Comparison of Nitroglycerin-Induced Pressure Ratio Drop and Resting Full-Cycle Ratio in a Pressure Wire Study
Abstract
1. Introduction
2. Materials and Methods
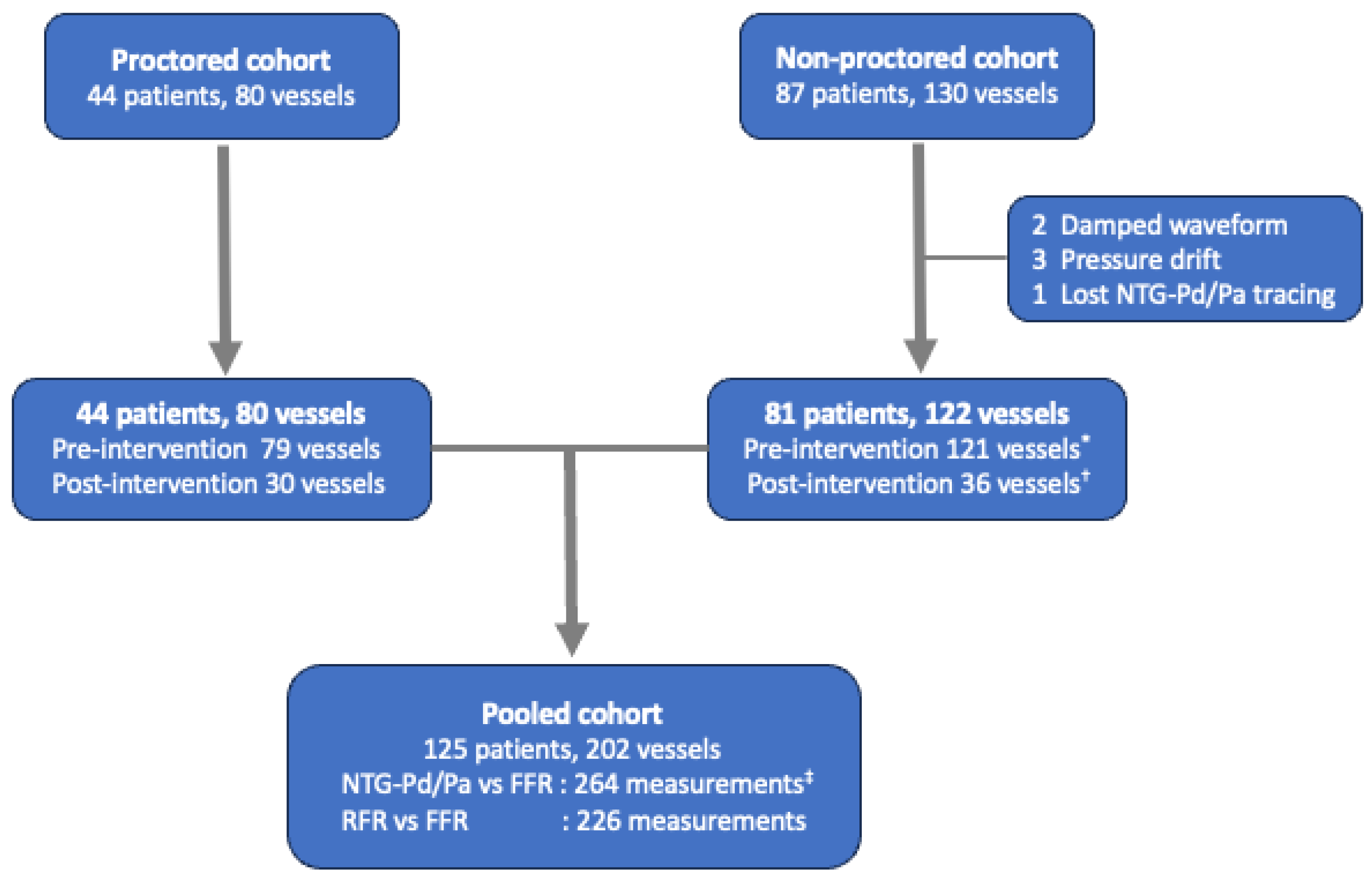
2.1. The Study Population
2.2. Study Protocol
2.3. Data Acquisition and Quality Check (NTG-Pd/Pa, FFR, and RFR)
2.4. Statistical Analysis
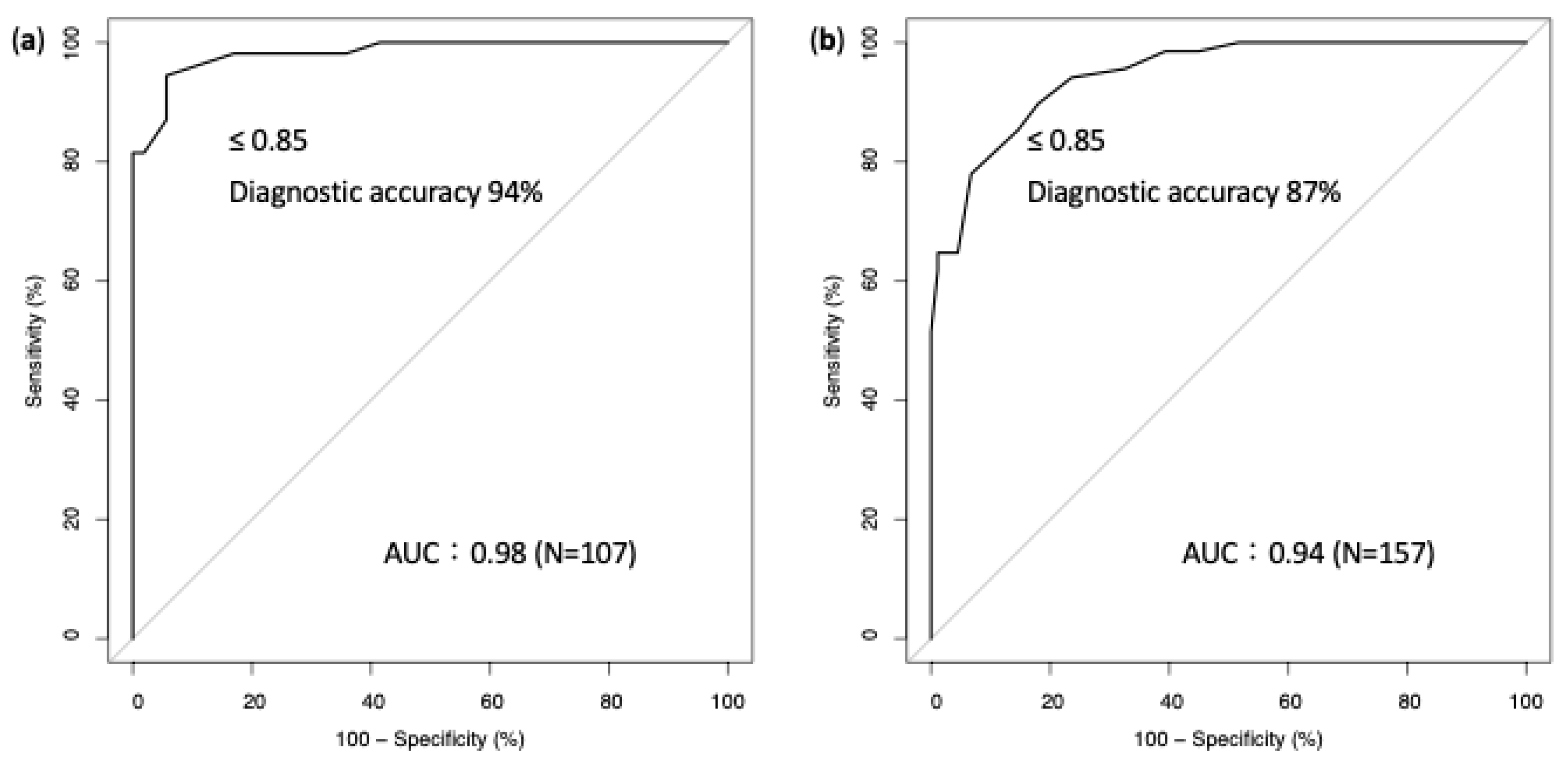
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Ueng, K.C.; Chiang, C.E.; Chao, T.H.; Wu, Y.W.; Lee, W.L.; Li, Y.H.; Ting, K.H.; Su, C.H.; Lin, H.J.; Su, T.C.; et al. 2023 Guidelines of the Taiwan Society of Cardiology on the diagnosis and management of chronic coronary syndrome. Acta Cardiol. Sin. 2023, 39, 4–96. [Google Scholar] [CrossRef] [PubMed]
- Gotberg, M.; Cook, C.M.; Sen, S.; Nijjer, S.; Escaned, J.; Davies, J.E. The evolving future of instantaneous wave-free ratio and fractional flow reserve. J. Am. Coll. Cardiol. 2017, 70, 1379–1402. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E.; Sen, S.; Dehbi, H.M.; Al-Lamee, R.; Petraco, R.; Nijjer, S.S.; Bhindi, R.; Lehman, S.J.; Walters, D.; Sapontis, J.; et al. Use of the instantaneous wave-free ratio or fractional flow reserve in PCI. N. Engl. J. Med. 2017, 376, 1824–1834. [Google Scholar] [CrossRef]
- Gotberg, M.; Christiansen, E.H.; Gudmundsdottir, I.J.; Sandhall, L.; Danielewicz, M.; Jakobsen, L.; Olsson, S.E.; Ohagen, P.; Olsson, H.; Omerovic, E.; et al. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N. Engl. J. Med. 2017, 376, 1813–1823. [Google Scholar] [CrossRef]
- Bernstein, L.; Friesinger, G.C.; Lichtlen, P.R.; Ross, R.S. The effect of nitroglycerin on the systemic and coronary circulation in man and dogs: Myocardial blood flow measured with xenon. Circulation 1966, 33, 107–116. [Google Scholar] [CrossRef]
- Martin-Reyes, R.; de la Torre Hernandez, J.M.; Franco-Pelaez, J.; Lopez-Palop, R.; Telleria Arrieta, M.; Amat Santos, I.J.; Carrillo Saez, P.; Sanchez-Recalde, A.; Sanmartin Pena, J.C.; Garcia Camarero, T.; et al. The use of the acute Pd/Pa drop after intracoronary nitroglycerin infusion to rule out significant FFR: CANICA (Can intracoronary nitroglycerin predict fractional flow reserve without adenosine?) multicenter study. Catheter. Cardiovasc. Interv. 2016, 87, 262–269. [Google Scholar] [CrossRef]
- Shah, S.P.; Waxman, S. Two cases of Bezold-Jarisch reflex induced by intra-arterial nitroglycerin in critical left main coronary artery stenosis. Tex. Heart Inst. J. 2013, 40, 484–486. [Google Scholar]
- De Bruyne, B.; Pijls, N.H.; Kalesan, B.; Barbato, E.; Tonino, P.A.; Piroth, Z.; Jagic, N.; Mobius-Winkler, S.; Rioufol, G.; Witt, N.; et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N. Engl. J. Med. 2012, 367, 991–1001. [Google Scholar] [CrossRef]
- Jong, C.B.; Liao, M.T.; Lu, T.S.; Meng, S.W.; Chen, C.K.; Tsai, Y.C.; Kuo, J.C.; Wu, C.C. Efficacy and safety of high-dose intracoronary adenosine injection in fractional flow reserve assessment. Acta Cardiol. Sin. 2022, 38, 553–563. [Google Scholar] [CrossRef]
- Jong, C.B.; Lu, T.S.; Lin, L.; Chen, T.Y.; Liao, M.T.; Kuo, J.C. Effect of prolonged pressure equalization on final drifting during pressure wire studies. Sci. Rep. 2024, 14, 11504. [Google Scholar] [CrossRef] [PubMed]
- Svanerud, J.; Ahn, J.M.; Jeremias, A.; van’t Veer, M.; Gore, A.; Maehara, A.; Crowley, A.; Pijls, N.H.J.; De Bruyne, B.; Johnson, N.P.; et al. Validation of a novel non-hyperaemic index of coronary artery stenosis severity: The Resting Full-cycle Ratio (VALIDATE RFR) study. EuroIntervention 2018, 14, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Leisenring, W.; Alonzo, T.; Pepe, M.S. Comparisons of predictive values of binary medical diagnostic tests for paired designs. Biometrics 2000, 56, 345–351. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Melenovsky, V.; Wichterle, D.; Malik, J.; Simek, J.; Hradec, J.; Ceska, R.; Malik, M. Nitroglycerin induced syncope occurs in subjects with delayed phase shift of baroreflex action. Pacing Clin. Electrophysiol. 2002, 25, 828–832. [Google Scholar] [CrossRef]
- Come, P.C.; Pitt, B. Nitroglycerin-induced severe hypotension and bradycardia in patients with acute myocardial infarction. Circulation 1976, 54, 624–628. [Google Scholar] [CrossRef]
- Egashira, K.; Inou, T.; Hirooka, Y.; Yamada, A.; Urabe, Y.; Takeshita, A. Evidence of impaired endothelium-dependent coronary vasodilatation in patients with angina pectoris and normal coronary angiograms. N. Engl. J. Med. 1993, 328, 1659–1664. [Google Scholar] [CrossRef]
- Habazettl, H.; Vollmar, B.; Christ, M.; Baier, H.; Conzen, P.F.; Peter, K. Heterogeneous microvascular coronary vasodilation by adenosine and nitroglycerin in dogs. J. Appl. Physiol. 1994, 76, 1951–1960. [Google Scholar] [CrossRef]
- Asrress, K.N.; Williams, R.; Lockie, T.; Khawaja, M.Z.; De Silva, K.; Lumley, M.; Patterson, T.; Arri, S.; Ihsan, S.; Ellis, H.; et al. Physiology of angina and its alleviation with nitroglycerin: Insights from invasive catheter laboratory measurements during exercise. Circulation 2017, 136, 24–34. [Google Scholar] [CrossRef]
- Patterson, T.; Rivolo, S.; Burkhoff, D.; Schreuder, J.; Briceno, N.; Allen, C.; Williams, R.; Arri, S.; Asrress, K.N.; Joseph, J.; et al. Physiological impact of afterload reduction on cardiac mechanics and coronary hemodynamics following isosorbide dinitrate administration in ischemic heart disease. J. Cardiovasc. Transl. Res. 2021, 14, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Desai, R.; Gore, A.; Rahim, H.; Maehara, A.; Matsumura, M.; Kirtane, A.; Jeremias, A.; Ali, Z. Real world validation of the nonhyperemic index of coronary artery stenosis severity-Resting full-cycle ratio-RE-VALIDATE. Catheter. Cardiovasc. Interv. 2020, 96, e53–e58. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Choi, K.H.; Park, J.; Hwang, D.; Rhee, T.M.; Kim, J.; Park, J.; Kim, H.Y.; Jung, H.W.; Cho, Y.K.; et al. Physiological and clinical assessment of resting physiological indexes. Circulation 2019, 139, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Escaned, J.; Malik, I.S.; Mikhail, G.W.; Foale, R.A.; Mila, R.; Tarkin, J.; Petraco, R.; Broyd, C.; Jabbour, R.; et al. Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis: Results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J. Am. Coll. Cardiol. 2012, 59, 1392–1402. [Google Scholar] [CrossRef]
- Escaned, J.; Echavarría-Pinto, M.; Garcia-Garcia, H.M.; van de Hoef, T.P.; de Vries, T.; Kaul, P.; Raveendran, G.; Altman, J.D.; Kurz, H.I.; Brechtken, J.; et al. Prospective assessment of the diagnostic accuracy of instantaneous wave-free ratio to assess coronary stenosis relevance: Results of ADVISE II International, Multicenter Study (Adenosine Vasodilator Independent Stenosis Evaluation II). Cardiovasc. Interv. 2015, 8, 824–833. [Google Scholar] [CrossRef]
- Goto, R.; Takashima, H.; Ohashi, H.; Ando, H.; Suzuki, A.; Sakurai, S.; Nakano, Y.; Sawada, H.; Fujimoto, M.; Suzuki, Y.; et al. Independent predictors of discordance between the resting full-cycle ratio and fractional flow reserve. Heart Vessel. 2021, 36, 790–798. [Google Scholar] [CrossRef]
- Malmberg, S.; Lauermann, J.; Karlström, P.; Gulin, D.; Barmano, N. Resting full-cycle ratio versus fractional flow reserve: A SWEDEHEART-registry-based comparison of two physiological indexes for assessing coronary stenosis severity. J. Interv. Cardiol. 2023, 2023, 6461691. [Google Scholar] [CrossRef]
- Kato, Y.; Dohi, T.; Chikata, Y.; Fukase, T.; Takeuchi, M.; Takahashi, N.; Endo, H.; Nishiyama, H.; Doi, S.; Okai, I.; et al. Predictors of discordance between fractional flow reserve and resting full-cycle ratio in patients with coronary artery disease: Evidence from clinical practice. J. Cardiol. 2021, 77, 313–319. [Google Scholar] [CrossRef]
- Johnson, N.P.; Jeremias, A.; Zimmermann, F.M.; Adjedj, J.; Witt, N.; Hennigan, B.; Koo, B.K.; Maehara, A.; Matsumura, M.; Barbato, E.; et al. Continuum of vasodilator stress from rest to contrast medium to adenosine hyperemia for fractional flow reserve assessment. Cardiovasc. Interv. 2016, 9, 757–767. [Google Scholar] [CrossRef]
- Jong, C.-B.; Kuo, J.-C.; Lin, I.C. Kidney protection strategy lowers the risk of contrast-associated acute kidney injury. PLoS ONE 2024, 19, e0312618. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jong, C.-B.; Lu, T.-S.; Liao, M.-T.; Xu, J.-L.; Chen, C.-K.; Kuo, J.-C.; Wu, C.-C. Comparison of Nitroglycerin-Induced Pressure Ratio Drop and Resting Full-Cycle Ratio in a Pressure Wire Study. J. Clin. Med. 2024, 13, 6716. https://doi.org/10.3390/jcm13226716
Jong C-B, Lu T-S, Liao M-T, Xu J-L, Chen C-K, Kuo J-C, Wu C-C. Comparison of Nitroglycerin-Induced Pressure Ratio Drop and Resting Full-Cycle Ratio in a Pressure Wire Study. Journal of Clinical Medicine. 2024; 13(22):6716. https://doi.org/10.3390/jcm13226716
Chicago/Turabian StyleJong, Chien-Boon, Tsui-Shan Lu, Min-Tsun Liao, Jia-Lang Xu, Chun-Kai Chen, Jui-Cheng Kuo, and Chih-Cheng Wu. 2024. "Comparison of Nitroglycerin-Induced Pressure Ratio Drop and Resting Full-Cycle Ratio in a Pressure Wire Study" Journal of Clinical Medicine 13, no. 22: 6716. https://doi.org/10.3390/jcm13226716
APA StyleJong, C.-B., Lu, T.-S., Liao, M.-T., Xu, J.-L., Chen, C.-K., Kuo, J.-C., & Wu, C.-C. (2024). Comparison of Nitroglycerin-Induced Pressure Ratio Drop and Resting Full-Cycle Ratio in a Pressure Wire Study. Journal of Clinical Medicine, 13(22), 6716. https://doi.org/10.3390/jcm13226716






