Impaired Kidney Function and 10-Year Outcome After Percutaneous Coronary Intervention—Interaction with Age, Sex, Diabetic Status and Clinical Presentation
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Definitions and Measurements
2.3. Outcomes and Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Baseline Data
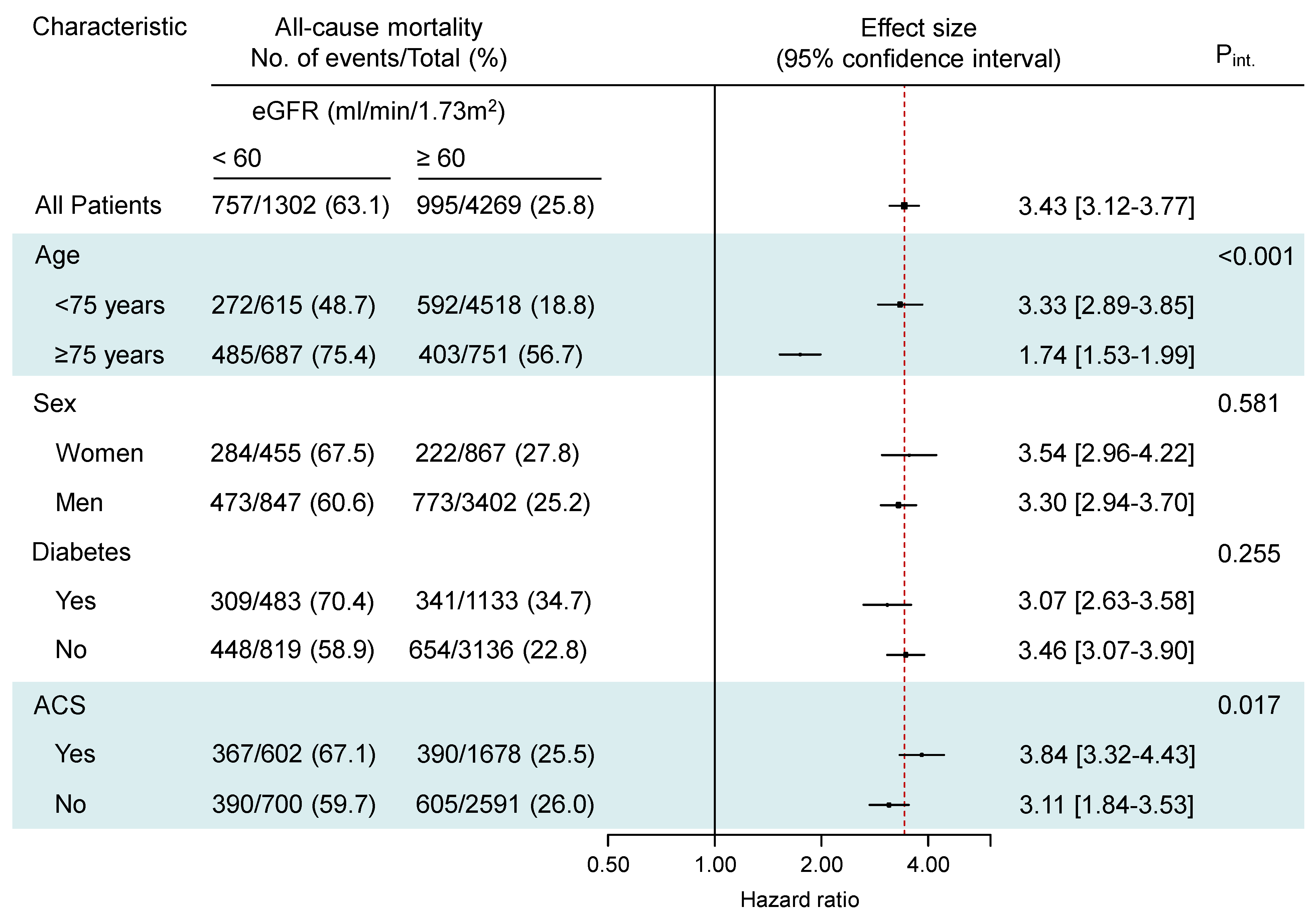
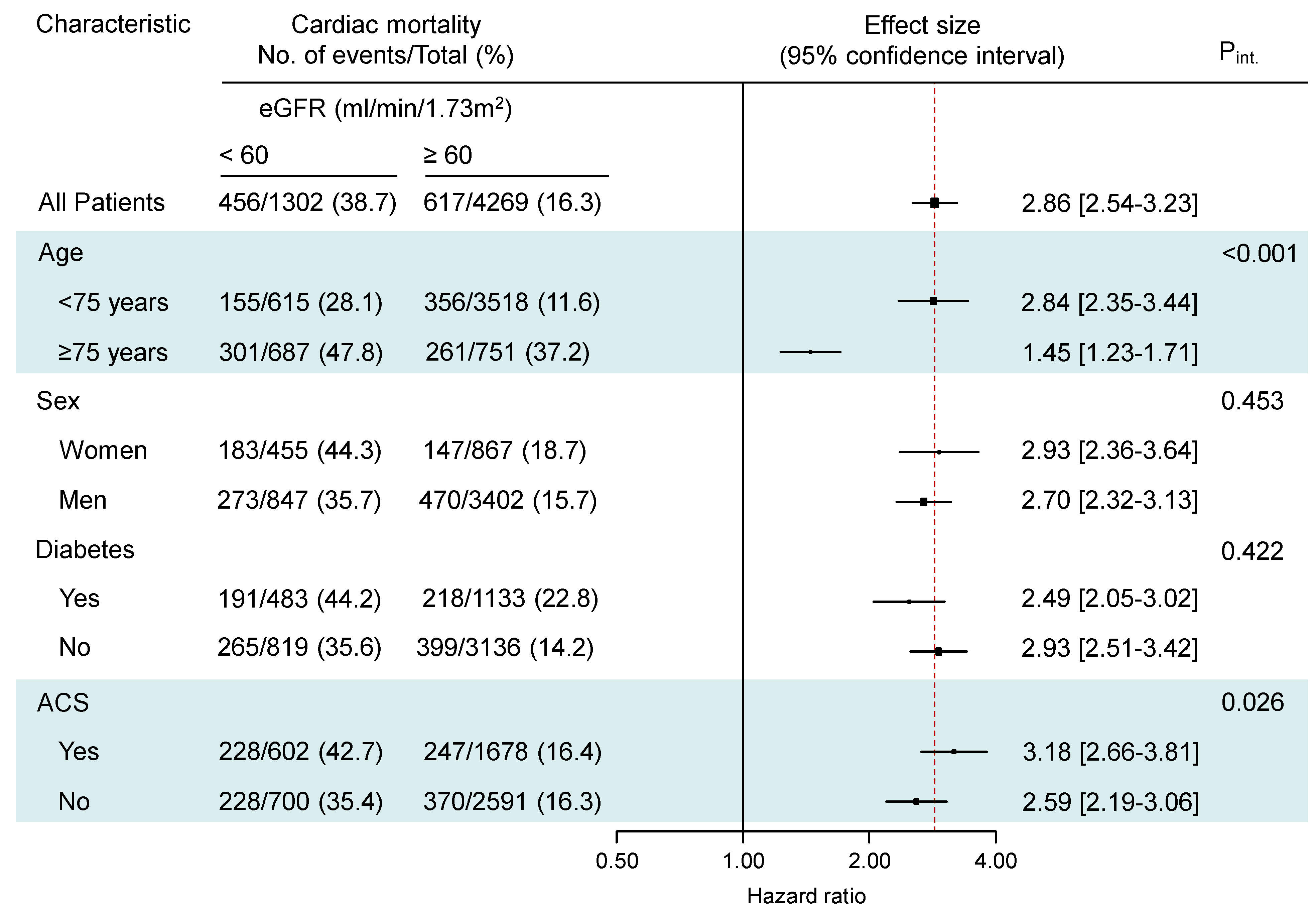
3.2. Clinical Outcome
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Francis, A.; Harhay, M.N.; Ong, A.C.M.; Tummalapalli, S.L.; Ortiz, A.; Fogo, A.B.; Fliser, D.; Roy-Chaudhury, P.; Fontana, M.; Nangaku, M.; et al. Chronic kidney disease and the global public health agenda: An international consensus. Nat. Rev. Nephrol. 2024, 20, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Anavekar, N.S.; McMurray, J.J.V.; Velazquez, E.J.; Solomon, S.D.; Kober, L.; Rouleau, J.; White, H.D.; Nordlander, R.; Maggioni, A.; Dickstein, K.; et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N. Engl. J. Med. 2004, 351, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Rai, N.K.; Wang, Z.; Drawz, P.E.; Connett, J.; Murphy, D.P. CKD Progression Risk and Subsequent Cause of Death: A Population-Based Cohort Study. Kidney Med. 2023, 5, 100604. [Google Scholar] [CrossRef]
- Shroff, G.R.; Chang, T.I. Risk Stratification and Treatment of Coronary Disease in Chronic Kidney Disease and End-Stage Kidney Disease. Semin. Nephrol. 2018, 38, 582–599. [Google Scholar] [CrossRef]
- Colombijn, J.M.T.; Idema, D.L.; van Beem, S.; Blokland, A.M.; van der Braak, K.; Handoko, M.L.; In ‘t Veld, L.F.H.; Kaul, T.; Kolagasigil-Akdemir, N.; Kusters, M.P.T.; et al. Representation of Patients With Chronic Kidney Disease in Clinical Trials of Cardiovascular Disease Medications: A Systematic Review. JAMA Netw. Open 2024, 7, e240427. [Google Scholar] [CrossRef]
- Bhatia, S.; Arora, S.; Bhatia, S.M.; Al-Hijji, M.; Reddy, Y.N.V.; Patel, P.; Rihal, C.S.; Gersh, B.J.; Deshmukh, A. Non-ST-Segment-Elevation Myocardial Infarction Among Patients With Chronic Kidney Disease: A Propensity Score-Matched Comparison of Percutaneous Coronary Intervention Versus Conservative Management. J. Am. Heart Assoc. 2018, 7, e007920. [Google Scholar] [CrossRef]
- Scott, J.; Bidulka, P.; Taylor, D.M.; Udayaraj, U.; Caskey, F.J.; Birnie, K.; Deanfield, J.; de Belder, M.; Denaxas, S.; Weston, C.; et al. Management and outcomes of myocardial infarction in people with impaired kidney function in England. BMC Nephrol. 2023, 24, 325. [Google Scholar] [CrossRef]
- Leszek, A.; Poli, L.; Zbinden, S.; Godoy, L.C.; Reny, J.L.; Farkouh, M.E.; Charytan, D.M.; Mavrakanas, T.A. Outcomes with revascularization and medical therapy in patients with coronary disease and chronic kidney disease: A meta-analysis. Atherosclerosis 2022, 351, 41–48. [Google Scholar] [CrossRef]
- Hemmelgarn, B.R.; Southern, D.; Culleton, B.F.; Mitchell, L.B.; Knudtson, M.L.; Ghali, W.A.; Investigators, A. Survival after coronary revascularization among patients with kidney disease. Circulation 2004, 110, 1890–1895. [Google Scholar] [CrossRef]
- Baber, U.; Li, S.X.; Pinnelas, R.; Pocock, S.J.; Krucoff, M.W.; Ariti, C.; Gibson, C.M.; Steg, P.G.; Weisz, G.; Witzenbichler, B.; et al. Incidence, Patterns, and Impact of Dual Antiplatelet Therapy Cessation Among Patients With and Without Chronic Kidney Disease Undergoing Percutaneous Coronary Intervention: Results From the PARIS Registry (Patterns of Non-Adherence to Anti-Platelet Regimens in Stented Patients). Circ. Cardiovasc. Interv. 2018, 11, e006144. [Google Scholar] [PubMed]
- Bangalore, S.; Guo, Y.; Samadashvili, Z.; Blecker, S.; Xu, J.; Hannan, E.L. Revascularization in Patients With Multivessel Coronary Artery Disease and Chronic Kidney Disease: Everolimus-Eluting Stents Versus Coronary Artery Bypass Graft Surgery. J. Am. Coll. Cardiol. 2015, 66, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Kang, J.; Yang, H.M.; Yang, S.; Park, J.; Han, J.K.; Kang, H.J.; Koo, B.K.; Kim, H.S. Better Prognosis After Complete Revascularization Using Contemporary Coronary Stents in Patients With Chronic Kidney Disease. Circ. Cardiovasc. Interv. 2019, 12, e007907. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.O.; Kang, D.Y.; Ahn, J.M.; Kim, S.O.; Lee, P.H.; Lee, J.; Kim, J.H.; Kim, H.J.; Kim, J.B.; Choo, S.J.; et al. Prognostic Impact of Mildly Impaired Renal Function in Patients Undergoing Multivessel Coronary Revascularization. J. Am. Coll. Cardiol. 2022, 79, 1270–1284. [Google Scholar] [CrossRef]
- Best, P.J.M.; Berger, P.B.; Davis, B.R.; Grines, C.L.; Sadeghi, H.M.; Williams, B.A.; Willerson, J.T.; Granett, J.R.; Holmes, D.R.; Investigators, P. Impact of mild or moderate chronic kidney disease on the frequency of restenosis—Results from the PRESTO trial. J. Am. Coll. Cardiol. 2004, 44, 1786–1791. [Google Scholar]
- Papafaklis, M.I.; Naka, K.K.; Papamichael, N.D.; Kolios, G.; Sioros, L.; Sclerou, V.; Katsouras, C.S.; Michalis, L.K. The impact of renal function on the long-term clinical course of patients who underwent percutaneous coronary intervention. Catheter. Cardiovasc. Interv. 2007, 69, 189–197. [Google Scholar] [CrossRef]
- Appleby, C.E.; Ivanov, J.; Lavi, S.; Mackie, K.; Horlick, E.M.; Ing, D.; Overgaard, C.B.; Seidelin, P.H.; von Harsdorf, R.; Dzavík, V. The Adverse Long-Term Impact of Renal Impairment in Patients Undergoing Percutaneous Coronary Intervention in the Drug-Eluting Stent Era. Circ.-Cardiovasc. Interv. 2009, 2, U309–U363. [Google Scholar] [CrossRef]
- Dohi, T.; Kasai, T.; Miyauchi, K.; Takasu, K.; Kajimoto, K.; Kubota, N.; Amano, A.; Daida, H. Prognostic impact of chronic kidney disease on 10-year clinical outcomes among patients with acute coronary syndrome. J. Cardiol. 2012, 60, 438–442. [Google Scholar] [CrossRef][Green Version]
- Kufner, S.; Joner, M.; Thannheimer, A.; Hoppmann, P.; Ibrahim, T.; Mayer, K.; Cassese, S.; Laugwitz, K.L.; Schunkert, H.; Kastrati, A.; et al. Ten-Year Clinical Outcomes From a Trial of Three Limus-Eluting Stents With Different Polymer Coatings in Patients With Coronary Artery Disease. Circulation 2019, 139, 325–333. [Google Scholar] [CrossRef]
- Kufner, S.; Ernst, M.; Cassese, S.; Joner, M.; Mayer, K.; Colleran, R.; Koppara, T.; Xhepa, E.; Koch, T.; Wiebe, J.; et al. 10-Year Outcomes From a Randomized Trial of Polymer-Free Versus Durable Polymer Drug-Eluting Coronary Stents. J. Am. Coll. Cardiol. 2020, 76, 146–158. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Cutlip, D.E.; Windecker, S.; Mehran, R.; Boam, A.; Cohen, D.J.; van Es, G.A.; Steg, P.G.; Morel, M.A.; Mauri, L.; Vranckx, P.; et al. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation 2007, 115, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; White, H.D.; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation 2007, 116, 2634–2653. [Google Scholar] [CrossRef]
- Best, P.J.M.; Lennon, R.; Ting, H.H.; Bell, M.R.; Rihal, C.S.; Holmes, D.R.; Berger, P.B. The impact of renal insufficiency on clinical outcomes in patients undergoing percutaneous coronary interventions. J. Am. Coll. Cardiol. 2002, 39, 1113–1119. [Google Scholar] [CrossRef]
- De Rosa, R.; Morici, N.; De Servi, S.; De Luca, G.; Galasso, G.; Piscione, F.; Ferri, L.A.; Piatti, L.; Grosseto, D.; Tortorella, G.; et al. Impact of renal dysfunction and acute kidney injury on outcome in elderly patients with acute coronary syndrome undergoing percutaneous coronary intervention. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 1160–1169. [Google Scholar] [CrossRef]
- Nikolsky, E.; Mehran, R.; Turcot, D.; Aymong, E.D.; Mintz, G.S.; Lasic, Z.; Lansky, A.J.; Tsounias, E.; Moses, J.W.; Stone, G.W.; et al. Impact of chronic kidney disease on prognosis of patients with diabetes mellitus treated with percutaneous coronary intervention. Am. J. Cardiol. 2004, 94, 300–305. [Google Scholar] [CrossRef]
- Panchal, H.B.; Zheng, S.M.; Devani, K.; White, C.J.; Leinaar, E.F.; Mukherjee, D.; Mamas, M.; Banerjee, S.; Bhatt, D.L.; Jneid, H.; et al. Impact of Chronic Kidney Disease on Revascularization and Outcomes in Patients with ST-Elevation Myocardial Infarction. Am. J. Cardiol. 2021, 150, 15–23. [Google Scholar] [CrossRef]
- Qi, Y.H.; He, J.Q.; Pan, M.J.; Yan, J. Impact of impaired renal function on outcomes of chronic total occlusion undergoing revascularization: A systemic review and meta-analysis. Int. Urol. Nephrol. 2022, 54, 3179–3191. [Google Scholar] [CrossRef]
- Saltzman, A.J.; Stone, G.W.; Claessen, B.E.; Narula, A.; Leon-Reyes, S.; Weisz, G.; Brodie, B.; Witzenbichler, B.; Guagliumi, G.; Kornowski, R.; et al. Long-Term Impact of Chronic Kidney Disease in Patients With ST-Segment Elevation Myocardial Infarction Treated With Primary Percutaneous Coronary Intervention The HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) Trial. JACC-Cardiovasc. Interv. 2011, 4, 1011–1019. [Google Scholar]
- Tomaniak, M.; Chichareon, P.; Klimczak-Tomaniak, D.; Takahashi, K.; Kogame, N.; Modolo, R.; Wang, R.T.; Ono, M.; Hara, H.; Gao, C.; et al. Impact of renal function on clinical outcomes after PCI in ACS and stable CAD patients treated with ticagrelor: A prespecified analysis of the GLOBAL LEADERS randomized clinical trial. Clin. Res. Cardiol. 2020, 109, 930–943. [Google Scholar] [CrossRef]
- Sarnak, M.J.; Amann, K.; Bangalore, S.; Cavalcante, J.L.; Charytan, D.M.; Craig, J.C.; Gill, J.S.; Hlatky, M.A.; Jardine, A.G.; Landmesser, U.; et al. Chronic Kidney Disease and Coronary Artery Disease. J. Am. Coll. Cardiol. 2019, 74, 1823–1838. [Google Scholar] [CrossRef] [PubMed]
- Lo Grandjean-Thomsen, N.; Marley, P.; Shadbolt, B.; Farshid, A. Impact of Mild-to-Moderate Chronic Kidney Disease on One Year Outcomes after Percutaneous Coronary Intervention. Nephron 2017, 137, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T.; Cons, C.K.D.P. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [PubMed]
- Raymond, N.T.; Zehnder, D.; Smith, S.C.; Stinson, J.A.; Lehnert, H.; Higgins, R.M. Elevated relative mortality risk with mild-to-moderate chronic kidney disease decreases with age. Nephrol. Dial. Transplant. 2007, 22, 3214–3220. [Google Scholar] [CrossRef]
- O’Hare, A.M.; Bertenthal, D.; Covinsky, K.E.; Landefeld, C.S.; Sen, S.; Mehta, K.; Steinman, M.A.; Borzecki, A.; Walter, L.C. Mortality risk stratification in chronic kidney disease: One size for all ages? J. Am. Soc. Nephrol. 2006, 17, 846–853. [Google Scholar] [CrossRef]
- Prakash, S.; O’Hare, A.M. Interaction of aging and chronic kidney disease. Semin. Nephrol. 2009, 29, 497–503. [Google Scholar] [CrossRef]
- Cardarelli, F.; Bellasi, A.; Ou, F.S.; Shaw, L.J.; Veledar, E.; Roe, M.T.; Morris, D.C.; Peterson, E.D.; Klein, L.W.; Raggi, P. Combined impact of age and estimated glomerular filtration rate on in-hospital mortality after percutaneous coronary intervention for acute myocardial infarction (from the American College of Cardiology National Cardiovascular Data Registry). Am. J. Cardiol. 2009, 103, 766–771. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, M.J.; Kang, Y.U.; Kim, C.S.; Bae, E.H.; Ma, S.K.; Ahn, Y.K.; Jeong, M.H.; Kim, Y.J.; Cho, M.C.; et al. Association of age and CKD with prognosis of myocardial infarction. Clin. J. Am. Soc. Nephrol. 2013, 8, 939–944. [Google Scholar] [CrossRef]
- Kotwal, S.; Ranasinghe, I.; Brieger, D.; Clayton, P.A.; Cass, A.; Gallagher, M. The influence of chronic kidney disease and age on revascularization rates and outcomes in acute myocardial infarction—A cohort study. Eur. Heart J. Acute Cardiovasc. Care 2017, 6, 291–298. [Google Scholar] [CrossRef]
- Go, A.S.; Bansal, N.; Chandra, M.; Lathon, P.V.; Fortmann, S.P.; Iribarren, C.; Hsu, C.Y.; Hlatky, M.A.; Investigators, A.S. Chronic kidney disease and risk for presenting with acute myocardial infarction versus stable exertional angina in adults with coronary heart disease. J. Am. Coll. Cardiol. 2011, 58, 1600–1607. [Google Scholar] [CrossRef]
- Antman, E.M.; Cohen, M.; Bernink, P.J.; McCabe, C.H.; Horacek, T.; Papuchis, G.; Mautner, B.; Corbalan, R.; Radley, D.; Braunwald, E. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA 2000, 284, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Davlouros, P.; Xanthopoulou, I.; Goudevenos, J.; Hamilos, M.; Vavuranakis, E.; Sitafidis, G.; Kanakakis, I.; Deftereos, S.; Alexopoulos, D. Contemporary Antiplatelet Treatment in Acute Coronary Syndrome Patients with Impaired Renal Function Undergoing Percutaneous Coronary Intervention. Cardiology 2017, 138, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Valdivielso, J.M.; Rodriguez-Puyol, D.; Pascual, J.; Barrios, C.; Bermudez-Lopez, M.; Sanchez-Nino, M.D.; Perez-Fernandez, M.; Ortiz, A. Atherosclerosis in Chronic Kidney Disease: More, Less, or Just Different? Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1938–1966. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, M.; Ishii, H.; Murakami, R.; Isobe, S.; Hayashi, M.; Amano, T.; Arai, K.; Yoshikawa, D.; Ohashi, T.; Uetani, T.; et al. Impact of renal function on coronary plaque composition. Nephrol. Dial. Transplant. 2010, 25, 175–181. [Google Scholar] [CrossRef]
- Baber, U.; Stone, G.W.; Weisz, G.; Moreno, P.; Dangas, G.; Maehara, A.; Mintz, G.S.; Cristea, E.; Fahy, M.; Xu, K.; et al. Coronary plaque composition, morphology, and outcomes in patients with and without chronic kidney disease presenting with acute coronary syndromes. JACC Cardiovasc. Imaging 2012, 5, S53–S61. [Google Scholar] [CrossRef]
- Kastrati, A.; Neumann, F.J.; Schomig, A. Operator volume and outcome of patients undergoing coronary stent placement. J. Am. Coll. Cardiol. 1998, 32, 970–976. [Google Scholar] [CrossRef]
- Karakasis, P.; Fragakis, N.; Kouskouras, K.; Karamitsos, T.; Patoulias, D.; Rizzo, M. Sodium-Glucose Cotransporter-2 Inhibitors in Patients With Acute Coronary Syndrome: A Modern Cinderella? Clin. Ther. 2024, in press. [Google Scholar] [CrossRef]
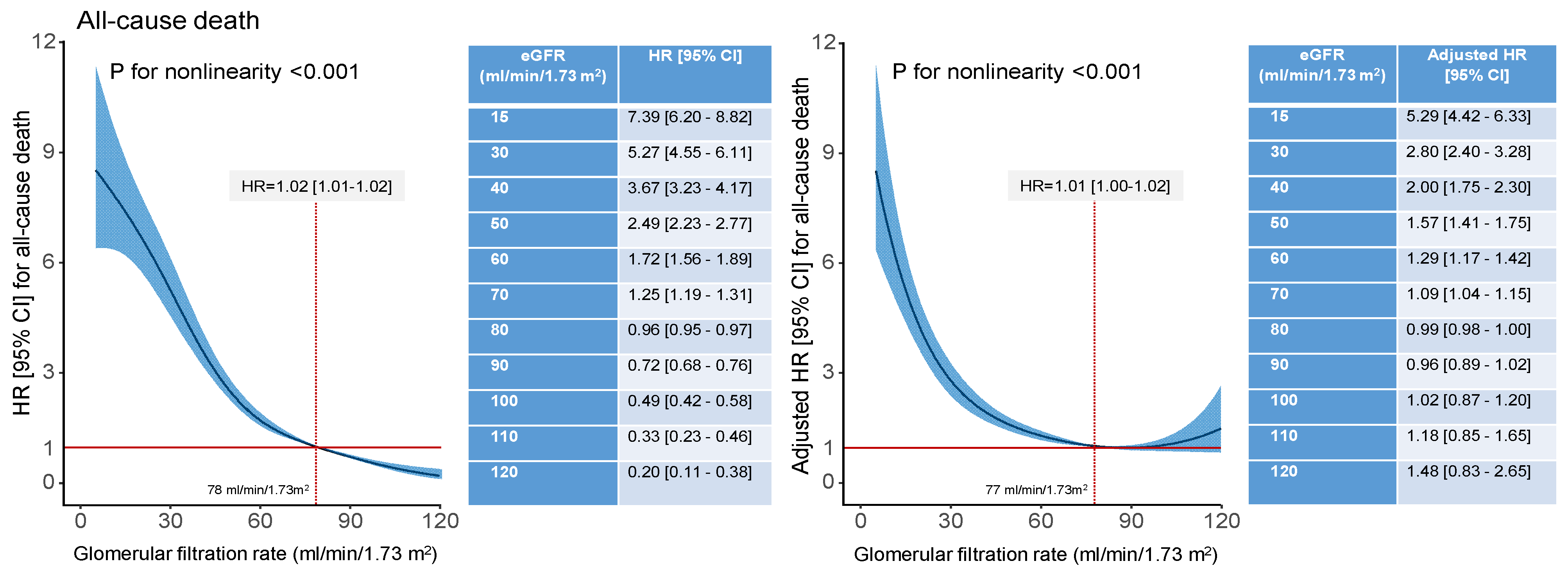
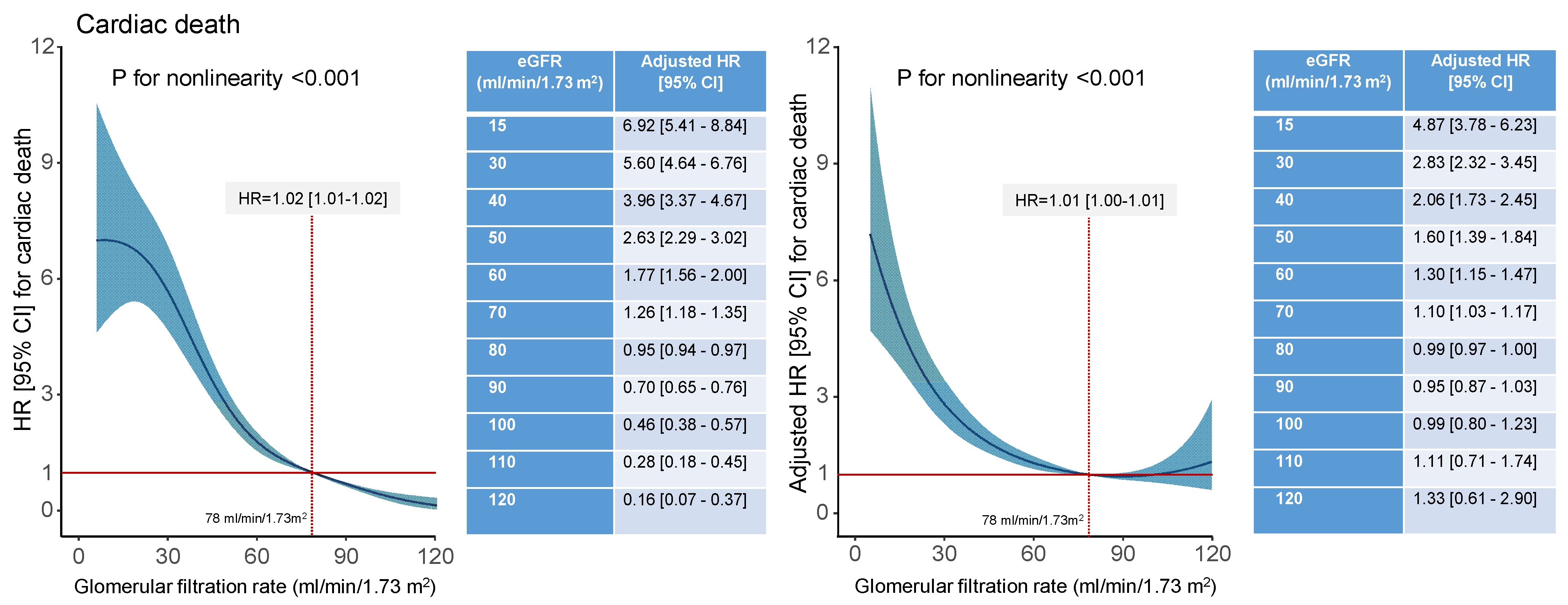
| Characteristic | Estimated Glomerular Filtration Rate (mL/min/1.73 m2) | p Value | |||
|---|---|---|---|---|---|
| <30 (n = 198) | 30 to <60 (n = 1104) | 60 to <90 (n = 2684) | ≥90 (n = 1585) | ||
| Estimated GFR (mL/min/1.73 m2) | 21.0 [10.1–26.7] | 50.1 [42.8–55.2] | 76.9 [68.8–84.5] | 96.9 [93.4–102] | <0.001 |
| Age (years) | 75.3 [68.1–81.0] | 75.5 [69.3–81.4] | 69.8 [64.3–75.5] | 59.2 [52.5–64.6] | <0.001 |
| Women | 68 (34.3%) | 387 (35.1%) | 630 (23.5%) | 237 (15.0%) | <0.001 |
| History of arterial hypertension | 127 (64.1%) | 792 (71.7%) | 1877 (69.9%) | 965 (60.9%) | <0.001 |
| History of hypercholesterolemia | 118 (59.6%) | 713 (64.6%) | 1735 (64.6%) | 1037 (65.4%) | 0.452 |
| Diabetes mellitus | 102 (51.5%) | 381 (34.5%) | 692 (25.8%) | 441 (27.8%) | <0.001 |
| On insulin therapy | 70 (35.4%) | 165 (14.9%) | 193 (7.2%) | 106 (6.7%) | <0.001 |
| On oral antidiabetic drugs | 22 (11.1%) | 165 (14.9%) | 377 (14.0%) | 256 (16.2%) | 0.130 |
| On diet alone | 10 (5.0) | 51 (4.6) | 122 (4.6) | 79 (5.0) | 0.919 |
| Body mass index (kg/m2) | 26.2 [23.8–29.7] | 26.9 [24.5–29.9] | 26.9 [24.5–29.8] | 27.4 [24.8–30.5] | <0.001 |
| Current smoker | 15 (7.6%) | 95 (8.6%) | 362 (13.5%) | 459 (29.0%) | <0.001 |
| Prior myocardial infarction | 70 (35.4%) | 355 (32.2%) | 768 (28.6%) | 430 (27.1%) | 0.007 |
| Prior coronary artery bypass surgery | 16 (8.1%) | 172 (15.6%) | 244 (9.1%) | 109 (6.9%) | <0.001 |
| Diagnosis at presentation | <0.001 | ||||
| Chronic coronary disease | 99 (50.0%) | 601 (54.4%) | 1655 (61.7%) | 936 (59.1%) | |
| Acute coronary syndrome | 99 (50.0%) | 503 (45.6%) | 1029 (38.3%) | 649 (40.9%) | |
| Serum creatinine (mg/dl) | 2.63 [2.12–4.81] | 1.30 [1.20–1.50] | 1.00 [0.85–1.07] | 0.80 [0.70–0.89] | <0.001 |
| Number of coronary arteries narrowed 1 2 3 | 21 (10.6%) 36 (18.2%) 141 (71.2%) | 121 (11.0%) 252 (22.8%) 731 (66.2%) | 418 (15.6%) 719 (26.8%) 1547 (57.6%) | 276 (17.4%) 480 (30.3%) 829 (52.3%) | <0.001 |
| Left ventricular ejection fraction (%) | 50.0 [36.8–59.2] | 52.0 [41.0–60.0] | 57.0 [48.0–62.0] | 58.0 [49.0–62.0] | <0.001 |
| Events | Estimated Glomerular Filtration Rate (mL/min/1.73 m2) | Hazard Ratio [95% Confidence Interval] | |||||
|---|---|---|---|---|---|---|---|
| <30 (n = 198) | 30 to <60 (n = 1104) | 60 to <90 (n = 2684) | ≥90 (n = 1585) | <30 vs. ≥90 mL/min/1.73 m2 | 30 to <60 vs. ≥90 mL/min/1.73 m2 | 60 to <90 vs. ≥90 mL/min/1.73 m2 | |
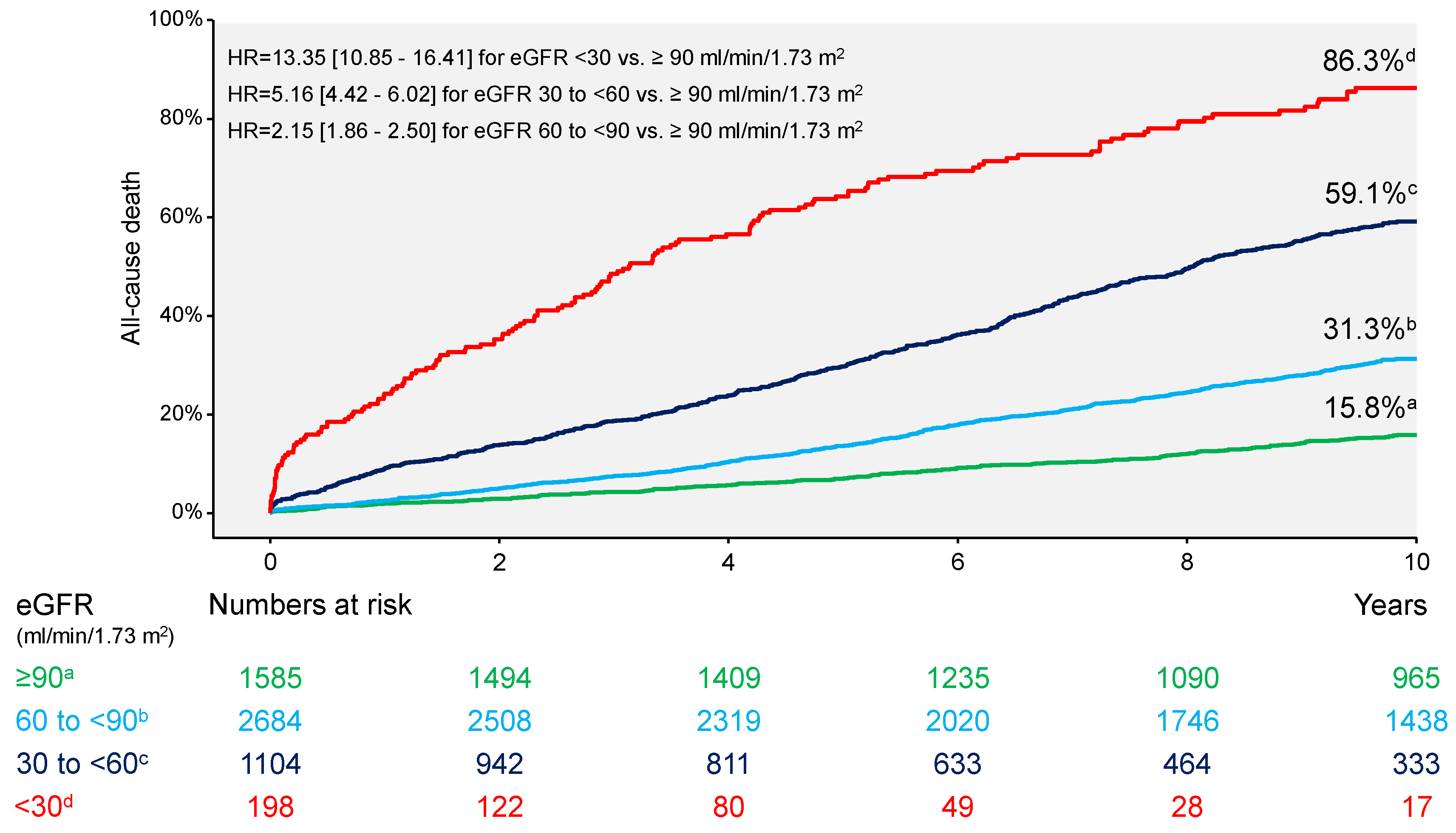
| All-cause death | 155 (86.3) | 602 (59.1) | 775 (31.3) | 220 (15.8) | 13.35 [10.85–16.41] | 5.16 [4.42–6.02] | 2.15 [1.86–2.50] |
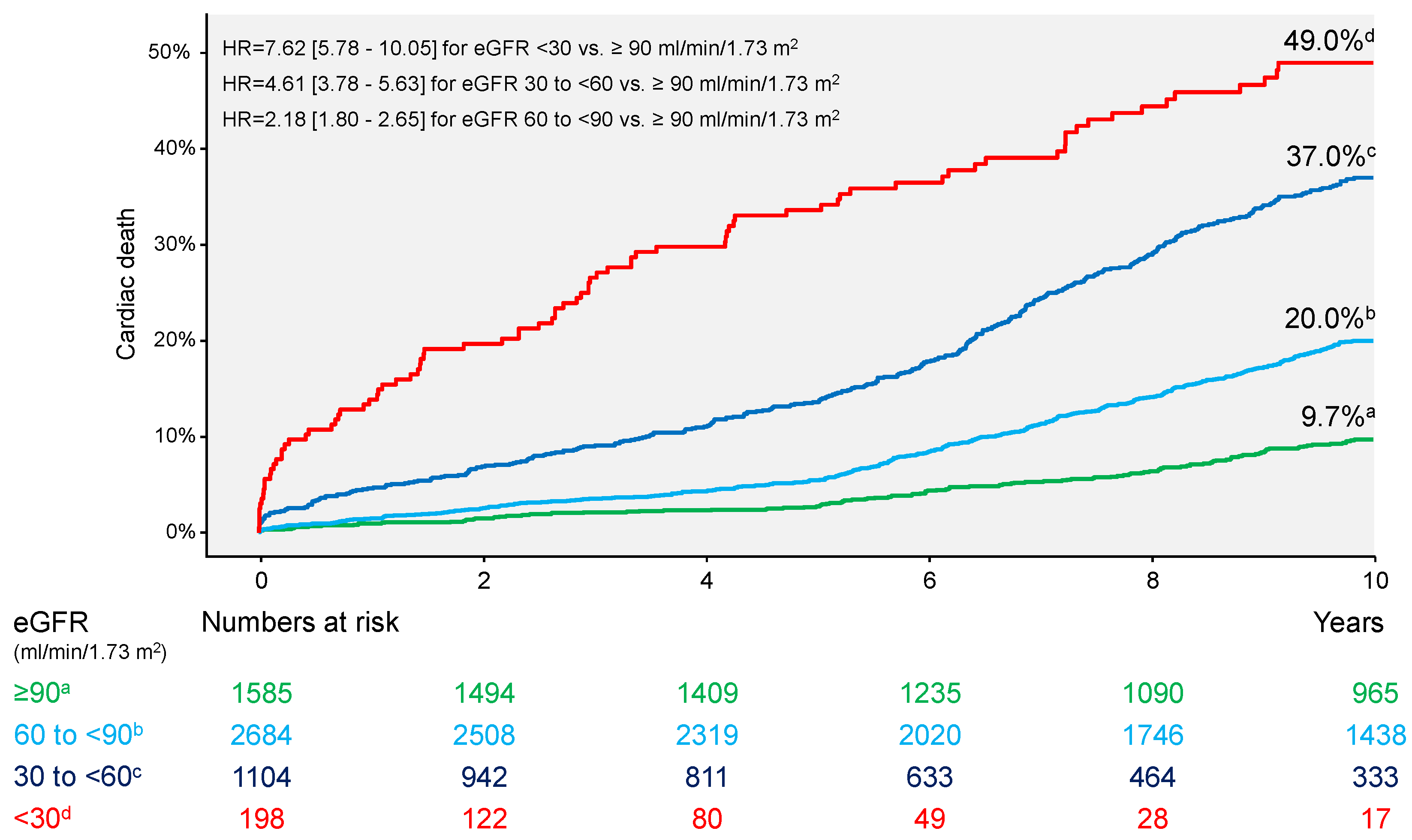
| Cardiac death | 87 (49.0) | 369 (37.0) | 486 (20.0) | 131 (9.7) | 7.62 [5.78–10.05] | 4.61 [3.78–5.63] | 2.18 [1.80–2.65] |
| Noncardiac death | 68 (37.3) | 233 (22.1) | 289 (11.3) | 89 (6.1) | 7.70 [5.61–10.57] | 4.00 [3.13–5.10] | 1.90 [1.50–2.42] |
| Myocardial infarction | 17 (8.7) | 84 (7.7) | 155 (6.0) | 78 (5.3) | 1.81 [1.07–3.05] | 1.55 [1.14–2.10] | 1.16 [0.88–1.52] |
| Definite stent thrombosis | 4 (2.1) | 8 (0.7) | 27 (1.0) | 14 (0.9) | 2.32 [0.76–7.04] | 0.81 [0.34–1.93] | 1.12 [0.59–2.13] |
| Target lesion revascularization | 32 (16.9) | 169 (15.7) | 493 (19.0) | 303 (20.7) | 0.87 [0.60–1.27] | 0.77 [0.64–0.93] | 0.94 [0.82–1.09] |
| Target vessel revascularization | 42 (21.8) | 232 (21.4) | 604 (23.1) | 379 (25.1) | 0.92 [0.67–1.28] | 0.85 [0.72–1.00] | 0.93 [0.82–1.06] |
| Nontarget vessel revascularization | 43 (22.9) | 287 (26.7) | 767 (29.4) | 462 (31.0) | 0.76 [0.55–1.04] | 0.87 [0.75–1.00] | 0.96 [0.86–1.08] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndrepepa, G.; Kufner, S.; Cassese, S.; Joner, M.; Sager, H.B.; Xhepa, E.; Laugwitz, K.-L.; Schunkert, H.; Kastrati, A. Impaired Kidney Function and 10-Year Outcome After Percutaneous Coronary Intervention—Interaction with Age, Sex, Diabetic Status and Clinical Presentation. J. Clin. Med. 2024, 13, 6833. https://doi.org/10.3390/jcm13226833
Ndrepepa G, Kufner S, Cassese S, Joner M, Sager HB, Xhepa E, Laugwitz K-L, Schunkert H, Kastrati A. Impaired Kidney Function and 10-Year Outcome After Percutaneous Coronary Intervention—Interaction with Age, Sex, Diabetic Status and Clinical Presentation. Journal of Clinical Medicine. 2024; 13(22):6833. https://doi.org/10.3390/jcm13226833
Chicago/Turabian StyleNdrepepa, Gjin, Sebastian Kufner, Salvatore Cassese, Michael Joner, Hendrik B. Sager, Erion Xhepa, Karl-Ludwig Laugwitz, Heribert Schunkert, and Adnan Kastrati. 2024. "Impaired Kidney Function and 10-Year Outcome After Percutaneous Coronary Intervention—Interaction with Age, Sex, Diabetic Status and Clinical Presentation" Journal of Clinical Medicine 13, no. 22: 6833. https://doi.org/10.3390/jcm13226833
APA StyleNdrepepa, G., Kufner, S., Cassese, S., Joner, M., Sager, H. B., Xhepa, E., Laugwitz, K.-L., Schunkert, H., & Kastrati, A. (2024). Impaired Kidney Function and 10-Year Outcome After Percutaneous Coronary Intervention—Interaction with Age, Sex, Diabetic Status and Clinical Presentation. Journal of Clinical Medicine, 13(22), 6833. https://doi.org/10.3390/jcm13226833







