Abstract
Background: There are cases of idiopathic interstitial pneumonias (IIPs) that do not meet the diagnostic criteria for connective tissue disease but have clinical features suggestive of autoimmune process. Interstitial pneumonia with autoimmune features (IPAF) was recently proposed as a research concept for these patients. Although several prospective studies on IPAF have been conducted, its clinical significance in cryptogenic organizing pneumonia (COP) remains unclear. Methods: Patients aged ≥20 years with suspected COP were prospectively enrolled between June 2018 and December 2022. Among the enrolled patients, those diagnosed with COP based on computed tomography (CT) and bronchoalveolar lavage (BAL) findings were compared between the IPAF and non-IPAF groups. Results: A total of 56 patients were enrolled in this study. Of these, 30 were diagnosed with COP and included in the analysis. Clinical and serological features were positive in two and six patients, respectively. Each feature was exclusive, and eight patients (26.7%) were diagnosed with IPAF. There were no differences between the IPAF and non-IPAF groups in terms of clinical features, including BAL findings, laboratory data, CT findings, and clinical course. During the one-year follow-up period, the frequency of COP exacerbation did not differ between the IPAF and non-IPAF groups, and no cases of systemic autoimmune disease or death occurred in either group. Conclusions: The COP characteristics of the IPAF and non-IPAF groups are similar in all aspects, and distinguishing between the two groups may be of little significance.
1. Introduction
There are cases of idiopathic interstitial pneumonias (IIPs) that do not meet the diagnostic criteria for connective tissue disease, but that have symptoms suggestive of autoimmune process, test positive for autoantibodies, or present histologically with findings suggestive of autoimmune disease [1]. Undifferentiated connective tissue disease was first proposed by Kinder et al. [2], lung-dominant connective tissue disease by Fischer et al. [3], and autoimmune-featured interstitial lung disease by Vij et al. [4]. However, since they all have slightly different diagnostic criteria, in 2015, Fischer et al. reported the concept of interstitial pneumonia with autoimmune features (IPAF) as an official statement of the European Respiratory Society (ERS) and American Thoracic Society (ATS), resulting in the unification of the diagnostic criteria [5]. IPAF is a disease concept that includes multiple types of interstitial pneumonia, including non-specific interstitial pneumonia (NSIP), organizing pneumonia (OP), NSIP with OP overlap, and lymphoid interstitial pneumonia.
A recent prospective observational study on IPAF reported that 70 of the 376 IIP cases (18.6%) met the IPAF criteria, and the IPAF group had a higher frequency of developing collagen disease, fewer acute exacerbations, and a better prognosis [6]. However, more than half of the patients in this study were classified as having unclassifiable idiopathic interstitial pneumonia, and it is unclear whether the same trend exists in each disease group (e.g., NSIP and OP). Therefore, this study focused specifically on cryptogenic organizing pneumonia (COP) and examined whether there are clinical differences in COP between IPAF and non-IPAF groups.
2. Materials and Methods
2.1. Study Design and Participants
This prospective multicenter observational study was conducted by the Okayama Respiratory Disease Study Group. The participating institutions included Okayama University Hospital, KKR Takamatsu Hospital, National Hospital Organization Iwakuni Medical Center, Japanese Red Cross Kobe Hospital, Kagawa Rosai Hospital, Okayama City Hospital, National Hospital Organization Minami-Okayama Medical Center, National Hospital Organization Fukuyama Medical Center, and Kurashiki Central Hospital. This study was approved by the Institutional Review Board of Okayama University Hospital (no. 1804-050, approval date: 7 May 2018) and all other participating hospitals.
Patients aged ≥ 20 years with suspected COP and scheduled for bronchoalveolar lavage (BAL) or surgical lung biopsy for diagnosing COP were prospectively enrolled between June 2018 and December 2022. Written informed consent was obtained from all the participants. Patients who did not provide consent were excluded from the study. The most reliable diagnosis was made by pathological examination of the lung tissue obtained by surgical lung biopsy. However, surgical lung biopsy is rarely performed for COP because of its good prognosis [7,8,9,10]. Although transbronchial biopsy (TBLB) may be able to diagnose OP [8,11,12], it can sometimes be inadequate due to a small sample size or may misdiagnose the disease due to partial OP of other diseases such as a different idiopathic interstitial pneumonia, vasculitis, or malignancy [7]. Therefore, we established the diagnostic criteria for COP in this study based on computed tomography (CT) images and BAL findings, with reference to a previous study [11]. The diagnostic criteria were as follows: (1) CT shows an OP pattern; (2) microbiological tests using BAL fluid are negative; (3) increase in BAL lymphocyte count (>25%); (4) BAL eosinophil count < 25%; and (5) there is no evidence of a possible cause of secondary OP (e.g., autoimmune disease, history of drug administration, history of radiotherapy). Patients meeting all criteria were diagnosed with COP and included in the analysis. We followed up these patients for one year.
IPAF was diagnosed according to the criteria proposed by the ERS and ATS in 2015, namely at least one positive feature from at least two of the three possible domains (Clinical, Serologic, Morphologic) [5]. In Japan, all the autoantibodies listed in the Serologic domain, except the anti-polymyositis-scleroderma (PM-Scl) antibody, can be measured under the Japanese health insurance system. Therefore, autoantibodies other than the anti-PM-Scl antibody were measured at each institution. Anti-tRNA synthetase antibody levels were measured using the MESACUP™ anti-aminoacyl tRNA synthetase test (Medical & Biological Laboratories Co., Ltd., Tokyo, Japan) detecting anti-Jo-1, anti-PL-7, anti-PL-12, anti-EJ, and anti-KS antibodies [13]. On the other hand, anti-OJ, anti-Zo, and anti-Ha antibodies, which were not included in the MESACUP™ anti-aminoacyl tRNA synthetase test, were not measured. Anti-PM-Scl antibody levels were measured by immunoprecipitation at the Department of Dermatology at Kanazawa University using sera stored at −30 °C at the time of registration.
A central review of the CT images was performed by three readers: two expert radiologists (KK: 20 years of experience and SM: 15 years of experience) and one expert pulmonologist (HH: 16 years of experience). The readers independently assessed whether the images were compatible with the OP. In addition, CT images were evaluated for distribution, predominant pattern, and the presence of a reversed halo sign [14]. All disagreements were resolved by consensus.
2.2. Statistical Analysis
Statistical analyses were performed using the EZR software, version 1.36 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [15]. Binary and continuous variables were evaluated using Fisher’s exact test and the Mann–Whitney U test, respectively. Statistical significance was set at p < 0.05.
3. Results
3.1. Patient Characteristics
A total of 56 patients were enrolled in this study. Since no patients underwent surgical lung biopsy, all were screened for COP based on the five criteria described in the Methods Section. Twenty-five patients were excluded because their BAL lymphocyte count was <25% and one patient was excluded because of loss of follow-up. Notably, 30 patients diagnosed with COP were included in the analysis (Figure 1). Regarding the BAL procedure, the volume of saline instilled was 150 mL (n = 20) or 100 mL (n = 10), and the median recovery rate was 46.3% (IQR: 35.5–54.8). Of the 30 cases, 17 were BAL fluid culture-positive, but the isolated bacteria were only endemic oral bacteria such as alpha-Streptococcus and Neisseria, and no bacteria suspected of causing bacterial pneumonia were detected. All BAL fluid smears and cultures for acid-fast bacilli were negative. TBLB was performed in 15 cases; however, diagnostic specimens were insufficient in eight cases. In the remaining seven cases, pathology findings consistent with OP were observed. The results of IPAF screening are shown in Tables S1–S3 (Supplementary Materials). Two patients had one feature from the clinical domain (distal digital fissure or Raynaud’s phenomenon). Features from the serologic domain were positive in six patients (antinuclear antibody nucleolar pattern, one patient; rheumatoid factor, three patients; anti-topoisomerase, one patient; and anti-tRNA synthetase, one patient). The measurement rate for each antibody was 100% for 7 of the 12 items, and the total measurement rate was 97.5% (351/360) (Table S2). Regarding the morphologic domain, all patients were judged to be positive due to an OP pattern on CT. Four patients had unexplained pleural effusion or thickening, and one had unexplained intrinsic airway disease. Finally, eight patients (26.7%) were diagnosed with IPAF, as all patients met the morphologic domain criteria, and eight patients had one positive feature in the clinical or serologic domain (Figure 1).
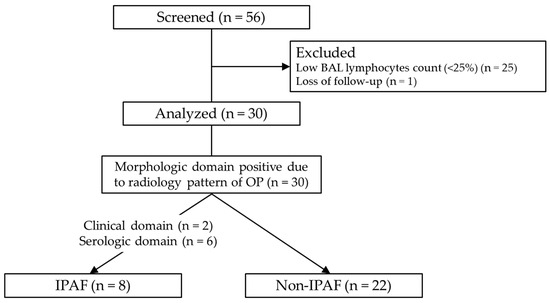
Figure 1.
Flow chart of the study. Of the 56 patients, 30 with cryptogenic organizing pneumonia were analyzed. Abbreviations: BAL, bronchoalveolar lavage; OP, organizing pneumonia; IPAF, interstitial pneumonia with autoimmune features.
3.2. Comparison of Clinical Characteristics Between IPAF and Non-IPAF Group
Table 1 shows a comparison between the characteristics of patients with and without IPAF. There were no significant differences in age, sex, body mass index, or smoking history between the two groups. The most frequent symptoms were coughing (70.0%) and dyspnea on exertion (73.3%), with similar frequencies between the two groups. The results of the laboratory examinations are shown in Table 1. The C-reactive protein was similarly elevated in both groups (5.4 mg/dL vs. 5.2 mg/dL), and the serum Krebs von den Lungen-6 was not elevated much in either group (299 U/mL vs. 422 U/mL). We evaluated all patients using BAL, and the total cell count (4.3 × 105 /mL vs. 5.0 × 105 /mL), percentage of lymphocytes (56.5% vs. 60.8%) and CD4/CD8 ratio (0.9 vs. 1.2) were comparable between each group.

Table 1.
Comparison of patient characteristics between patients with and without IPAF.
Representative CT images of the study participants are shown in Figure 2. Figure 2A–C show cases from the IPAF group and Figure 2D–F show cases from the non-IPAF group. Figure 2A,B,D,E show consolidation with a peripheral predominance. The red arrowheads in Figure 2C,F indicate the reversed halo sign. Table 2 shows the results of the central review of CT patterns for both groups. Bilateral and peripherally dominant consolidations were the major patterns observed in our study. A reversed halo sign was observed in four patients. There were no significant differences in the distribution or opacity patterns on CT between the IPAF and non-IPAF groups.
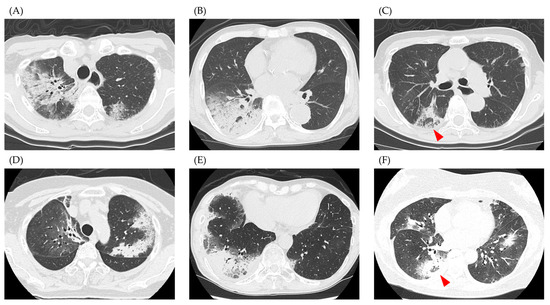
Figure 2.
Representative computed tomography images. (A–C) show cases from the IPAF group, and (D–F) show cases from the non-IPAF group. (A,B,D,E) depict cases with peripheral dominant consolidation, while images (C,F) show cases with a reversed halo sign (red arrowhead). Abbreviations: IPAF, interstitial pneumonia with autoimmune features.

Table 2.
Computed tomography pattern.
Table 3 shows the treatment of the COP. Notably, 76.7% of patients (23/30) were treated with corticosteroids, and the starting dose was similar between the two groups (25 mg/day vs. 30 mg/day). None of the patients received immunosuppressive drugs. Figure 3 shows the rate of corticosteroid use in the IPAF and non-IPAF groups during a one-year follow-up period. The rate of corticosteroid tapering was similar between the IPAF and non-IPAF groups. At 12 months, 0% and 15.8% of patients in the IPAF and non-IPAF groups were receiving corticosteroid therapy, respectively, and six patients never received corticosteroids during the observational period (IPAF group, one; non-IPAF group, five). A small number of cases in both groups developed COP exacerbation during the one-year follow-up period. The frequency of COP exacerbation did not differ between the IPAF and non-IPAF groups (Figure 4).

Table 3.
Treatment of organizing pneumonia.
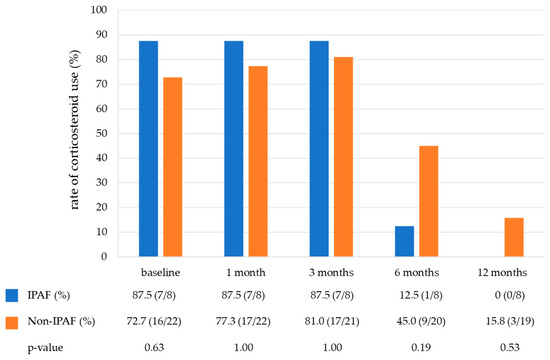
Figure 3.
The rate of corticosteroid use in the IPAF group and non-IPAF group during a one-year follow-up period. Abbreviations: IPAF, interstitial pneumonia with autoimmune features.
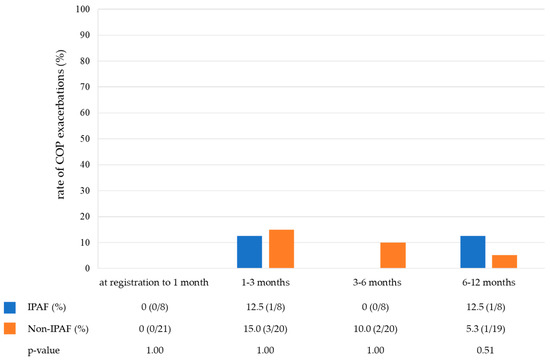
Figure 4.
The rate of COP exacerbations in the IPAF group and non-IPAF group during the one-year follow-up period. Abbreviations: COP, cryptogenic organizing pneumonia; IPAF, interstitial pneumonia with autoimmune features.
During the one-year follow-up period, no patients developed systemic autoimmune disease or further lung disease in either group. In addition, there were no cases of death in this study.
4. Discussion
In this study, we focused on COP among IIPs to determine the frequency and clinical presentation of IPAF. Several prospective observational studies on IPAF have been conducted; however, COP was not their primary population, and IPAF in COP has not been adequately studied [6,16,17,18]. The strengths of this study include a detailed review of clinical features of patients, including BAL, laboratory data, CT findings, clinical course, and high coverage of the serologic domain. In particular, the anti-PM-Scl antibody and anti-melanoma differentiation-associated protein-5 antibody, which have often not been measured in previous reports [6,17,19,20,21,22], were measured in most cases in this study, and the total autoantibody measurement rate was very high at 97.5%.
There are limited data on the frequency of IPAF in COP. A previous prospective study including 21 COP cases reported that 47.8% of the patients met the IPAF criteria [6]. Another retrospective study showed that 17 of the 142 patients with COP (12.0%) met the IPAF criteria [23]. In this study, 26.7% of the patients with COP met the IPAF criteria, suggesting a relatively high frequency of IPAF in COP.
One reason for distinguishing IPAF from IIPs is that the prognosis may differ between the two groups. Among IIPs, the IPAF group has been reported to have a significantly better prognosis than the non-IPAF group in NSIP and unclassifiable IIPs [20,21]. Regarding idiopathic pulmonary fibrosis, several studies have shown a better survival rate in the IPAF group, although the difference was not significant due to the small sample sizes [6,19,20]. Is the IPAF group also associated with a better prognosis in COP, which generally has a good prognosis? Since COP is rarely fatal, the prognosis is expected to be good in both groups. However, is there a difference in response to steroid therapy or in the relapse rate? No deaths occurred in either group, and no differences in steroid tapering or relapse were observed during the 1-year follow-up period.
Another reason for distinguishing IPAF from IIPs is the higher incidence of development of systemic autoimmune diseases [18,24]. Enomoto et al. reported a significantly higher 1-year cumulative incidence of systemic autoimmune diseases in the IPAF group (4.42% vs. 0.89%) [6]. Although it is unclear whether the IPAF group is more likely to develop systemic autoimmune disease with COP, no patients in this study developed these diseases during the 1-year follow-up period, even in the IPAF group. However, considering the reported 1-year cumulative incidence of systemic autoimmune diseases (4.42%/year) [6], the absence of systemic autoimmune diseases in this study may have been due to the insufficient number of cases. Another study reported that 4 of the 20 IPAF patients with an OP pattern on CT developed connective tissue disease [25]. In this report, two of the four patients developed connective tissue disease after more than one year. Therefore, if we had a longer follow-up period, it is possible that our patients would have developed a systemic autoimmune disease. Larger and longer follow-up studies are needed to determine the susceptibility of patients with COP and IPAF to systemic autoimmune diseases.
Our study revealed no differences between the IPAF and non-IPAF groups in terms of clinical background, symptoms, pulmonary function, laboratory examinations, BAL, and CT images as well as the prognosis and incidence of systemic autoimmune disease. Therefore, when treating patients with COP, screening for IPAF using a large number of autoantibody tests may be of limited clinical significance [5].
Our study has several limitations. First, the sample size was relatively small, especially for examining the incidence of developing systemic autoimmune diseases in patients with COP and IPAF. Therefore, larger studies are needed to confirm our results. Second, the diagnosis of COP was essentially based on BAL findings and CT images, with pathological confirmation obtained in only seven cases. Cases diagnosed using surgical lung biopsy should be studied; however, surgical lung biopsy is rarely performed in clinical practice [7,8,9,10]. Relatively high diagnostic accuracy (79%) has been reported with CT alone [26], and BAL has a high positive predictive value of 85% to 90% [11,27]. Because of the high reliability of COP diagnosis by CT and BAL, many studies include cases diagnosed without pathological confirmation [6,25,27,28]. The good treatment response to corticosteroids and favorable prognosis in our cases are consistent with COP [7]. However, the results of this study should only be interpreted as the results of clinically diagnosed COP based on CT and BAL.
5. Conclusions
In conclusion, despite having some limitations, we believe that this study is important because no previous study has examined IPAF in COP in as much detail. The characteristics of the IPAF and non-IPAF groups in COP are similar in all aspects, and distinguishing between the two groups may be of little significance. Larger studies are warranted to confirm these results.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13226870/s1, Table S1: clinical domain, Table S2: serologic domain, Table S3: morphologic domain.
Author Contributions
Conceptualization, H.H.; formal analysis, H.H., S.M. and K.K.; investigation, H.H., H.I., Y.A., Y.M. (Yoshihiro Mori), T.T., S.K., C.M., K.S., N.H., T.S., J.I., Y.T., S.S., A.T., Y.I., M.A., S.M., K.K. and T.M.; resources, H.H., H.I., Y.A., Y.M. (Yoshihiro Mori), T.T., S.K., C.M., K.S., N.H., T.S., J.I., Y.T., S.S., A.T., Y.I. and M.A.; writing—original draft preparation, H.H.; writing—review and editing, H.I., Y.A., Y.M. (Yoshihiro Mori), T.T., S.K., C.M., K.S., N.H., T.S., J.I., Y.T., S.S., A.T., Y.I., M.A., S.M., K.K., T.M., Y.M. (Yoshinobu Maeda) and N.M.; resources, H.H., H.I., Y.A., Y.M. (Yoshihiro Mori), T.T., S.K., C.M., K.S., N.H., T.S., J.I., Y.T., S.S., A.T., Y.I. and M.A.; data curation, H.H., H.I., Y.A., Y.M. (Yoshihiro Mori), T.T., S.K., C.M., K.S., N.H., T.S., J.I., Y.T., S.S., A.T., Y.I., M.A., S.M., K.K., T.M., Y.M. (Yoshinobu Maeda) and N.M.; supervision, Y.M. (Yoshinobu Maeda) and N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was carried out according to the principles of the Declaration of Helsinki for human investigations and approved by the Institutional Review Board of Okayama University Hospital (no. 1804-050, approval date: 7 May 2018) and all other participating hospitals.
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank all the members of the Okayama Respiratory Disease Study Group for their willingness to support this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Graney, B.A.; Fischer, A. Interstitial Pneumonia with Autoimmune Features. Ann. Am. Thorac. Soc. 2019, 16, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Kinder, B.W.; Collard, H.R.; Koth, L.; Daikh, D.I.; Wolters, P.J.; Elicker, B.; Jones, K.D.; King, T.E., Jr. Idiopathic nonspecific interstitial pneumonia: Lung manifestation of undifferentiated connective tissue disease? Am. J. Respir. Crit. Care Med. 2007, 176, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; West, S.G.; Swigris, J.J.; Brown, K.K.; du Bois, R.M. Connective tissue disease-associated interstitial lung disease: A call for clarification. Chest 2010, 138, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Vij, R.; Noth, I.; Strek, M.E. Autoimmune-featured interstitial lung disease: A distinct entity. Chest 2011, 140, 1292–1299. [Google Scholar] [CrossRef]
- Fischer, A.; Antoniou, K.M.; Brown, K.K.; Cadranel, J.; Corte, T.J.; du Bois, R.M.; Lee, J.S.; Leslie, K.O.; Lynch, D.A.; Matteson, E.L.; et al. An official European Respiratory Society/American Thoracic Society research statement: Interstitial pneumonia with autoimmune features. Eur. Respir. J. 2015, 46, 976–987. [Google Scholar] [CrossRef]
- Enomoto, N.; Homma, S.; Inase, N.; Kondoh, Y.; Saraya, T.; Takizawa, H.; Inoue, Y.; Ishii, H.; Taguchi, Y.; Izumi, S.; et al. Prospective nationwide multicentre cohort study of the clinical significance of autoimmune features in idiopathic interstitial pneumonias. Thorax 2022, 77, 143–153. [Google Scholar] [CrossRef]
- Cottin, V.; Cordier, J.F. Cryptogenic organizing pneumonia. Semin. Respir. Crit. Care Med. 2012, 33, 462–475. [Google Scholar]
- Taniguchi, Y.; Arai, T.; Tsuji, T.; Sugimoto, C.; Tachibana, K.; Akira, M.; Inoue, Y. Cryptogenic organizing pneumonia: Clinical outcomes of 60 consecutive cases. J. Thorac. Dis. 2024, 16, 3129–3141. [Google Scholar] [CrossRef]
- Akira, M.; Yamamoto, S.; Sakatani, M. Bronchiolitis obliterans organizing pneumonia manifesting as multiple large nodules or masses. Am. J. Roentgenol. 1998, 170, 291–295. [Google Scholar] [CrossRef]
- Cazzato, S.; Zompatori, M.; Baruzzi, G.; Schiattone, M.; Burzi, M.; Rossi, A.; Ratta, L.; Terzuolo, G.; Falcone, F.; Poletti, V. Bronchiolitis obliterans-organizing pneumonia: An Italian experience. Respir. Med. 2000, 94, 702–708. [Google Scholar] [CrossRef]
- Poletti, V.; Cazzato, S.; Minicuci, N.; Zompatori, M.; Burzi, M.; Schiattone, M. The diagnostic value of bronchoalveolar lavage and transbronchial lung biopsy in cryptogenic organizing pneumonia. Eur. Respir. J. 1996, 9, 2513–2516. [Google Scholar] [CrossRef] [PubMed]
- Dina, R.; Sheppard, M. The histological diagnosis of clinically documented cases of cryptogenic organizing pneumonia: Diagnostic features in transbronchial biopsies. Histopathology 1993, 23, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, R.; Imura, Y.; Hosono, Y.; Seto, M.; Murakami, A.; Watanabe, K.; Handa, T.; Mishima, M.; Hirakata, M.; Takeuchi, T.; et al. The multicenter study of a new assay for simultaneous detection of multiple anti-aminoacyl-tRNA synthetases in myositis and interstitial pneumonia. PLoS ONE 2014, 9, e85062. [Google Scholar] [CrossRef]
- King, T.E., Jr.; Lee, J.S. Cryptogenic Organizing Pneumonia. N. Engl. J. Med. 2022, 386, 1058–1069. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, M.; Cassone, G.; De Pasquale, L.; Cerri, S.; Della Casa, G.; Vacchi, C.; Luppi, F.; Salvarani, C.; Manfredi, A. Interstitial pneumonia with autoimmune features: A single center prospective follow-up study. Autoimmun. Rev. 2020, 19, 102451. [Google Scholar] [CrossRef]
- Sambataro, G.; Sambataro, D.; Torrisi, S.E.; Vancheri, A.; Colaci, M.; Pavone, M.; Pignataro, F.; Del Papa, N.; Palmucci, S.; Vancheri, C. Clinical, serological and radiological features of a prospective cohort of Interstitial Pneumonia with Autoimmune Features (IPAF) patients. Respir. Med. 2019, 150, 154–160. [Google Scholar] [CrossRef]
- Sambataro, G.; Sambataro, D.; Spicuzza, L.; Meloni, F.; Lorini, G.; Malatino, L.; Colaci, M.; Sebastiani, G.; Iuliano, A.; Canofari, C.; et al. Progression and prognosis of interstitial pneumonia with autoimmune features: A longitudinal, prospective, multi-centre study. Clin. Exp. Rheumatol. 2023, 41, 1140–1148. [Google Scholar] [CrossRef]
- Kelly, B.T.; Moua, T. Overlap of interstitial pneumonia with autoimmune features with undifferentiated connective tissue disease and contribution of UIP to mortality. Respirology 2018, 23, 600–605. [Google Scholar] [CrossRef]
- Yoshimura, K.; Kono, M.; Enomoto, Y.; Nishimoto, K.; Oyama, Y.; Yasui, H.; Hozumi, H.; Karayama, M.; Suzuki, Y.; Furuhashi, K.; et al. Distinctive characteristics and prognostic significance of interstitial pneumonia with autoimmune features in patients with chronic fibrosing interstitial pneumonia. Respir. Med. 2018, 137, 167–175. [Google Scholar] [CrossRef]
- Enomoto, N.; Yazawa, S.; Mochizuka, Y.; Fukada, A.; Tanaka, Y.; Naoi, H.; Aono, Y.; Inoue, Y.; Yasui, H.; Karayama, M.; et al. Radiological and histopathological features and treatment response by subtypes of interstitial pneumonia with autoimmune features: A prospective, multicentre cohort study. Respir. Med. 2024, 224, 107577. [Google Scholar] [CrossRef]
- Oldham, J.M.; Adegunsoye, A.; Valenzi, E.; Lee, C.; Witt, L.; Chen, L.; Husain, A.N.; Montner, S.; Chung, J.H.; Cottin, V.; et al. Characterisation of patients with interstitial pneumonia with autoimmune features. Eur. Respir. J. 2016, 47, 1767–1775. [Google Scholar] [CrossRef]
- Dai, J.; Wang, L.; Yan, X.; Li, H.; Zhou, K.; He, J.; Meng, F.; Xu, S.; Liang, G.; Cai, H. Clinical features, risk factors, and outcomes of patients with interstitial pneumonia with autoimmune features: A population-based study. Clin. Rheumatol. 2018, 37, 2125–2132. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Lee, J.H.; Chae, E.J.; Song, J.S.; Song, J.W. Long-term clinical course and outcome of interstitial pneumonia with autoimmune features. Respirology 2020, 25, 636–643. [Google Scholar] [CrossRef]
- Ito, Y.; Arita, M.; Kumagai, S.; Takei, R.; Noyama, M.; Tokioka, F.; Nishimura, K.; Koyama, T.; Notohara, K.; Ishida, T. Serological and morphological prognostic factors in patients with interstitial pneumonia with autoimmune features. BMC Pulm. Med. 2017, 17, 111. [Google Scholar] [CrossRef] [PubMed]
- Johkoh, T.; Müller, N.L.; Cartier, Y.; Kavanagh, P.V.; Hartman, T.E.; Akira, M.; Ichikado, K.; Ando, M.; Nakamura, H. Idiopathic interstitial pneumonias: Diagnostic accuracy of thin-section CT in 129 patients. Radiology 1999, 211, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Jara-Palomares, L.; Gomez-Izquierdo, L.; Gonzalez-Vergara, D.; Rodriguez-Becerra, E.; Marquez-Martin, E.; Barrot-Cortés, E.; Martin-Juan, J. Utility of high-resolution computed tomography and BAL in cryptogenic organizing pneumonia. Respir. Med. 2010, 104, 1706–1711. [Google Scholar] [CrossRef]
- Papakosta, D.; Manika, K.; Gounari, E.; Kyriazis, G.; Kontakiotis, T.; Spyropoulos, G.; Kontakioti, E.; Zarogoulidis, K. Bronchoalveolar lavage fluid and blood natural killer and natural killer T-like cells in cryptogenic organizing pneumonia. Respirology 2014, 19, 748–754. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).