Salvage Treatment for Extragonadal Germ Cell Tumours: High-Dose Chemotherapy and Autologous Stem Cell Transplantation Outcomes—A Single-Centre Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. High-Dose Chemotherapy Regimen and ASCT
2.3. Outcomes
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Akdag, G.; Alan, O.; Dogan, A.; Yuksel, Z.; Yildirim, S.; Kinikoglu, O.; Kudu, E.; Surmeli, H.; Odabas, H.; Yildirim, M.E.; et al. Outcomes of surveillance versus adjuvant treatment for patients with stage-I seminoma: A single-center experience. World J. Urol. 2023, 41, 2201–2207. [Google Scholar] [CrossRef]
- Hartmann, J.T.; Nichols, C.R.; Droz, J.P.; Horwich, A.; Gerl, A.; Fossa, S.D.; Beyer, J.; Pont, J.; Kanz, L.; Einhorn, L.; et al. Prognostic variables for response and outcome in patients with extragonadal germ-cell tumors. Ann. Oncol. 2002, 13, 1017–1028. [Google Scholar] [CrossRef]
- Rivera, C.; Arame, A.; Jougon, J.; Velly, J.F.; Begueret, H.; Dahan, M.; Riquet, M. Prognostic factors in patients with primary mediastinal germ cell tumors, a surgical multicenter retrospective study. Interact. Cardiovasc. Thorac. Surg. 2010, 11, 585–589. [Google Scholar] [CrossRef]
- Krege, S.; Beyer, J.; Souchon, R.; Albers, P.; Albrecht, W.; Algaba, F.; Bamberg, M.; Bodrogi, I.; Bokemeyer, C.; Cavallin-Ståhl, E.; et al. European consensus conference on diagnosis and treatment of germ cell cancer: A report of the second meeting of the European Germ Cell Cancer Consensus group (EGCCCG): Part I. Eur. Urol. 2008, 53, 478–496. [Google Scholar] [CrossRef]
- Schmoll, H.J. Extragonadal germ cell tumors. Ann. Oncol. 2002, 13 (Suppl. S4), 265–272. [Google Scholar] [CrossRef]
- Bokemeyer, C.; Nichols, C.R.; Droz, J.P.; Schmoll, H.J.; Horwich, A.; Gerl, A.; Fossa, S.D.; Beyer, J.; Pont, J.; Kanz, L.; et al. Extragonadal germ cell tumors of the mediastinum and retroperitoneum: Results from an international analysis. J. Clin. Oncol. 2002, 20, 1864–1873. [Google Scholar] [CrossRef]
- Fizazi, K.; Culine, S.; Droz, J.P.; Le Chevalier, T.; Ruffié, P.; Théodore, C. Primary mediastinal non-seminomatous germ-cell tumors: From clinics to biology. Bull. Cancer 1997, 84, 313–327. [Google Scholar]
- Toner, G.C.; Geller, N.L.; Lin, S.Y.; Bosl, G.J. Extragonadal and poor risk nonseminomatous germ cell tumors. Survival and prognostic features. Cancer 1991, 67, 2049–2057. [Google Scholar] [CrossRef]
- Beyer, J.; Albers, P.; Altena, R.; Aparicio, J.; Bokemeyer, C.; Busch, J.; Cathomas, R.; Cavallin-Stahl, E.; Clarke, N.W.; Claßen, J.; et al. Maintaining success, reducing treatment burden, focusing on survivorship: Highlights from the third European consensus conference on diagnosis and treatment of germ-cell cancer. Ann. Oncol. 2013, 24, 878–888. [Google Scholar] [CrossRef]
- Patrikidou, A.; Cazzaniga, W.; Berney, D.; Boormans, J.; de Angst, I.; Di Nardo, D.; Fankhauser, C.; Fischer, S.; Gravina, C.; Gremmels, H.; et al. European Association of Urology Guidelines on Testicular Cancer: 2023 Update. Eur. Urol. 2023, 84, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Loehrer, P.J., Sr.; Einhorn, L.H.; Williams, S.D. VP-16 plus ifosfamide plus cisplatin as salvage therapy in refractory germ cell cancer. J. Clin. Oncol. 1986, 4, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Loehrer, P.J., Sr.; Gonin, R.; Nichols, C.R.; Weathers, T.; Einhorn, L.H. Vinblastine plus ifosfamide plus cisplatin as initial salvage therapy in recurrent germ cell tumor. J. Clin. Oncol. 1998, 16, 2500–2504. [Google Scholar] [CrossRef]
- Adra, N.; Abonour, R.; Althouse, S.K.; Albany, C.; Hanna, N.H.; Einhorn, L.H. High-Dose Chemotherapy and Autologous Peripheral-Blood Stem-Cell Transplantation for Relapsed Metastatic Germ Cell Tumors: The Indiana University Experience. J. Clin. Oncol. 2017, 35, 1096–1102. [Google Scholar] [CrossRef]
- Kilari, D.; D’Souza, A.; Fraser, R.; Qayed, M.; Davila, O.; Agrawal, V.; Diaz, M.A.; Chhabra, S.; Cerny, J.; Copelan, E.; et al. Autologous Hematopoietic Stem Cell Transplantation for Male Germ Cell Tumors: Improved Outcomes over 3 Decades. Biol. Blood Marrow Transpl. 2019, 25, 1099–1106. [Google Scholar] [CrossRef]
- Sharma, A.; Babra, D.S.; Joshi, P.V.; Hall, M.; Gogbashian, A.; Vasdev, N.; Joseph, M.; Yazdan, A.; Kanfer, E. Survival Outcomes After High-dose Chemotherapy and Stem Cell Transplantation in the Salvage Setting for Relapsed or Refractory Germ Cell Cancers. In Vivo 2020, 34, 3675–3679. [Google Scholar] [CrossRef]
- Chovanec, M.; Adra, N.; Abu Zaid, M.; Abonour, R.; Einhorn, L. High-dose chemotherapy for relapsed testicular germ cell tumours. Nat. Rev. Urol. 2023, 20, 217–225. [Google Scholar] [CrossRef]
- De Giorgi, U.; Richard, S.; Badoglio, M.; Kanfer, E.; Bourrhis, J.H.; Nicolas-Virelizier, E.; Vettenranta, K.; Lioure, B.; Martin, S.; Dreger, P.; et al. Salvage high-dose chemotherapy in female patients with relapsed/refractory germ-cell tumors: A retrospective analysis of the European Group for Blood and Marrow Transplantation (EBMT). Ann. Oncol. 2017, 28, 1910–1916. [Google Scholar] [CrossRef]
- Saxman, S.B.; Nichols, C.R.; Einhorn, L.H. Salvage chemotherapy in patients with extragonadal nonseminomatous germ cell tumors: The Indiana University experience. J. Clin. Oncol. 1994, 12, 1390–1393. [Google Scholar] [CrossRef]
- Broun, E.R.; Nichols, C.R.; Einhorn, L.H.; Tricot, G.J. Salvage therapy with high-dose chemotherapy and autologous bone marrow support in the treatment of primary nonseminomatous mediastinal germ cell tumors. Cancer 1991, 68, 1513–1515. [Google Scholar] [CrossRef]
- Feldman, D.R.; Sheinfeld, J.; Bajorin, D.F.; Fischer, P.; Turkula, S.; Ishill, N.; Patil, S.; Bains, M.; Reich, L.M.; Bosl, G.J.; et al. TI-CE high-dose chemotherapy for patients with previously treated germ cell tumors: Results and prognostic factor analysis. J. Clin. Oncol. 2010, 28, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Beyer, J.; Kramar, A.; Mandanas, R.; Linkesch, W.; Greinix, A.; Droz, J.P.; Pico, J.L.; Diehl, A.; Bokemeyer, C.; Schmoll, H.J.; et al. High-dose chemotherapy as salvage treatment in germ cell tumors: A multivariate analysis of prognostic variables. J. Clin. Oncol. 1996, 14, 2638–2645. [Google Scholar] [CrossRef] [PubMed]
- Pico, J.L.; Rosti, G.; Kramar, A.; Wandt, H.; Koza, V.; Salvioni, R.; Theodore, C.; Lelli, G.; Siegert, W.; Horwich, A.; et al. A randomised trial of high-dose chemotherapy in the salvage treatment of patients failing first-line platinum chemotherapy for advanced germ cell tumours. Ann. Oncol. 2005, 16, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, U.; Demirer, T.; Wandt, H.; Taverna, C.; Siegert, W.; Bornhauser, M.; Kozak, T.; Papiani, G.; Ballardini, M.; Rosti, G. Second-line high-dose chemotherapy in patients with mediastinal and retroperitoneal primary non-seminomatous germ cell tumors: The EBMT experience. Ann. Oncol. 2005, 16, 146–151. [Google Scholar] [CrossRef]
- Richardson, N.H.; Taza, F.; Abonour, R.; Althouse, S.K.; Ashkar, R.; Abu Zaid, M.; Hanna, N.H.; Kesler, K.A.; Adra, N.; Einhorn, L.H. High-dose chemotherapy and peripheral blood stem cell transplantation as salvage therapy in primary mediastinal nonseminomatous germ cell tumors: The Indiana University experience. Cancer 2024, 130, 3115–3122. [Google Scholar] [CrossRef]
- Josefsen, D.; Ous, S.; Høie, J.; Stenwig, A.E.; Fosså, S.D. Salvage treatment in male patients with germ cell tumours. Br. J. Cancer 1993, 67, 568–572. [Google Scholar] [CrossRef][Green Version]
- Kumano, M.; Miyake, H.; Hara, I.; Furukawa, J.; Takenaka, A.; Fujisawa, M. First-line high-dose chemotherapy combined with peripheral blood stem cell transplantation for patients with advanced extragonadal germ cell tumors. Int. J. Urol. 2007, 14, 336–338. [Google Scholar] [CrossRef]
- Rosti, G.; De Giorgi, U.; Wandt, H.; Lioure, B.; Leyvraz, S.; Kolbe, K.; Papiani, G.; Ballardini, M.; Kulekci, A.; Demirer, T. First-line high-dose chemotherapy for patients with poor prognosis extragonadal germ cell tumors: The experience of the European Bone Marrow Transplantation (EBMT) Solid Tumors Working Party. Bone Marrow Transpl. 2004, 34, 1033–1037. [Google Scholar] [CrossRef][Green Version]
- Motzer, R.J.; Geller, N.L.; Tan, C.C.; Herr, H.; Morse, M.; Fair, W.; Sheinfeld, J.; Sogani, P.; Russo, P.; Bosl, G.J. Salvage chemotherapy for patients with germ cell tumors. The Memorial Sloan-Kettering Cancer Center experience (1979–1989). Cancer 1991, 67, 1305–1310. [Google Scholar] [CrossRef]
- Beyer, J.; Collette, L.; Sauvé, N.; Daugaard, G.; Feldman, D.R.; Tandstad, T.; Tryakin, A.; Stahl, O.; Gonzalez-Billalabeitia, E.; De Giorgi, U.; et al. Survival and New Prognosticators in Metastatic Seminoma: Results From the IGCCCG-Update Consortium. J. Clin. Oncol. 2021, 39, 1553–1562. [Google Scholar] [CrossRef]
- Vaena, D.A.; Abonour, R.; Einhorn, L.H. Long-term survival after high-dose salvage chemotherapy for germ cell malignancies with adverse prognostic variables. J. Clin. Oncol. 2003, 21, 4100–4104. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.A.; Weickhardt, A.; Grimison, P.; Asher, R.; Heller, G.Z.; Lewin, J.; Liow, E.; Toner, G.; Tung, I.L.Y.; Tran, B.; et al. High-dose chemotherapy for relapsed germ cell tumours: Outcomes in low-volume specialized centres. BJU Int. 2022, 130 (Suppl. S1), 5–16. [Google Scholar] [CrossRef] [PubMed]
- Lorch, A.; Bascoul-Mollevi, C.; Kramar, A.; Einhorn, L.; Necchi, A.; Massard, C.; De Giorgi, U.; Fléchon, A.; Margolin, K.; Lotz, J.P.; et al. Conventional-dose versus high-dose chemotherapy as first salvage treatment in male patients with metastatic germ cell tumors: Evidence from a large international database. J. Clin. Oncol. 2011, 29, 2178–2184. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, T.D.; Seidenfeld, J.; Basch, E.M.; Einhorn, L.H.; Fancher, T.; Smith, D.C.; Stephenson, A.J.; Vaughn, D.J.; Cosby, R.; Hayes, D.F. American Society of Clinical Oncology Clinical Practice Guideline on uses of serum tumor markers in adult males with germ cell tumors. J. Clin. Oncol. 2010, 28, 3388–3404. [Google Scholar] [CrossRef]
- Rodney, A.J.; Tannir, N.M.; Siefker-Radtke, A.O.; Liu, P.; Walsh, G.L.; Millikan, R.E.; Swisher, S.G.; Tu, S.M.; Pagliaro, L.C. Survival outcomes for men with mediastinal germ-cell tumors: The University of Texas M. D. Anderson Cancer Center experience. Urol. Oncol. 2012, 30, 879–885. [Google Scholar] [CrossRef]
- McHugh, D.J.; Feldman, D.R. Conventional-Dose versus High-Dose Chemotherapy for Relapsed Germ Cell Tumors. Adv. Urol. 2018, 2018, 7272541. [Google Scholar] [CrossRef]
- Hainsworth, J.D.; Einhorn, L.H.; Williams, S.D.; Stewart, M.; Greco, F.A. Advanced extragonadal germ-cell tumors. Successful treatment with combination chemotherapy. Ann. Intern. Med. 1982, 97, 7–11. [Google Scholar] [CrossRef]
- Einhorn, L.H.; Williams, S.D.; Chamness, A.; Brames, M.J.; Perkins, S.M.; Abonour, R. High-dose chemotherapy and stem-cell rescue for metastatic germ-cell tumors. N. Engl. J. Med. 2007, 357, 340–348. [Google Scholar] [CrossRef]
| Age | Histology | Location | Tumour Markers at HDCT (IU/L) | IPFSG Group | Outcomes | PFS (Months) | OS (Months) | Status | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 21 | Teratoma | Mediastinum | AFP: 2.75 B-HCG: 0.6 | very high | PD | 3 | 7.1 | DOD |
| 2 | 25 | Yolk sac | Mediastinum | AFP: 429 B-HCG: 0.5 | very high | PD | 2.4 | 12.2 | DOD |
| 3 | 28 | Mixed Germ cell | Retroperitoneum | AFP: 1.3 B-HCG: 0.7 | high risk | PR | 10.6 | 10.6 | DOD |
| 4 | 34 | Yolk sac | Retroperitoneum | AFP: 2232 B-HCG: 0.1 | very high | PD | 2.4 | 6.1 | DOD |
| 5 | 22 | Seminoma | Mediastinum | AFP: 3.1 B-HCG: 0.5 | high risk | CR | 4.1 | 9.4 | DOD |
| 6 * | 29 | Yolk sac | Mediastinum | AFP: 33 B-HCG: 1.54 | very high | PR | 12.5 | 16.1 | DOD |
| 7 | 26 | Mixed Germ cell | Mediastinum | AFP: 4 B-HCG: 4.23 | very high | PD | 14.1 | 16 | DOD |
| 8 | 31 | Mixed Germ cell | Retroperitoneum | AFP: 3.3 B-HCG: 0.2 | intermediate | PR | 10.2 | 10.2 | PD |
| 9 | 45 | Mixed Germ cell | Retroperitoneum | AFP: 3.53 B-HCG: 4.33 | high risk | PR | 7.3 | 7.3 | PD |
| 10 | 28 | Mixed Germ cell | Mediastinum | AFP: 81.5 B-HCG: 0.5 | very high | CR | 19 | 21.8 | DOD |
| 11 | 48 | Mixed Germ cell | Retroperitoneum | AFP: 2.5 B-HCG: 8950 | very high | PR | 9.8 | 9.8 | DOD |
| 12 | 31 | Mixed Germ cell | Mediastinum | AFP: 6900 B-HCG: 0.5 | very high | PD | 1.4 | 3.4 | DOD |
| 13 | 27 | Yolk sac | Retroperitoneum | AFP: 3.4 B-HCG: 1.2 | intermediate | CR | 4.5 | 23.8 | PD |
| 14 | 25 | Choriocarcinoma | Mediastinum | AFP: 1.05 B-HCG: 100,000 | very high | PD | 2.4 | 2.4 | DOD |
| 15 | 31 | Mixed Germ cell | Retroperitoneum | AFP: 10 B-HCG: 13,800 | intermediate | PD | 2.6 | 4.9 | DOD |
| 16 * | 32 | Embryonal Carcinoma | Retroperitoneum | AFP: 3.54 B-HCG: 0.1 | intermediate | PR | 7.8 | 24.1 | DOD |
| 17 | 25 | Mixed Germ cell | Retroperitoneum | AFP: 4.84 B-HCG: 2.22 | intermediate | CR | 25.4 | 25.4 | NED |
| 18 | 32 | Yolk sac | Retroperitoneum | AFP: 115 B-HCG: 0.1 | intermediate | CR | 65 | 65 | NED |
| 19 | 22 | Mixed Germ cell | Brain | AFP: 7 B-HCG: 241 | high risk | CR | 5 | 24 | DOD |
| 20 * | 27 | Mixed Germ cell | Retroperitoneum | AFP: 4 B-HCG: 0.5 | intermediate | PR | 4.8 | 30 | DOD |
| 21 | 25 | Yolk sac | Mediastinum | AFP: 9 B-HCG: 1 | very high | PR | 6.1 | 6.1 | DOD |
| 22 | 25 | Mixed Germ cell | Retroperitoneum | AFP: 30.7 B-HCG: 0.5 | high risk | PR | 3.6 | 14 | DOD |
| 23 | 34 | Mixed Germ cell | Retroperitoneum | AFP: 10 B-HCG: 1.3 | intermediate | CR | 11 | 14 | PD |
| 24 | 43 | Choriocarcinoma | Mediastinum | AFP: 2.4 B-HCG: 19,991 | very high | PD | 1.9 | 1.9 | DOD |
| 25 * | 46 | Teratoma | Mediastinum | AFP: 7.3 B-HCG: 0.7 | very high | SD | 7.2 | 12 | DOD |
| n, (%) | ||
|---|---|---|
| Number of Patients, n | 25 (100) | |
| Age, Median | 28 (21–48) | |
| Gender | Male | 25 (100) |
| Tumour Location | Retroperitoneum | 13 (52) |
| Mediastineum | 11 (44) | |
| Brain | 1 (4) | |
| Histology | Seminoma | 1 (4) |
| Yolk Sac | 6 (24) | |
| Embryonal Carcinoma | 1 (4) | |
| Choriocarcinoma | 2(8) | |
| Teratoma | 2 (8) | |
| Mixed Germ Cell Tumour | 13 (52) | |
| Metastatic Site | Retroperitoneal Lymph Node | 17 (68) |
| Lung | 12 (48) | |
| Liver | 3 (12) | |
| Brain | 2 (8) | |
| Bone | 1 (4) | |
| IPFSG Classification | Low | 0 (0) |
| Intermediate | 8 (32) | |
| High | 5 (20) | |
| Very High | 12 (48) | |
| Platin Sensitivity | Sensitive | 18 (72) |
| refractory | 7 (28) | |
| AFP Before HDCT | <1000 IU/L | 23 (92) |
| ≥1000 IU/L | 2 (8) | |
| Beta-HCG Before HDCT | <1000 IU/L | 21 (84) |
| ≥1000 IU/L | 4 (16) | |
| Number of Treatment Lines Before HDCT | 1 | 24 (96) |
| ≥2 | 1 (4) | |
| Tumour Response After HDCT | CR | 7 (28) |
| PR | 9 (36) | |
| SD | 1 (4) | |
| PD | 8 (32) | |
| ORR After HDCT | Present | 16 (64) |
| Absent | 9 (36) | |
| DCR | Present | 17 (68) |
| Absent | 8 (32) | |
| CR STATUS | CR | 7 (28) |
| Non-CR | 18 (72) | |
| STATUS | Alive | 6 (24) |
| Dead | 19 (76) | |
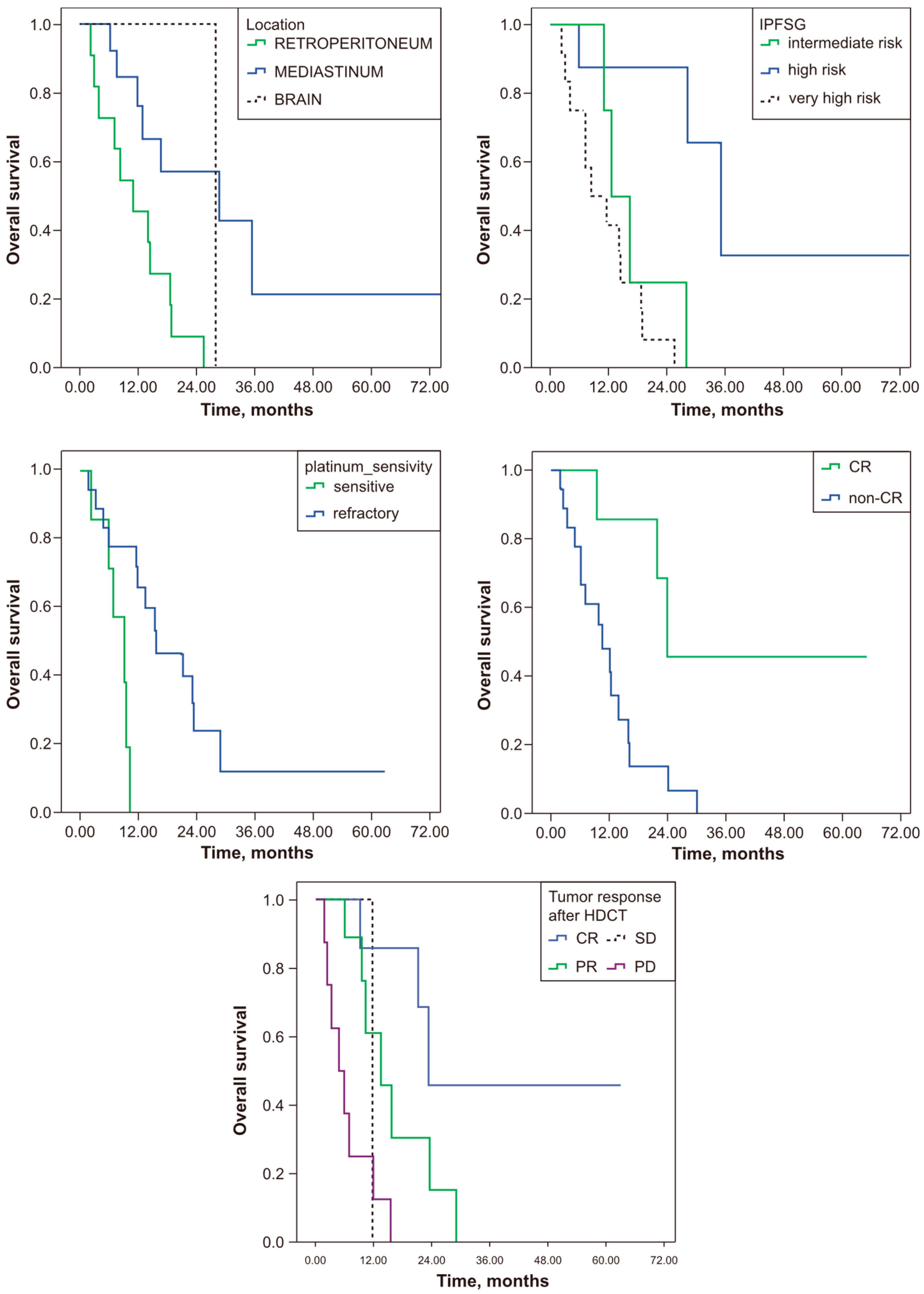
| Median PFS (Months) (95% CI) | p | OS (Months) (95% CI) | p | ||
|---|---|---|---|---|---|
| PFS, OS (Months) | 6.1 (2.28–10.0) | 12.25 (6.51–17.99) | |||
| Tumour Location | |||||
| Retroperitoneum | 9.79 (1.90–17.67) | 0.35 | 24.14 (12.72–42.83) | 0.013 | |
| Mediastineum | 4.41 (0.13–8.14) | 9.42 (6.06–13.64) | |||
| Brain | 4.92 (4.92–4.92) | 23.95 (23.95–23.95) | |||
| Histology | |||||
| Seminoma | 4.14 (4.14–4.14) | 0.023 | 9.42 (9.42–9.42) | <0.0001 | |
| Yolk Sac | 4.46 (0.12–8.92) | 12.25 (7.55–49.27) | |||
| Embryonal Carcinoma | 7.75 (7.75–7.75) | 24.14 (24.14–24.14) | |||
| Choriocarcinoma | 1.87 (1.87–1.87) | 1.87 (1.87–1.87) | |||
| Teratoma | 2.95 (2.95–2.95) | 7.09 (4.73–14.39) | |||
| Mixed Germ Cell Tumour | 10.61 (2.12–19.09) | 15.90 (5.34–26.46) | |||
| Metastatic Site | |||||
| Retroperitoneal Lymph Node | Present | 9.79 (3.02–16.55) | 0.034 | 15.90 (3.17–28.63) | 0.027 |
| Absent | 2.43 (0.0–5.25) | 6.14 (0.0–18.16) | |||
| Lung | Present | 4.46 (1.06–7.87) | 0.36 | 9.42 (4.00–14.85) | 0.059 |
| Absent | 7.19 (3.97–10.41) | 23.95 (5.87–42.03) | |||
| Liver | Present | 2.95 (2.43–3.48) | 0.23 | 7.09 (3.57–10.61) | 0.023 |
| Absent | 6.14 (2.87–9.41) | 15.90 (10.26–27.73) | |||
| Brain | Present | 4.92 (4.92–4.92) | 0.86 | 10.61 (4.20–30.35) | 0.91 |
| Absent | 6.14 (1.87–10.41) | 13.89 (8.67–19.12) | |||
| Bone | Present | 2.43 (2.43–2.43) | 0.08 | 12.25 (12.25–12.25) | 0.68 |
| Absent | 6.14 (2.72–9.56) | 13.89 (10.77–26.29) | |||
| IPFSG Classification | |||||
| Low | 0 (0–0) | 0.23 | 0 (0–0) | 0.001 | |
| Intermediate | 7.75 (0.37–15.13) | 29.69 (21.12–38.8) | |||
| High | 4.92 (3.23–6.62) | 10.61 (6.23–14.99) | |||
| Very High | 2.95 (0.0–9.25) | 7.09 (0.90–13.28) | |||
| Platin Sensitivity | |||||
| Sensitive | 6.14 (1.43–10.85) | 0.26 | 16.09 (6.51–25.68) | 0.004 | |
| Refractory | 4.14 (1.10–7.17) | 9.42 (4.64–14.21) | |||
| AFP Before HDCT | |||||
| <1000 IU/L | 7.19 (2.91–11.47) | <0.0001 | 13.9 (8.51–19.27) | 0.003 | |
| ≥1000 IU/L | 1.38 (1.38–1.38) | 3.35 (3.35–3.35) | |||
| Beta-HCG Before HDCT | |||||
| <1000 IU/L | 7.19 (3.12–11.26) | 0.039 | 15.9(10.90–20.90) | <0.0001 | |
| ≥1000 IU/L | 2.43 (1.69–3.17) | 2.43 (0–5.39) | |||
| Number of Lines Before HDCT | |||||
| 1 | 4.92 (1.65–8.20) | 0.9 | 12.25 (7.49–17.01) | 0.61 | |
| ≥2 | 7.75 (7.75–7.75) | 24.18 (24.18–24.18) | |||
| Tumour Response After HDCT | |||||
| CR | 11.03 (0.0–26.72) | 0.012 | 23.95 (23.95–23.95) | <0.0001 | |
| PR | 9.79 (5.13–14.45) | 13.89 (7.22–20.56) | |||
| SD | 7.19 (7.19–7.19) | 12.02 (12.02–12.02) | |||
| PD | 2.43 (2.34–2.51) | 4.89 (1.11–8.67) | |||
| CR Status | |||||
| CR | 11.03 (0.0–26.72) | 0.049 | 23.95 (23.95–23.95) | 0.009 | |
| Non-CR | 3.54 (0–10.22) | 10.61 (6.72–14.49) | |||
| DCR | |||||
| Present | 9.79 (5.79–13.78) | 0.001 | 21.78 (11.11–32.45) | <0.0001 | |
| Absent | 2.43 (2.34–2.52) | 4.89 (1.11–8.67) | |||
| ORR After HDCT | |||||
| Present | 9.79 (5.03–14.55) | 0.003 | 21.78 (10.06–33.50) | <0.0001 | |
| Absent | 2.43 (2.33–2.52) | 6.07 (2.62–9.53) | |||
| Median Follow-up Time (Months) | 25.42 | ||||
| 12 Months PFS% | 25 | ||||
| 24 Months PFS% | 10 | ||||
| 12 Months OS% | 59 | ||||
| 24 Months OS% | 30 | ||||
| CR | Non-CR | p | ||
|---|---|---|---|---|
| n, % | n, % | |||
| Location | Retroperitoneum | 4 (16) | 9 (36) | 0.2 |
| Mediastineum | 2 (8) | 9 (36) | ||
| Brain | 1 (4) | 0 (0) | ||
| Histology | Seminoma | 1 (4) | 0 (0) | 0.46 |
| Yolk Sac | 2 (8) | 4 (16) | ||
| Embryonal Carcinoma | 0 (0) | 1 (4) | ||
| Choriocarcinoma | 0 (0) | 2 (8) | ||
| Teratoma | 0 (0) | 2 (8) | ||
| Mixed Germ Cell Tumour | 4 (16) | 9 (36) | ||
| Number of lines before HDCT | 1 | 7 (28) | 17 (68) | 0.72 |
| ≥2 | 0 (0) | 1 (4) | ||
| IPFSG Classification | Low | 0 (0) | 0 (0) | 0.1 |
| Intermediate | 4 (16) | 4 (16) | ||
| High | 2 (8) | 3 (12) | ||
| Very High | 1 (4) | 11 (44) | ||
| AFP Before HDCT | <1000 IU/L | 7 (28) | 16 (64) | 0.51 |
| ≥1000 IU/L | 0 (0) | 2 (8) | ||
| Beta-HCG Before HDCT | <1000 IU/L | 7 (28) | 14 (68) | 0.29 |
| ≥1000 IU/L | 0 (0) | 4 (16) | ||
| Metastatic site | Retroperitoneal Lymph Node | 6 (24) | 11 (44) | 0.24 |
| Lung | 3 (12) | 9 (36) | 0.55 | |
| Liver | 0 (0) | 3 (12) | 0.35 | |
| Brain | 1 (4) | 1 (4) | 0.49 | |
| Bone | 0 (0) | 1 (4) | 0.52 | |
| Platinum Sensitivity | Sensitive | 6 (24) | 12 (48) | 0.33 |
| Refractory | 1 (4) | 6 (24) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Topal, A.; Erturk, I.; Koseoglu, C.; Dumludag, A.; Kuzu, Ö.F.; Karadurmus, B.; Kaplan Tuzun, E.; Atacan, H.; Mammadzada, N.; Yildirim, G.; et al. Salvage Treatment for Extragonadal Germ Cell Tumours: High-Dose Chemotherapy and Autologous Stem Cell Transplantation Outcomes—A Single-Centre Experience. J. Clin. Med. 2024, 13, 6494. https://doi.org/10.3390/jcm13216494
Topal A, Erturk I, Koseoglu C, Dumludag A, Kuzu ÖF, Karadurmus B, Kaplan Tuzun E, Atacan H, Mammadzada N, Yildirim G, et al. Salvage Treatment for Extragonadal Germ Cell Tumours: High-Dose Chemotherapy and Autologous Stem Cell Transplantation Outcomes—A Single-Centre Experience. Journal of Clinical Medicine. 2024; 13(21):6494. https://doi.org/10.3390/jcm13216494
Chicago/Turabian StyleTopal, Alper, Ismail Erturk, Caglar Koseoglu, Aysegul Dumludag, Ömer Faruk Kuzu, Berkan Karadurmus, Esmanur Kaplan Tuzun, Huseyin Atacan, Nurlan Mammadzada, Gizem Yildirim, and et al. 2024. "Salvage Treatment for Extragonadal Germ Cell Tumours: High-Dose Chemotherapy and Autologous Stem Cell Transplantation Outcomes—A Single-Centre Experience" Journal of Clinical Medicine 13, no. 21: 6494. https://doi.org/10.3390/jcm13216494
APA StyleTopal, A., Erturk, I., Koseoglu, C., Dumludag, A., Kuzu, Ö. F., Karadurmus, B., Kaplan Tuzun, E., Atacan, H., Mammadzada, N., Yildirim, G., Acar, R., & Karadurmus, N. (2024). Salvage Treatment for Extragonadal Germ Cell Tumours: High-Dose Chemotherapy and Autologous Stem Cell Transplantation Outcomes—A Single-Centre Experience. Journal of Clinical Medicine, 13(21), 6494. https://doi.org/10.3390/jcm13216494






