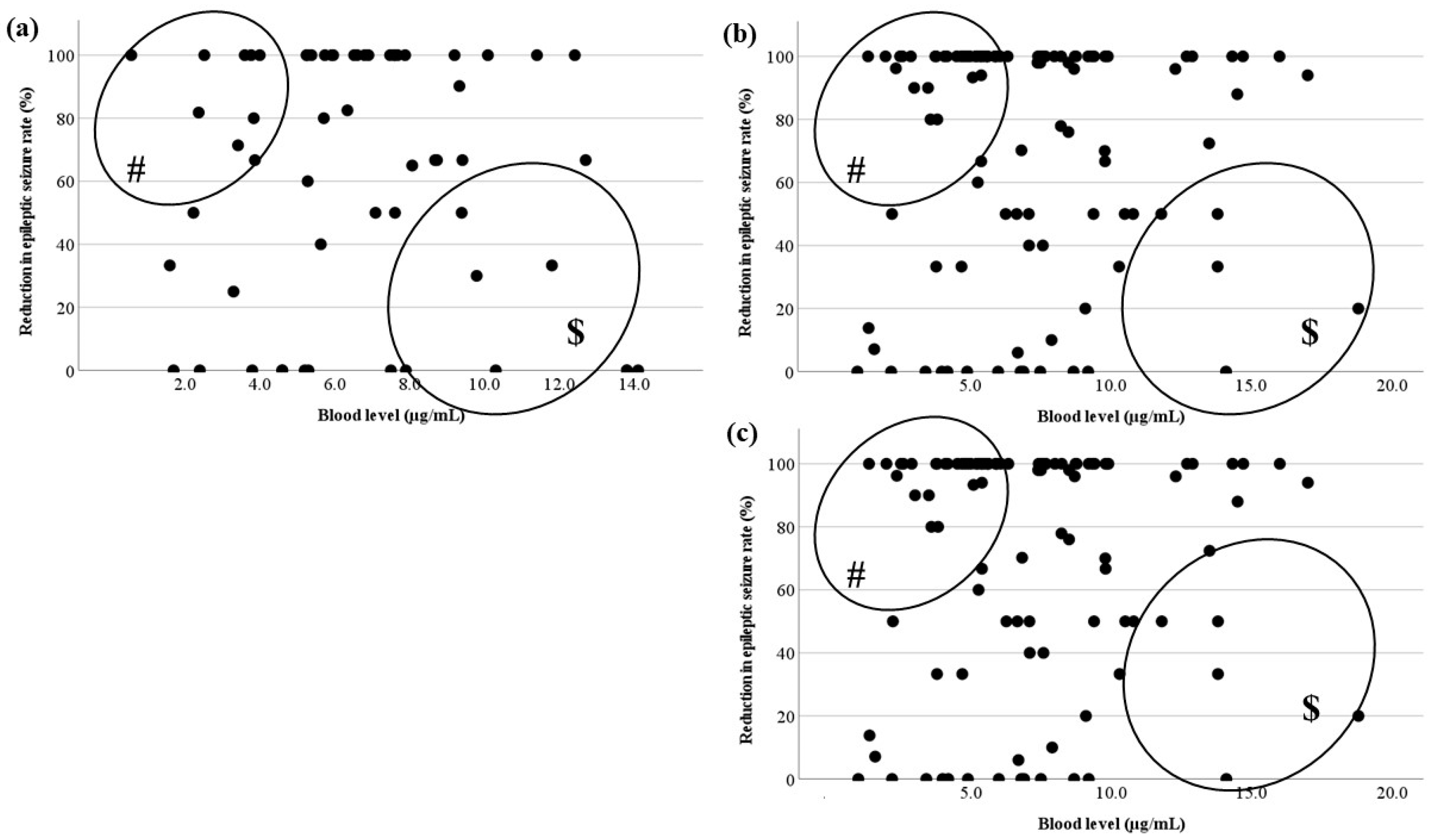
3.3. Relationship Between the Blood Level and RR
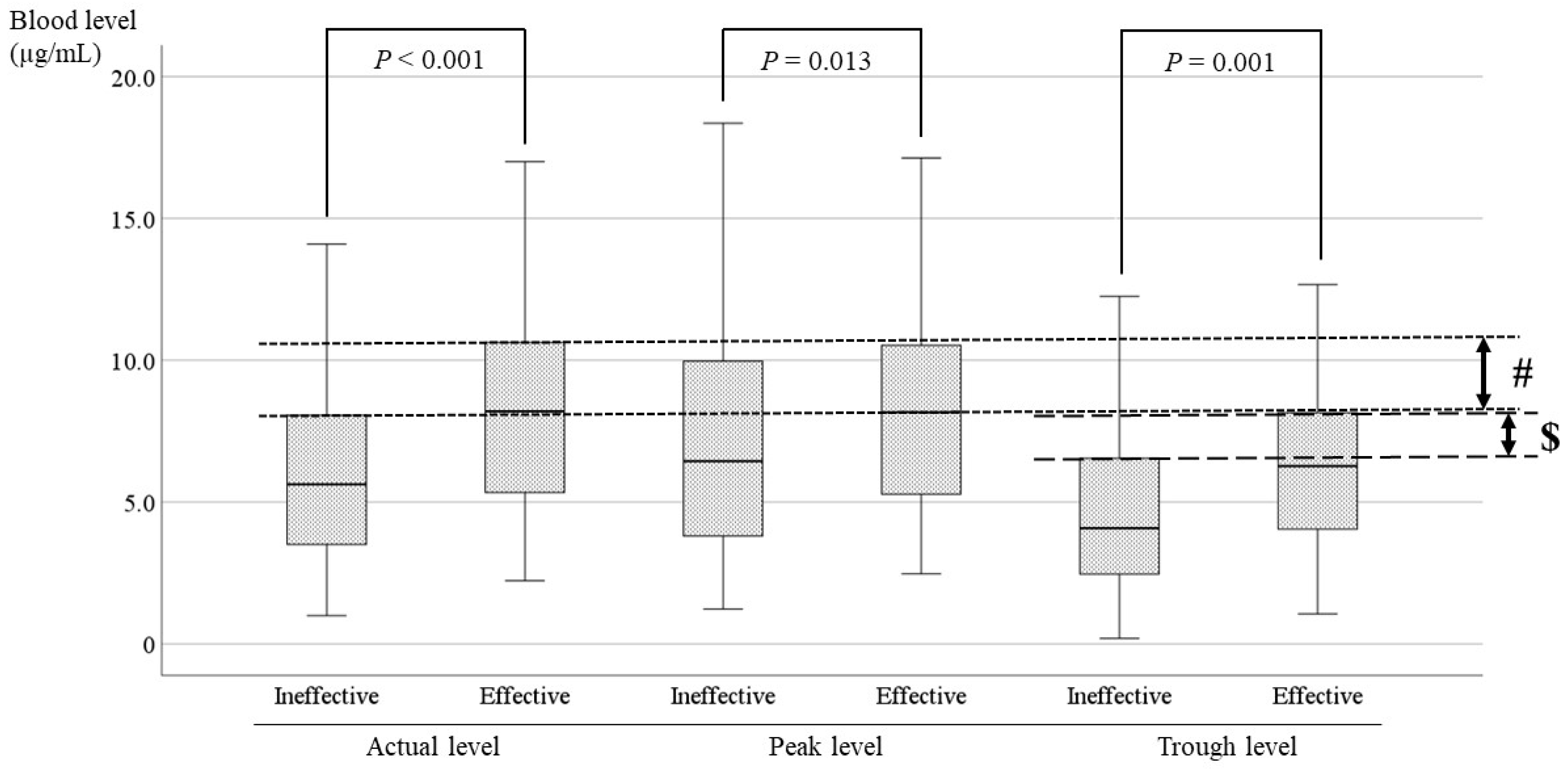
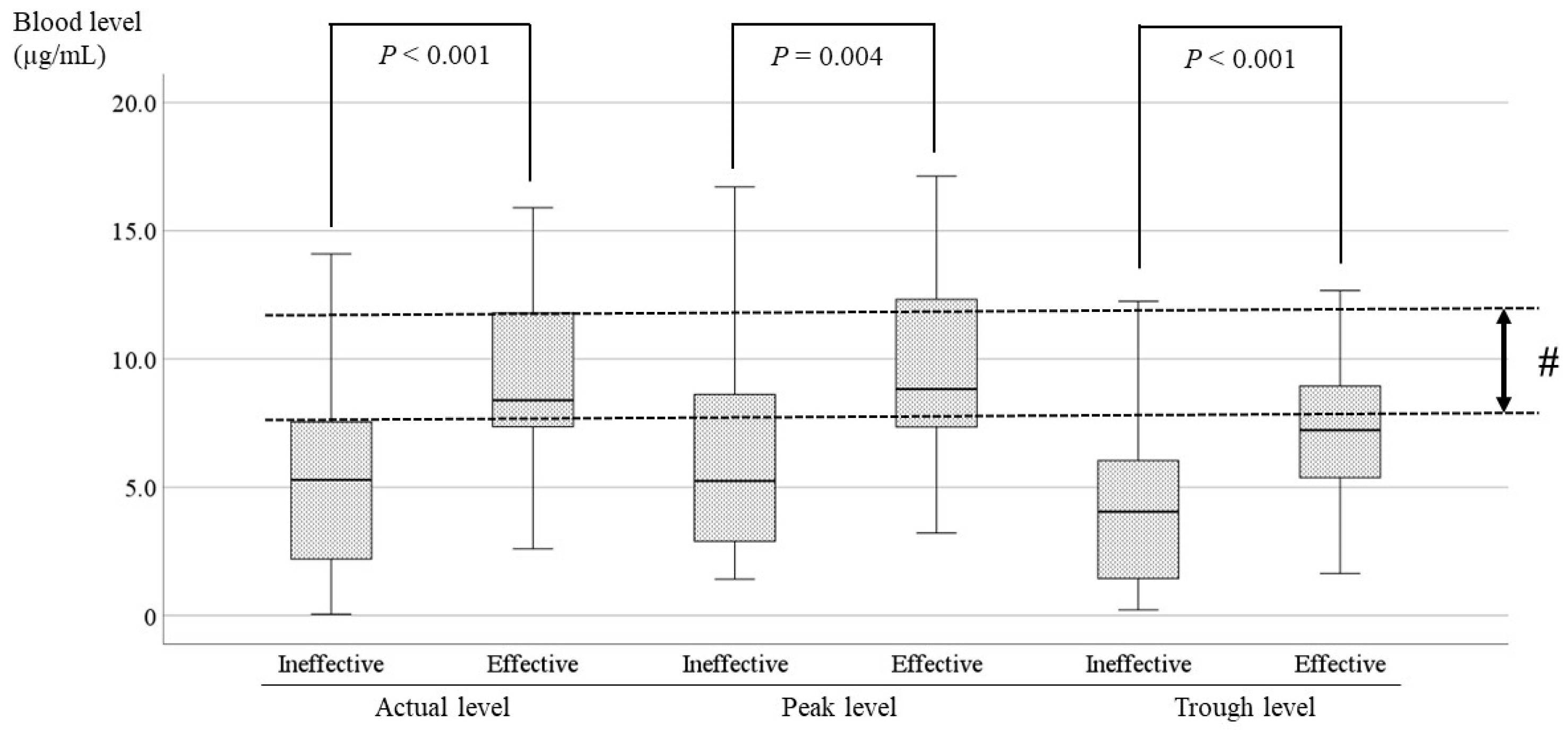
3.4. Comparisons of the LCM Blood Level Between the Effective and Ineffective Cases and Identification of the Optimal Range
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Errington, A.C.; Coyne, L.; Stöhr, T.; Selve, N.; Lees, G. Seeking a mechanism of action for the novel anticonvulsant lacosamide. Neuropharmacology 2006, 50, 1016–1029. [Google Scholar] [CrossRef] [PubMed]
- Errington, A.C.; Stöhr, T.; Heers, C.; Lees, G. The investigational anticonvulsant lacosamide selectively enhances slow inactivation of voltage-gated sodium channels. Mol. Pharmacol. 2008, 73, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Niespodziany, I.; Leclère, N.; Vandenplas, C.; Foerch, P.; Wolff, C. Comparative study of lacosamide and classical sodium channel blocking antiepileptic drugs on sodium channel slow inactivation. J. Neurosci. Res. 2013, 91, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Kohn, E.; Lezinger, M.; Daniel, S.; Masarwi, M.; Brandriss, N.; Bar-Chaim, A.; Berkovitch, M.; Heyman, E.; Komargodski, R. Therapeutic drug monitoring of lacosamide among children: Is it helpful? Front. Pharmacol. 2023, 14, 1164902. [Google Scholar] [CrossRef]
- Steinhoff, B.J.; Hübers, E.; Kurth, C.; Jürges (Kehl-Kork), U. Plasma concentration and clinical effects of perampanel-The Kork experience. Seizure 2019, 67, 18–22. [Google Scholar] [CrossRef]
- Giroux, P.C.; Salas-Prato, M.; Théorêt, Y.; Carmant, L. Levetiracetam in children with refractory epilepsy: Lack of correlation between plasma concentration and efficacy. Seizure 2009, 18, 559–563. [Google Scholar] [CrossRef]
- Cawello, W. Clinical pharmacokinetic and pharmacodynamic profile of lacosamide. Clin. Pharmacokinet. 2015, 54, 901–914. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef]
- Fisher, R.S.; Cross, J.H.; French, J.A.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshé, S.L.; Peltola, J.; Perez, E.R.; et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 522–530. [Google Scholar] [CrossRef]
- Casas-Fernández, C.; Martínez-Bermejo, A.; Rufo-Campos, M.; Smeyers-Durá, P.; Herranz-Fernández, J.L.; Ibáñez-Micó, S.; Campistol-Plana, J.; Alarcón-Martínez, H.; Campos-Castelló, J. Efficacy and tolerability of lacosamide in the concomitant treatment of 130 patients under 16 years of age with refractory epilepsy: A prospective, open-label, observational, multicenter study in Spain. Drugs R D 2012, 12, 187–197. [Google Scholar] [CrossRef]
- Gavatha, M.; Ioannou, I.; Papavasiliou, A.S. Efficacy and tolerability of oral lacosamide as adjunctive therapy in pediatric patients with pharmacoresistant focal epilepsyy. Epilepsy Behav. 2011, 20, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Yorns, W.R., Jr.; Khurana, D.S.; Carvalho, K.S.; Hardison, H.H.; Legido, A.; Valencia, I. Efficacy of lacosamide as adjunctive therapy in children with refractory epilepsy. J. Child. Neurol. 2014, 29, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Cawello, W.; Nickel, B.; Eggert-Formella, A. No pharmacokinetic interaction between lacosamide and carbamazepine in healthy volunteers. J. Clin. Pharmacol. 2010, 50, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Cawello, W.; Bonn, R. No pharmacokinetic interaction between lacosamide and valproic acid in healthy volunteers. J. Clin. Pharmacol. 2012, 52, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Oh, J.; Kim, D.Y.; Son, H.; Hwang, S.; Shin, H.R.; Kim, E.Y.; Lee, H.S.; Lee, W.J.; Moon, J.; et al. Effects of CYP2C19 genetic polymorphisms on the pharmacokinetics of lacosamide in Korean patients with epilepsy. Epilepsia 2022, 63, 2958–2969. [Google Scholar] [CrossRef]
- Beyreuther, B.K.; Freitag, J.; Heers, C.; Krebsfänger, N.; Scharfenecker, U.; Stöhr, T. Lacosamide: A review of preclinical properties. CNS Drug Rev. 2007, 13, 21–42. [Google Scholar] [CrossRef]
- Wolff, C.; Carrington, B.; Varrin-Doyer, M.; Vandendriessche, A.; Van der Perren, C.; Famelart, M.; Gillard, M.; Foerch, P.; Rogemond, V.; Honnorat, J.; et al. Drug binding assays do not reveal specific binding of lacosamide to collapsin response mediator protein 2 (CRMP-2). CNS Neurosci. Ther. 2012, 18, 493–500. [Google Scholar] [CrossRef]
- Shiloh-Malawsky, Y.; Fan, Z.; Greenwood, R.; Tennison, M. Successful treatment of childhood prolonged refractory status epilepticus with lacosamide. Seizure 2011, 20, 586–588. [Google Scholar] [CrossRef]
- Ishikawa, N.; Eguchi, Y.; Izumo, H.; Tateishi, Y.; Tani, H.; Kobayashi, Y.; Okada, S. Clinical impact of the dose and blood concentration of lacosamide in Japanese pediatric patients with epilepsy: A cohort study. Epilepsy Behav. 2022, 129, 108614. [Google Scholar] [CrossRef]
- Zhao, T.; Yu, L.H.; Zhang, H.L.; Yu, J.; Feng, J.; Wang, T.T.; Sun, Y.; Li, H.J. Long-term effectiveness and safety of lacosamide as adjunctive therapy in children and adolescents with refractory epilepsy: A real-world study. BMC Pediatr. 2023, 23, 249. [Google Scholar] [CrossRef]
- Hiemke, C.; Baumann, P.; Bergemann, N.; Conca, A.; Dietmaier, O.; Egberts, K.; Fric, M.; Gerlach, M.; Greiner, C.; Gründer, G.; et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: Update 2011. Pharmacopsychiatry 2011, 44, 195–235. [Google Scholar] [CrossRef] [PubMed]
- Vossler, D.G.; Knake, S.; O’Brien, T.J.; Watanabe, M.; Brock, M.; Steiniger-Brach, B.; Williams, P.; Roebling, R.; SP0982 co-investigators. Efficacy and safety of adjunctive lacosamide in the treatment of primary generalised tonic-clonic seizures: A double-blind, randomised, placebo-controlled trial. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, H.L.; Zhang, Y.Y.; Dong, N.; Hu, Y.H.; Chen, J.; Lu, X.P.; Chen, F. Plasma lacosamide monitoring in children with epilepsy: Focus on reference therapeutic range and influencing factors. Front. Pediatr. 2022, 10, 949783. [Google Scholar] [CrossRef]
- Terada, K.; Yamamoto, T.; Yokoyama, T.; Ikeda, K.; Hoshii, N. Long-term Efficacy and Safety of Conversion to Lacosamide Monotherapy in Japanese Adults with Partial-onset Seizures: A Multicenter, Open-label, Phase 3 Trial. Shinryo Shinyaku 2019, 56, 1–10. (In Japanese) [Google Scholar]
- Inoue, Y.; Osakabe, T.; Hirano, K.; Shimizu, S. Tolerability of adjunctive therapy with lacosamide, a novel antiepileptic drug, in adult epilepsy patiens: Secondary analysis of data from a double-blind comparative trial and an ongoing long-term open trial. Jpn. J. Clin. Psychophaemacol. 2017, 20, 439–453. (In Japanese) [Google Scholar]
- Aícua-Rapún, I.; André, P.; Rossetti, A.O.; Ryvlin, P.; Hottinger, A.F.; Decosterd, L.A.; Buclin, T.; Novy, J. Therapeutic Drug Monitoring of Newer Antiepileptic Drugs: A Randomized Trial for Dosage Adjustment. Ann. Neurol. 2020, 87, 22–29. [Google Scholar] [CrossRef]
- Patsalos, P.N.; Spencer, E.P.; Berry, D.J. Therapeutic Drug Monitoring of Antiepileptic Drugs in Epilepsy: A 2018 Update. Ther Drug Monit. 2018, 40, 526–548. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B. An Updated Overview on Therapeutic Drug Monitoring of Recent Antiepileptic Drugs. Drugs R D 2016, 16, 303–316. [Google Scholar] [CrossRef]
- Asadi-Pooya, A.A.; Farazdaghi, M. Idiopathic generalized epilepsies: Which seizure type is more difficult to control? J. Clin. Neurosci. 2023, 114, 93–96. [Google Scholar] [CrossRef]
- Duy, P.Q.; Krauss, G.L.; Crone, N.E.; Ma, M.; Johnson, E.L. Antiepileptic drug withdrawal and seizure severity in the epilepsy monitoring unit. Epilepsy Behav. 2020, 109, 107128. [Google Scholar] [CrossRef]



| Age (y) | Dose (mg/kg/day) | Blood Level (µg/mL) | 1 p Value | |
|---|---|---|---|---|
| All samples | 15.3 ± 6.0 | 5.1 ± 2.2 | 7.1 ± 3.5 | |
| Timing of sampling | ||||
| 1 month | 14.4 ± 6.1 | 4.6 ± 2.1 | 6.1 ± 3.2 | – |
| 6 months after | 16.2 ± 6.5 | 5.4 ± 2.1 | 8.2 ± 3.5 | p = 0.006 |
| 12 months after | 15.9 ± 5.7 | 5.6 ± 1.9 | 8.1 ± 3.3 | p = 0.027 |
| Seizure type | ||||
| Focal aware seizure | 15.7 ± 4.9 | 5.2 ± 2.3 | 6.7 ± 3.2 | – |
| Focal impaired awareness seizure | 14.9 ± 5.6 | 5.1 ± 2.3 | 7.2 ± 3.7 | p = 0.331 |
| Focal to bilateral tonic–clonic seizure | 15.5 ± 6.6 | 4.9 ± 2.0 | 7.1 ± 3.4 | p = 0.611 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasaki, T.; Kobayashi, T.; Miyamoto, Y.; Imaizumi, T.; Kaku, S.; Udagawa, N.; Yamamoto, H.; Shimizu, N. Efficacy and Blood Levels of Lacosamide in Patients with Focal Epilepsy. J. Clin. Med. 2024, 13, 6958. https://doi.org/10.3390/jcm13226958
Iwasaki T, Kobayashi T, Miyamoto Y, Imaizumi T, Kaku S, Udagawa N, Yamamoto H, Shimizu N. Efficacy and Blood Levels of Lacosamide in Patients with Focal Epilepsy. Journal of Clinical Medicine. 2024; 13(22):6958. https://doi.org/10.3390/jcm13226958
Chicago/Turabian StyleIwasaki, Toshiyuki, Toshihiro Kobayashi, Yusaku Miyamoto, Taichi Imaizumi, Shotaro Kaku, Noriko Udagawa, Hitoshi Yamamoto, and Naoki Shimizu. 2024. "Efficacy and Blood Levels of Lacosamide in Patients with Focal Epilepsy" Journal of Clinical Medicine 13, no. 22: 6958. https://doi.org/10.3390/jcm13226958
APA StyleIwasaki, T., Kobayashi, T., Miyamoto, Y., Imaizumi, T., Kaku, S., Udagawa, N., Yamamoto, H., & Shimizu, N. (2024). Efficacy and Blood Levels of Lacosamide in Patients with Focal Epilepsy. Journal of Clinical Medicine, 13(22), 6958. https://doi.org/10.3390/jcm13226958




