Consequences of the Poor Anticoagulation Control of Patients with Non-Valvular Atrial Fibrillation Treated with Vitamin K Antagonists
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Study Population
Study Cohorts
2.3. Study Variables
2.3.1. Sociodemographic Characteristics and Comorbidities
2.3.2. Pharmacological Treatment
2.3.3. Cardiovascular Events and Mortality
2.3.4. CHA2DS2-VASc and HAS-BLED Scores
2.3.5. Mortality
2.3.6. Healthcare Resources and Healthcare Costs
2.4. Statistical Analysis
3. Results
3.1. Study Population
Characteristics of the Study Population
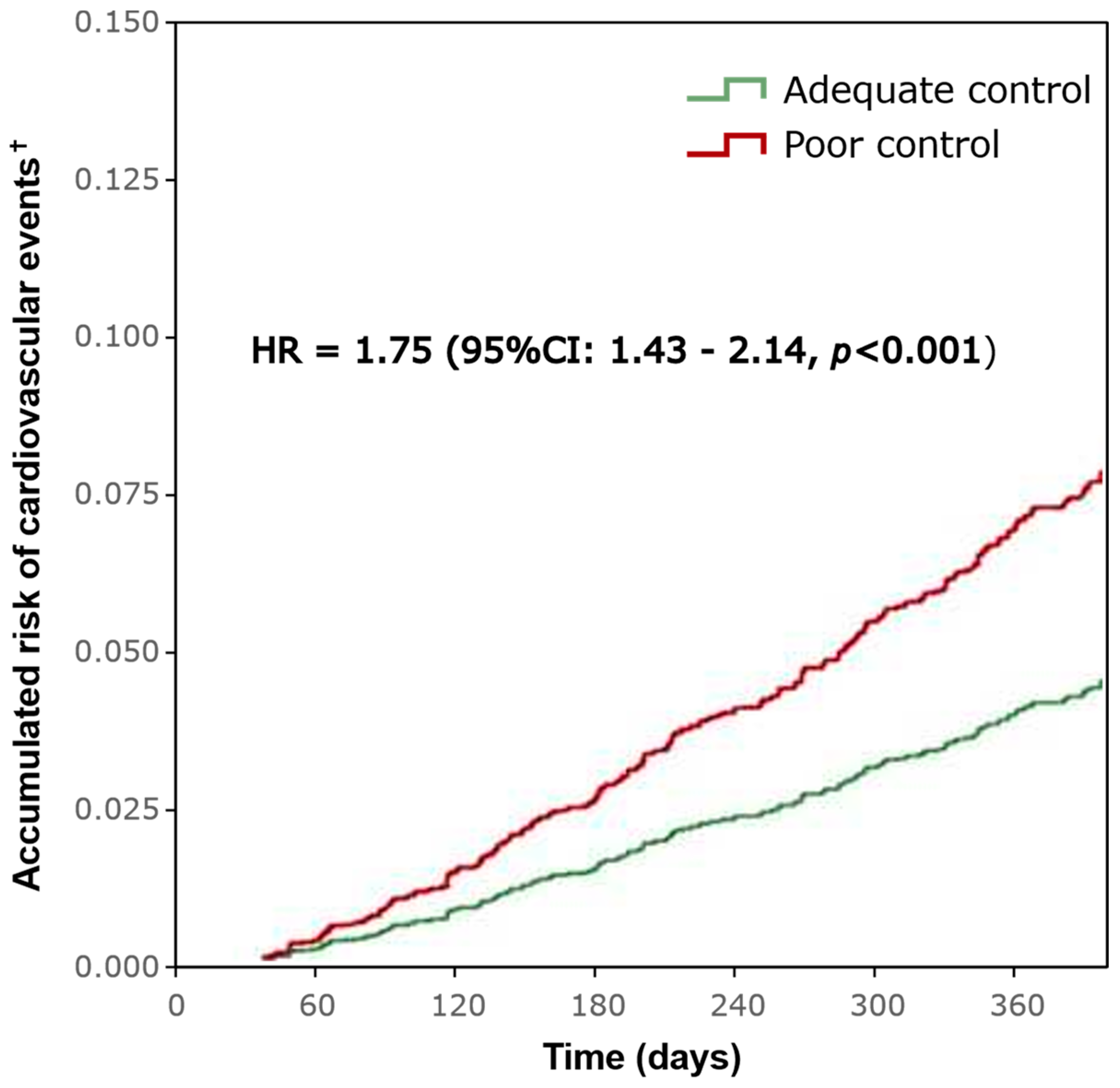
3.2. Cardiovascular Events and Mortality
Variations in CHA2DS2-VASc and HAS-BLED Scores
3.3. Treatment of the Study Population
3.3.1. Anticoagulant Treatment
3.3.2. Variations in Concomitant Medication
3.4. Use of Healthcare Resources and Costs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014, 130, 2071–2104. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Estes, N.A.M., 3rd; Fonarow, G.C.; Jurgens, C.Y.; Kittleson, M.M.; Marine, J.E.; McManus, D.D.; McNamara, R.L. 2020 Update to the 2016 ACC/AHA Clinical Performance and Quality Measures for Adults with Atrial Fibrillation or Atrial Flutter: A Report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. Circ. Cardiovasc. Qual. Outcomes 2021, 14, e000100. [Google Scholar] [CrossRef]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Kardiol. Pol. 2016, 74, 1359–1469. [Google Scholar] [CrossRef]
- Gómez-Doblas, J.J.; Muñiz, J.; Martin, J.J.A.; Rodríguez-Roca, G.; Lobos, J.M.; Awamleh, P.; Permanyer-Miralda, G.; Chorro, F.J.; Anguita, M.; Roig, E. Prevalencia de fibrilación auricular en España. Resultados del estudio OFRECE Prevalence of Atrial Fibrillation in Spain. OFRECE Study Results. Rev. Esp. Cardiol. 2014, 67, 259–269. [Google Scholar] [CrossRef]
- Polo Garcia, J.; Vargas Ortega, D.; Formiga, F.; Unzueta, I.; Fernandez de Cabo, S.; Chaves, J. Profiling of patients with non-valvular atrial fibrillation and moderate-to-high risk of stroke not receiving oral anticoagulation in Spain. Semergen 2019, 45, 396–405. [Google Scholar] [CrossRef]
- Mora-Llabata, V.; Dubois-Marqués, D.; Roldán-Torres, I.; Mateu-Navarro, C.; Sanz-García, J.J.; Moreno-Ballester, V.; Mira-Gimeno, S.; Albiñana-Fernández, F. Prevalencia de fibrilación auricular y características de la fibrilación auricular no valvular en la población general. Registro AFINVA. Rev. Colomb. Cardiol. 2017, 24, 26–33. [Google Scholar] [CrossRef]
- Schafer, A.; Flierl, U.; Berliner, D.; Bauersachs, J. Anticoagulants for Stroke Prevention in Atrial Fibrillation in Elderly Patients. Cardiovasc. Drugs Ther. 2020, 34, 555–568. [Google Scholar] [CrossRef]
- Lip, G.Y.; Potpara, T.; Boriani, G.; Blomstrom-Lundqvist, C. A tailored treatment strategy: A modern approach for stroke prevention in patients with atrial fibrillation. J. Intern. Med. 2016, 279, 467–476. [Google Scholar] [CrossRef]
- Escobar, C.; Borras, X.; Bover Freire, R.; Gonzalez-Juanatey, C.; Morillas, M.; Munoz, A.V.; Gomez-Doblas, J.J. A Delphi consensus on the management of oral anticoagulation in patients with non-valvular atrial fibrillation in Spain: ACOPREFERENCE study. PLoS ONE 2020, 15, e0231565. [Google Scholar] [CrossRef] [PubMed]
- Shikdar, S.; Vashisht, R.; Bhattacharya, P.T. International Normalized Ratio (INR) [Updated 2023 May 1]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507707/ (accessed on 1 January 2024).
- Dorgalaleh, A.; Favaloro, E.J.; Bahraini, M.; Rad, F. Standardization of Prothrombin Time/International Normalized Ratio (PT/INR). Int. J. Lab. Hematol. 2021, 43, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Witt, D.M. Approaches to optimal dosing of vitamin K antagonists. Semin. Thromb. Hemost. 2012, 38, 667–672. [Google Scholar] [CrossRef]
- Moreau, C.; Loriot, M.A.; Siguret, V. Vitamin K antagonists: From discovery to pharmacogenetics. Ann. Biol. Clin. 2012, 70, 539–551. [Google Scholar] [CrossRef]
- Self, T.H.; Wallace, J.L.; Sakaan, S.; Sands, C.W. Effect of Body Weight on Dose of Vitamin K Antagonists. South. Med. J. 2015, 108, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Schulman, S.; Witt, D.M.; Vandvik, P.O.; Qayyum, F.; Holbrook, A.M. Anticoagulation control with daily low-dose vitamin k to reduce clinically adverse outcomes and international normalized ratio variability: A systematic review and meta-analysis. Pharmacotherapy 2013, 33, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- AEMPS. Criterios y Recomendaciones Generales Para el Uso de los Anticoagulantes Orales Directos (ACOD) en la Prevención del Ictus y la Embolia Sistémica en Pacientes con Fibrilación Auricular no Valvular. Available online: https://www.aemps.gob.es/medicamentosUsoHumano/informesPublicos/docs/criterios-anticoagulantes-orales.pdf (accessed on 1 January 2024).
- Barrios, V.; Escobar, C.; Prieto, L.; Osorio, G.; Polo, J.; Lobos, J.M.; Vargas, D.; García, N. Control de la anticoagulación en pacientes con fibrilación auricular no valvular asistidos en atención primaria en España. Estudio PAULA. Rev. Esp. Cardiol. 2015, 68, 769–776. [Google Scholar] [CrossRef]
- Barrios, V.; Cinza-Sanjurjo, S.; Gavin, O.; Egocheaga, I.; Burgos-Pol, R.; Soto, J.; Polanco, C.; Suarez, J.; Casado, M.A. Cost and burden of poor anticoagulation control with vitamin K antagonists in patients with nonvalvular atrial fibrillation in Spain. Rev. Esp. Cardiol. 2021, 74, 773–780. [Google Scholar] [CrossRef]
- Suarez Fernandez, C.; Mostaza, J.M.; Castilla Guerra, L.; Cantero Hinojosa, J.; Surinach, J.M.; Acosta de Bilbao, F.; Tamarit, J.J.; Diaz Diaz, J.L.; Hernandez, J.L.; Cazorla, D.; et al. Adherence to recommendations of the Therapeutic Positioning Report about treatment with oral anticoagulants in elderly patients with atrial fibrillation. The ESPARTA study. Med. Clin. 2018, 151, 8–15. [Google Scholar] [CrossRef]
- Zirlik, A.; Bode, C. Vitamin K antagonists: Relative strengths and weaknesses vs. direct oral anticoagulants for stroke prevention in patients with atrial fibrillation. J. Thromb. Thrombolysis 2017, 43, 365–379. [Google Scholar] [CrossRef]
- Cano, L.M.; Cardona, P.; Quesada, H.; Lara, B.; Rubio, F. Ischaemic stroke in patients treated with oral anticoagulants. Neurologia 2016, 31, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Sendon, J.; Merino, J.L. Poor Anticoagulation Control in Atrial Fibrillation: How Much Longer? Rev. Esp. Cardiol. 2015, 68, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Caravaca, J.M.; Roldan, V.; Esteve-Pastor, M.A.; Valdes, M.; Vicente, V.; Marin, F.; Lip, G.Y.H. Reduced Time in Therapeutic Range and Higher Mortality in Atrial Fibrillation Patients Taking Acenocoumarol. Clin. Ther. 2018, 40, 114–122. [Google Scholar] [CrossRef] [PubMed]
- White, H.D.; Gruber, M.; Feyzi, J.; Kaatz, S.; Tse, H.F.; Husted, S.; Albers, G.W. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: Results from SPORTIF III and V. Arch. Intern. Med. 2007, 167, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Dalmau Llorca, M.R.; Aguilar Martin, C.; Carrasco-Querol, N.; Hernandez Rojas, Z.; Forcadell Drago, E.; Rodriguez Cumplido, D.; Pepio Vilaubi, J.M.; Castro Blanco, E.; Goncalves, A.Q.; Fernandez-Saez, J. Oral Anticoagulant Adequacy in Non-Valvular Atrial Fibrillation in Primary Care: A Cross-Sectional Study Using Real-World Data (Fantas-TIC Study). Int. J. Environ. Res. Public Health 2021, 18, 2244. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Pol, R.; Barrios, V.; Cinza-Sanjurjo, S.; Gavin, O.; Egocheaga, I.; Soto, J.; Polanco, C.; Suarez, J.; Casado, M.A. P3815Cost and burden of poor control of the level of anticoagulation in patients with non-valvular atrial fibrillation treated with vitamin K antagonist in the Spanish National Health Service. Eur. Heart J. 2019, 40, 2403. [Google Scholar] [CrossRef]
- Suh, D.C.; Nelson, W.W.; Choi, J.C.; Choi, I. Risk of hemorrhage and treatment costs associated with warfarin drug interactions in patients with atrial fibrillation. Clin. Ther. 2012, 34, 1569–1582. [Google Scholar] [CrossRef]
- Catalogues for Real-World Data Sources, Studies, Institutions, and Networks. HMA-EMA Catalogues of Real-World Data Sources and Studies Big-Pac. Available online: https://catalogues.ema.europa.eu/node/4136/administrative-details (accessed on 1 October 2024).
- Gobierno de España. Boletín Oficial del Estado. In Ley Orgánica 3/2018, de 5 de Diciembre, de Protección de Datos Personales y Garantía de los Derechos Digitales; Boletín Oficial del Estado: Madrid, Spain, 2018; pp. 119788–119857. Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-2018-16673 (accessed on 1 January 2024).
- Sicras-Mainar, A.; Enriquez, J.L.; Hernández, I.; Sicras-Navarro, A.; Aymerich, T.; Leon, M. Pmu146 Validation and Representativeness of the Spanish Big-Pac Database: Integrated Computerized Medical Records for Research into Epidemiology, Medicines and Health Resource Use (Real Word Evidence). Value Health 2019, 22, S734. [Google Scholar] [CrossRef]
- Ministerio de Sanidad, Consumo y Bienestar Social. Clasificación Internacional de Enfermedades. 10a Revisión. Modificación Clínica. 3a Edición. Available online: https://www.mscbs.gob.es/estadEstudios/estadisticas/normalizacion/CIE10/Clasif_Inter_Enfer_CIE_10_rev_3_ed.diag.pdf (accessed on 16 January 2024).
- Deyo, R.A.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef]
- World Health Organization (WHO). The Anatomical Therapeutic Chemical Classification System with Defined Daily Doses (ATC/DDD). Available online: https://www.who.int/standards/classifications/other-classifications/the-anatomical-therapeutic-chemical-classification-system-with-defined-daily-doses (accessed on 1 January 2024).
- Dzeshka, M.S.; Lane, D.A.; Lip, G.Y. Stroke and bleeding risk in atrial fibrillation: Navigating the alphabet soup of risk-score acronyms (CHADS2, CHA2 DS2-VASc, R2 CHADS2, HAS-BLED, ATRIA, and more). Clin. Cardiol. 2014, 37, 634–644. [Google Scholar] [CrossRef]
- Sicras-Mainar, A.; Capel, M.; Navarro-Artieda, R.; Nuevo, J.; Orellana, M.; Resler, G. Real-life retrospective observational study to determine the prevalence and economic burden of severe asthma in Spain. J. Med. Econ. 2020, 23, 492–500. [Google Scholar] [CrossRef]
- Consejo General de Colegios Oficiales de Farmacéuticos. BOT Plus 2. Base de Datos de Medicamentos. Available online: https://botplusweb.portalfarma.com/ (accessed on 1 January 2024).
- Instituto Nacional de Estadística (INE). Ganancia Media Laboral por edad y Sexo. Available online: https://www.ine.es/dynt3/inebase/index.htm?padre=4563&capsel=4563 (accessed on 16 January 2024).
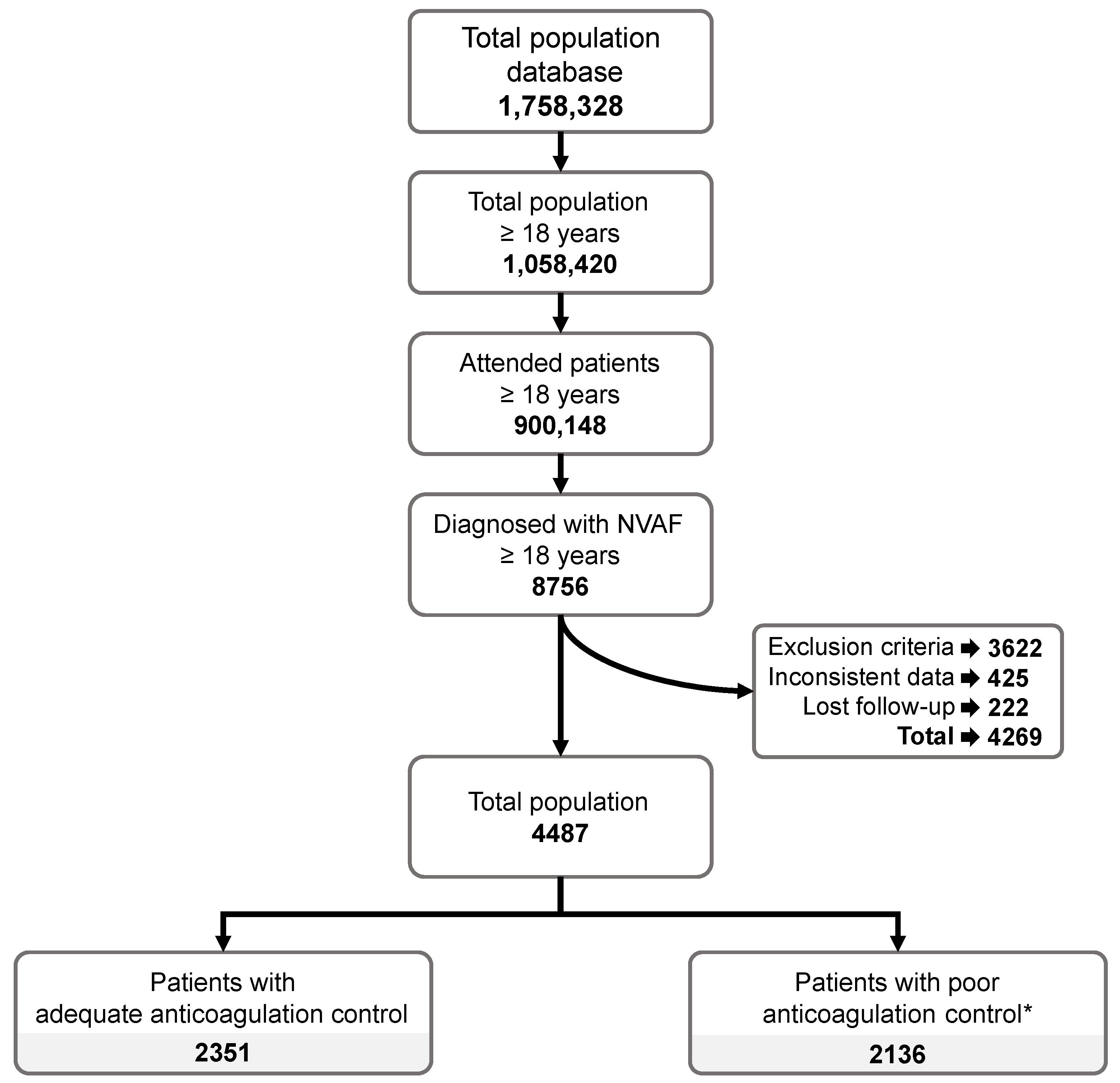
| Study Cohort | Adequate Anticoagulation Control | Poor Anticoagulation Control | Total | p-Value |
|---|---|---|---|---|
| N (%) | 2351 (52.4) | 2136 (47.6) | 4487 (100) | |
| Demographic characteristics | ||||
| Age, mean (SD) | 69.5 (11.4) | 70.6 (7.9) | 70.0 (9.9) | <0.001 |
| Age range, N (%) | <0.001 | |||
| 18–49 years | 76 (3.2) | 9 (0.4) | 85 (1.9) | |
| 50–64 years | 547 (23.3) | 71 (3.3) | 618 (13.8) | |
| 65–74 years | 638 (27.1) | 709 (33.2) | 1347 (30) | |
| 75–84 years | 765 (32.5) | 944 (44.2) | 1709 (38.1) | |
| ≥85 years | 325 (13.8) | 403 (18.9) | 728 (16.2) | |
| Sex, males, N (%) | 1129 (48) | 1012 (47.4) | 2141 (47.7) | 0.666 |
| Smoke active, N (%) | 114 (4.8) | 148 (6.9) | 262 (5.8) | 0.003 |
| BMI, kg/m2, mean (SD) | 29.9 (5.2) | 30.1 (5.1) | 30 (5.1) | 0.157 |
| Comorbidities, mean (SD) | 2.7 (1.8) | 2.9 (1.4) | 2.8 (1.6) | <0.001 |
| Comorbidities, N (%) | ||||
| Hypertension | 1675 (71.2) | 1534 (71.8) | 3209 (71.5) | 0.673 |
| Diabetes | 697 (29.6) | 650 (30.4) | 1347 (30) | 0.567 |
| Dyslipidemia | 1044 (44.4) | 1002 (46.9) | 2046 (45.6) | 0.093 |
| Obesity | 543 (23.1) | 512 (24) | 1055 (23.5) | 0.491 |
| Alcoholism | 50 (2.1) | 51 (2.4) | 101 (2.3) | 0.556 |
| Myocardial infraction | 142 (6) | 123 (5.8) | 265 (5.9) | 0.690 |
| Hemorrhagic stroke | 7 (0.3) | 4 (0.2) | 11 (0.2) | 0.455 |
| Ischemic stroke | 170 (7.2) | 180 (8.4) | 350 (7.8) | 0.136 |
| Transient ischemic attack | 72 (3.1) | 79 (3.7) | 151 (3.4) | 0.238 |
| Peripheral artery disease | 128 (5.4) | 165 (7.7) | 293 (6.5) | 0.002 |
| Heart insufficiency | 433 (18.4) | 426 (19.9) | 859 (19.1) | 0.194 |
| Renal insufficiency | 181 (7.7) | 186 (8.7) | 367 (8.2) | 0.218 |
| Asthma | 166 (7.1) | 176 (8.2) | 342 (7.6) | 0.137 |
| COPD | 392 (16.7) | 380 (17.8) | 772 (17.2) | 0.322 |
| Dementia | 55 (2.3) | 42 (2) | 97 (2.2) | 0.391 |
| Depression | 203 (8.6) | 196 (9.2) | 399 (8.9) | 0.525 |
| Neoplasm | 179 (7.6) | 172 (8.1) | 351 (7.8) | 0.585 |
| Hepatic insufficiency | 113 (4.8) | 125 (5.9) | 238 (5.3) | 0.119 |
| Scores | ||||
| Charlson comorbidity index, mean (SD) | 1.4 (1.4) | 1.6 (1.5) | 1.5 (1.5) | <0.001 |
| Patients with a score of 0, N (%) | 690 (29.3) | 560 (26.2) | 1250 (27.9) | <0.001 |
| Patients with a score of 1, N (%) | 796 (33.9) | 667 (31.2) | 1463 (32.6) | |
| Patients with a score of 2, N (%) | 467 (19.9) | 441 (20.6) | 908 (20.2) | |
| Patients with a score ≥ 3, N (%) | 398 (16.9) | 468 (21.9) | 866 (19.3) | |
| CHA2DS2VASc score, mean (SD) | 2.6 (1.6) | 3.4 (1.2) | 3 (1.5) | <0.001 |
| Patients with a score of 0, N (%) | 276 (11.7) | 32 (1.5) | 308 (6.9) | <0.001 |
| Patients with a score of 1, N (%) | 406 (17.3) | 99 (4.6) | 505 (11.3) | |
| Patients with a score of 2, N (%) | 444 (18.9) | 331 (15.5) | 775 (17.3) | |
| Patients with a score ≥ 3, N (%) | 1225 (52.1) | 1674 (78.4) | 2899 (64.5) | |
| HAS-BLED score, mean (SD) | 2.8 (1.0) | 3.4 (0.7) | 3.1 (0.9) | <0.001 |
| Patients with a score of 0, N (%) | 14 (0.6) | 0 (0) | 14 (0.3) | <0.001 |
| Patients with a score of 1–2, N (%) | 950 (40.4) | 48 (2.3) | 998 (22.2) | |
| Patients with a score of 3–5, N (%) | 1372 (58.4) | 2079 (97.3) | 3451 (76.9) | |
| Patients with a score ≥ 6, N (%) | 15 (0.6) | 9 (0.4) | 24 (0.6) | |
| Patients per type of event *, N (%) | ||||
| Minor bleeding | 159 (6.8) | 195 (9.1) | 354 (7.9) | 0.003 |
| Major bleeding | 129 (5.5) | 101 (4.7) | 230 (5.1) | 0.250 |
| Study Cohort | Adequate Anticoagulation Control | Poor Anticoagulation Control | Total | p-Value |
|---|---|---|---|---|
| N (%) | 2351 (52.4) | 2136 (47.6) | 4487 (100) | |
| Cardiovascular events | ||||
| For patients with cardiovascular events, N (%) | 486 (20.7) | 651 (30.5) | 1137 (25.3) | <0.001 |
| 95% CI | (19.1–22.3) | (28.5–32.5) | (24.0–26.6) | |
| Mean number of events, N (SD) | 0.2 (0.5) | 0.3 (0.5) | 0.3 (0.5) | <0.001 |
| Number of events, N (%) | ||||
| Patients with 1 cardiovascular event | 440 (18.7) | 589 (27.6) | 1029 (22.9) | |
| Patients with ≥ 2 cardiovascular events | 46 (2.0) | 62 (2.9) | 108 (2.4) | <0.001 |
| Type of event, N (%) | ||||
| Ischemic strokes | 62 (2.6) | 67 (3.1) | 129 (2.8) | 0.025 |
| Systemic thromboembolisms | 16 (0.7) | 21 (0.9) | 37 (0.8) | 0.003 |
| Minor bleeding | 337 (14.3) | 434 (20.3) | 771 (17.2) | <0.001 |
| Major bleeding | 118 (5.0) | 198 (9.3) | 316 (7.0) | <0.001 |
| Mortality, N (%) | 172 (7.3) | 231 (10.8) | 404 (9) | <0.001 |
| 95% CI | (6.2–8.4) | (9.5–12.1) | (8.2–9.8) |
| Study Cohort | Adequate Anticoagulation Control | Poor Anticoagulation Control | Total | p-Value |
|---|---|---|---|---|
| N (%) | 2351 (52.4) | 2136 (47.6) | 4487 (100) | |
| Index date | ||||
| Scores | ||||
| CHA2DS2-VASc index, mean (SD) | 2.6 (1.6) | 3.4 (1.2) | 3 (1.5) | <0.001 |
| HAS-BLED (SD), mean (SD) | 2.8 (1) | 3.5 (0.7) | 3.1 (0.9) | <0.001 |
| Concomitant medication | ||||
| Patients with concomitant medication, % | 96.4% | 99.3% | 97.8% | <0.001 |
| Concomitant medication, N (SD) | 3.2 (1.6) | 3.3 (1.4) | 3.2 (1.5) | 0.029 |
| Percentage of use, N (%) | ||||
| NSAIDs | 1559 (66.3) | 1449 (67.8) | 3008 (67.0) | 0.278 |
| Antiplatelet agents | 953 (40.5) | 898 (42.0) | 1851 (41.3) | 0.306 |
| Antidiabetics | 688 (29.3) | 618 (28.9) | 1306 (29.1) | 0.807 |
| Beta-blockers | 1372 (58.4) | 1269 (59.4) | 2641 (58.9) | 0.475 |
| Agents acting in the renin–angiotensin system | 1592 (67.7) | 1495 (70) | 3087 (68.8) | 0.100 |
| Lipid-lowering agent | 1335 (56.8) | 1290 (60.4) | 2625 (58.5) | 0.014 |
| End of the study | ||||
| Scores | ||||
| CHA2DS2-VASc index, mean (SD) | 2.7 (1.7) | 3.6 (1.4) | 3.1 (1.6) | <0.001 |
| HAS-BLED (SD), mean (SD) | 3.1 (1.2) | 3.8 (0.9) | 3.43 (1.1) | <0.001 |
| Concomitant medication | ||||
| Patients with concomitant medication, % | 96.4% | 99.3% | 97.8% | <0.001 |
| Mean of concomitant medication, N (SD) | 3.2 (1.6) | 3.4 (1.4) | 3.3 (1.5) | 0.002 |
| Percentage of use, N (%) | ||||
| NSAIDs | 1577 (67.1) | 1484 (69.5) | 3061 (68.2) | 0.085 |
| Antiplatelet agents | 970 (41.3) | 921 (43.1) | 1891 (42.1) | 0.208 |
| Antidiabetics | 704 (29.9) | 654 (30.6) | 1358 (30.3) | 0.624 |
| Beta-blockers | 1396 (59.4) | 1305 (61.1) | 2701 (60.2) | 0.241 |
| Agents acting in the renin–angiotensin system | 1615 (68.7) | 1526 (71.4) | 3141 (70) | 0.045 |
| Lipid-lowering agents | 1351 (57.5) | 1325 (62) | 2676 (59.6) | 0.002 |
| Difference (end of the study—index date) | ||||
| Scores | ||||
| CHA2DS2-VASc index, mean (SD) | 0.1 (0.4) | 0.2 (0.5) | 0.2 (0.5) | <0.001 |
| HAS-BLED (SD), mean (SD) | 0.3 (0.5) | 0.4 (0.59 | 0.3 (0.5) | <0.001 |
| Concomitant medication, variation (SD) | ||||
| NSAID | 0.8 (8.7) | 1.6 (12.7) | 1.2 (10.8) | 0.013 |
| p-value of variation | <0.001 | <0.001 | <0.001 | |
| Antiplatelet agents | 0.7 (8.5) | 1.1 (10.3) | 0.9 (9.4) | 0.154 |
| p-value of variation | <0.001 | <0.001 | <0.001 | |
| Antidiabetics | 0.7 (8.2) | 1.7 (12.9) | 1.2 (10.7) | 0.001 |
| p-value of variation | <0.001 | <0.001 | <0.001 | |
| Beta-blockers | 1 (10.1) | 1.7 (12.9) | 1.3 (11.5) | 0.041 |
| p-value of variation | <0.001 | <0.001 | <0.001 | |
| Agents acting in the renin–angiotensin system | 1 (9.8) | 1.5 (12) | 1.2 (10.9) | 0.130 |
| p-value of variation | <0.001 | <0.001 | <0.001 | |
| Lipid-lowering agents | 0.7 (8.2) | 1.6 (12.7) | 1.1 (10.6) | <0.001 |
| p-value of variation | <0.001 | <0.001 | <0.001 |
| Study Cohort | Adequate Anticoagulation Control | Poor Anticoagulation Control | Total | p-Value |
|---|---|---|---|---|
| N (%) | 2351 (52.4) | 2136 (47.6) | 4487 (100) | |
| Time from diagnosis to first prescription | ||||
| Mean (SD) | 13.2 (23.6) | 12.5 (23) | 12.8 (23.3) | 0.352 |
| Median (P25–P75) | 1 (0–13) | 1 (0–12) | 1 (0–12) | |
| Treatment duration, days | ||||
| Mean (SD) | 476.8 (262.8) | 432.8 (259.4) | 455.8 (262.1) | <0.001 |
| Median (P25–P75) | 576 (214–730) | 355 (194–730) | 461 (205–730) | |
| Medicine prescribed | ||||
| Use of acenocoumarol, N (%) | 2121 (90.2) | 1944 (91) | 4065 (90.6) | 0.338 |
| Mean initial dose prescribed, mg (SD) | 0.8 (0.7) | 0.8 (0.7) | 0.8 (0.7) | |
| Mean treatment duration, days (SD) | 476.4 (263) | 432.3 (260) | 455.3 (263) | |
| Median treatment duration, days (P25–P75) | 579.5 (213–730) | 354 (194–730) | 457.5 (205–730) | |
| Use of warfarin, N (%) | 230 (9.8) | 192 (9) | 422 (9.4) | 0.338 |
| Mean initial dose prescribed, mg (SD) | 1.3 (1.1) | 1.3 (0.8) | 1.3 (1) | |
| Mean treatment duration, days (SD) | 480.4 (260.6) | 436.9 (253.4) | 460.6 (258) | |
| Median treatment duration, days (P25–P75) | 564 (246–730) | 376.5 (204–730) | 465 (218–730) | |
| Discontinuation | ||||
| Patients for whom treatment was discontinued, N (%) | 1321 (56.2) | 1393 (65.2) | 2719 (60.6) | <0.001 |
| CI 95% | (54.2–58.2) | (63.2–67.2) | (59.2–62.0) | |
| Causes, N (%) | 1322 (56.2) | 1531 (71.7) | 1425 (31.6) | <0.001 |
| New events | 487 (20.7) | 722 (30.7) | 600 (25.5) | |
| Medication switch | 362 (15.4) | 360 (15.3) | 362 (15.4) | |
| Abandonment | 315 (13.4) | 214 (9.1) | 268 (11.4) | |
| Mortality | 158 (6.7) | 235 (10) | 195 (8.3) | |
| Persistence | ||||
| at 12 months, N (%) | 1359 (57.8) | 1053 (49.3) | 2410 (53.7) | <0.001 |
| CI 95% | (55.8–59.8) | (47.2–51.4) | (52.2–55.2) | |
| at 24 months, N (%) | 1030 (43.8) | 745 (34.9) | 1772 (39.5) | <0.001 |
| CI 95% | (41.8–45.8) | (32.9–36.9) | (38.1–40.9) |
| Study Cohort | Adequate Anticoagulation Control | Poor Anticoagulation Control | Total | p-Value |
|---|---|---|---|---|
| N (%) | 2351 (52.4) | 2136 (47.6) | 4487 (100) | |
| Visits | ||||
| Primary care visits, mean (SD) | 15.8 (11.5) | 16.6 (11.4) | 16.2 (11.4) | 0.019 |
| Nurse visits, mean (SD) | 14.3 (12.7) | 17 (9.3) | 15.6 (11.3) | <0.001 |
| Specialist care visits, mean (SD) | 2.1 (1.3) | 2.2 (1.4) | 2.2 (1.3) | 0.002 |
| Emergency visits, mean (SD) | 0.8 (2) | 0.8 (2.3) | 0.8 (2.2) | 0.203 |
| Hospitalizations | ||||
| Mean (SD) number of hospitalizations | 0.2 (0.7) | 0.3 (0.7) | 0.3 (0.7) | 0.006 |
| Hospitalization, N (%) | 366 (15.6) | 429 (20.1) | 795 (17.7) | <0.001 |
| Duration of hospitalizations, mean (SD) days, | 1.7 (4.7) | 2.6 (5.8) | 2.1 (5.3) | <0.001 |
| Tests, mean (SD) | ||||
| Laboratory | 0.06 (0.46) | 0.15 (0.76) | 0.1 (0.62) | <0.001 |
| Radiology | 0.01 (0.14) | 0.02 (0.16) | 0.02 (0.15) | 0.218 |
| Computed tomography | 0.01 (0.07) | 0.01 (0.08) | 0.01 (0.07) | 0.659 |
| Magnetic resonance | 0 (0.04) | 0 (0.04) | 0 (0.04) | 0.801 |
| Catheterization | 0 (0.03) | 0 (0.03) | 0 (0.03) | 0.924 |
| Angioplasty | 0 (0.09) | 0 (0.05) | 0 (0.07) | 0.648 |
| Endarterectomy | 0.01 (0.08) | 0.01 (0.11) | 0.01 (0.1) | 0.120 |
| Study Cohort | Adequate Anticoagulation Control | Poor Anticoagulation Control | Total | p-Value |
|---|---|---|---|---|
| N (%) | 2351 (52.4) | 2136 (47.6) | 4487 (100) | |
| Visits, mean (SD) | ||||
| Primary care visits | 367 (266) | 385 (264) | 376 (265) | 0.019 |
| Nurse visits | 243 (215) | 290 (158) | 265 (192) | <0.001 |
| Specialist care visits | 193 (120) | 204 (126) | 198 (123) | 0.002 |
| Emergency visits | 89 (238) | 99 (268) | 94 (253) | 0.203 |
| Hospitalizations | 725 (1996) | 1094 (2450) | 901 (2231) | <0.001 |
| Test, mean (SD) | ||||
| Laboratory | 1 (10) | 3 (17) | 2 (14) | <0.001 |
| Radiology | 0 (0.3) | 0 (0.3) | 0 (0.3) | 0.218 |
| Computed tomography | 0 (7) | 1 (7) | 1 (7) | 0.659 |
| Magnetic resonance | 0 (7) | 0 (7) | 0 (7) | 0.801 |
| Catheterization | 0 (5) | 0 (6) | 0 (6) | 0.924 |
| Angioplasty | 1 (17) | 1 (10) | 1 (14) | 0.648 |
| Endarterectomy | 1 (16) | 2 (20) | 2 (18) | 0.120 |
| Medication, mean (SD) | ||||
| Vitamin K antagonists | 44 (32) | 41 (30) | 42 (31) | 0.007 |
| Concomitant medication | 344 (206) | 356 (182) | 350 (195) | 0.045 |
| Total healthcare costs, mean (SD) | 2010 (2103) | 2477 (2554) | 2232 (2340) | <0.001 |
| Difference * | ||||
| Total healthcare costs, mean ** | 2015 | 2470 | −455 | <0.001 |
| 95%CI | (1920–2109) | (2371–2569) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sicras Mainar, A.; Salazar-Mendiguchía, J.; del Campo Alonso, M.I.; Echeto, A.; Vilanova Larena, D.; Comín Colet, J. Consequences of the Poor Anticoagulation Control of Patients with Non-Valvular Atrial Fibrillation Treated with Vitamin K Antagonists. J. Clin. Med. 2024, 13, 6495. https://doi.org/10.3390/jcm13216495
Sicras Mainar A, Salazar-Mendiguchía J, del Campo Alonso MI, Echeto A, Vilanova Larena D, Comín Colet J. Consequences of the Poor Anticoagulation Control of Patients with Non-Valvular Atrial Fibrillation Treated with Vitamin K Antagonists. Journal of Clinical Medicine. 2024; 13(21):6495. https://doi.org/10.3390/jcm13216495
Chicago/Turabian StyleSicras Mainar, Antoni, Joel Salazar-Mendiguchía, María Isabel del Campo Alonso, Ainara Echeto, David Vilanova Larena, and Josep Comín Colet. 2024. "Consequences of the Poor Anticoagulation Control of Patients with Non-Valvular Atrial Fibrillation Treated with Vitamin K Antagonists" Journal of Clinical Medicine 13, no. 21: 6495. https://doi.org/10.3390/jcm13216495
APA StyleSicras Mainar, A., Salazar-Mendiguchía, J., del Campo Alonso, M. I., Echeto, A., Vilanova Larena, D., & Comín Colet, J. (2024). Consequences of the Poor Anticoagulation Control of Patients with Non-Valvular Atrial Fibrillation Treated with Vitamin K Antagonists. Journal of Clinical Medicine, 13(21), 6495. https://doi.org/10.3390/jcm13216495






