Impact of the Prepectoral Breast Reconstruction Assessment Score on Expander-Based Reconstruction Success
Abstract
1. Introduction
2. Materials and Methods
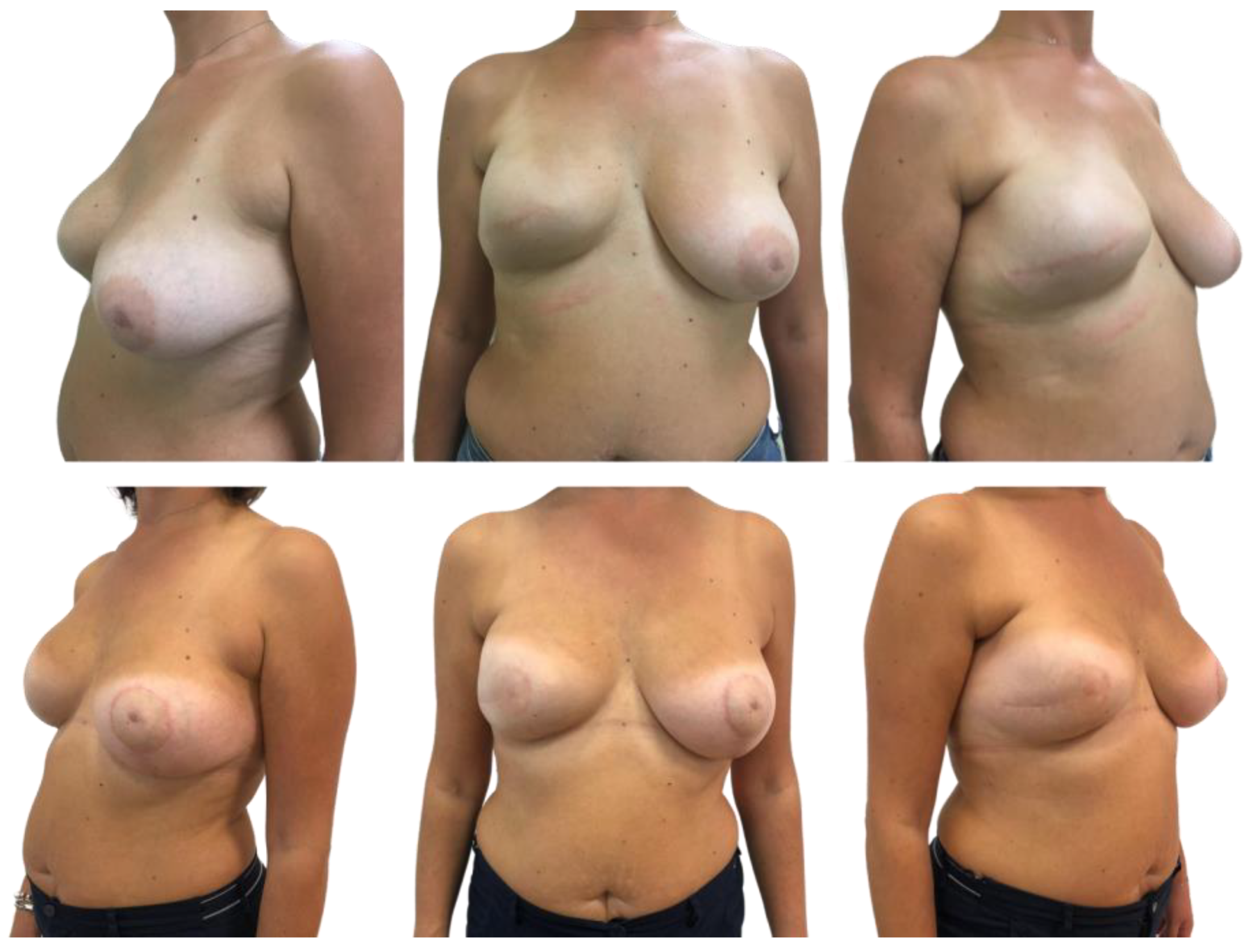
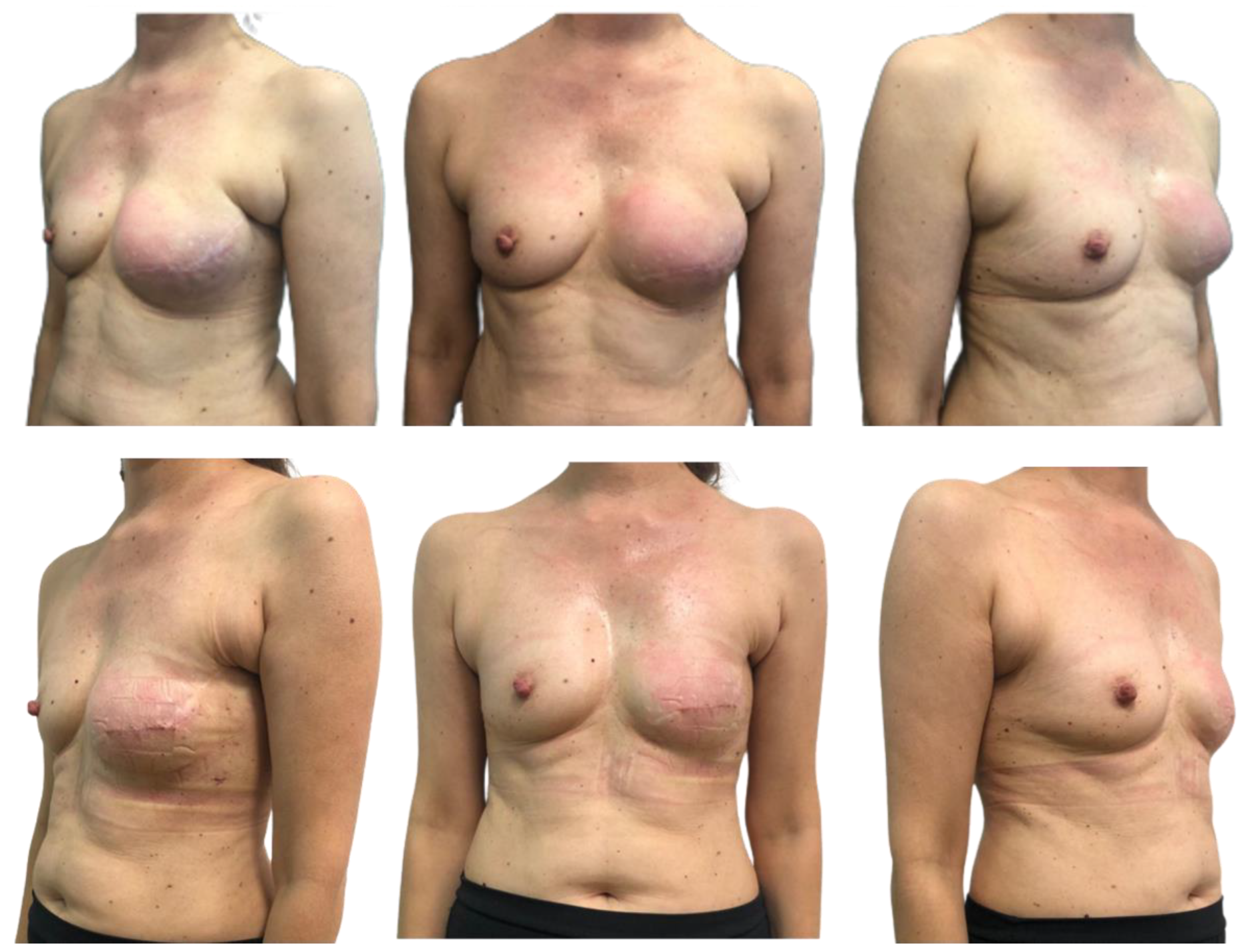
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lucas, D.J.; Sabino, J.; Shriver, C.D.; Pawlik, T.M.; Singh, D.P.; Vertrees, A.E. Doing more: Trends in breast cancer surgery, 2005 to 2011. Am. Surg. 2015, 81, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Jagsi, R.; Jiang, J.; Momoh, A.O.; Alderman, A.; Giordano, S.H.; Buchholz, T.A.; Kronowitz, S.J.; Smith, B.D. Trends and variation in use of breast reconstruction in patients with breast cancer undergoing mastectomy in the United States. J. Clin. Oncol. 2014, 32, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.G.B.; Irri, R.M.; MacCallum, V.; Chattopadhyay, R.M.; Murphy, J.F.; Harvey, J.R.F. A prospective comparison of short-term outcomes of subpectoral and prepectoral strattice-based immediate breast reconstruction. Plast. Reconstr. Surg. 2018, 141, 1077–1084. [Google Scholar] [CrossRef]
- Bernini, M.; Calabrese, C.; Cecconi, L.; Santi, C.; Gjondedaj, U.; Roselli, J.; Nori, J.; Fausto, A.; Orzalesi, L.; Casella, D. Subcutaneous direct-to-implant breast reconstruction: Surgical, functional, and aesthetic results after long-term follow-up. Plast. Reconstr. Surg. Glob. Open 2015, 3, e574. [Google Scholar] [CrossRef]
- Nahabedian, M.Y.; Cocilovo, C. Two-stage prosthetic breast reconstruction: A comparison between prepectoral and partial subpectoral techniques. Plast. Reconstr. Surg. 2017, 140, 22S–30S. [Google Scholar] [CrossRef]
- Casella, D.; Torto, F.L.; Marcasciano, M.; Barellini, L.; Frattaroli, J.M.; Turriziani, G.; Ribuffo, D. Breast Animation Deformity: A Ret-rospective Study on Long-Term and Patient-Reported Breast-Q Outcomes. Ann. Plast. Surg. 2021, 86, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Snyderman, R.K.; Starzynski, T. Breast Reconstruction. Surg. Clin. N. Am. 1969, 49, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Gruber, R.P.; Kahn, R.A.; Lash, H.; Maser MR Apfelberg, D.B.; Laub, D.R. Breast reconstruction following mastectomy: A comparison ofsubmuscular and subcutaneous techniques. Plast. Reconstr. Surg. 1981, 67, 312–317. [Google Scholar] [CrossRef]
- Torto, F.L.; Marcasciano, M.; Kaciulyte, J.; Redi, U.; Barellini, L.; De Luca, A.; Perra, A.; Frattaroli, J.M.; Cavalieri, E.; Di Taranto, G.; et al. Prepectoral breast reconstruction with TiLoop® Bra Pocket: A single center prospective study. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Breuing, K.H.; Colwell, A.S. Immediate Breast Tissue Expander-Implant Reconstruction with Inferolateral AlloDerm Hammock and Postoperative Radiation: A Preliminary Report. ePlasty 2009, 9, e16. [Google Scholar]
- Murphy, D.M.; O’donnell, J.P.M.; Ryan, J.; O’neill, B.L.; Boland, M.R.M.M.; Lowery, A.J.; Kerin, M.J.F.; McInerney, N.M. Immediate Breast Cancer Reconstruction with or without Dermal Matrix or Synthetic Mesh Support: A Review and Network Meta-Analysis. Plast. Reconstr. Surg. 2023, 151, 563e–574e. [Google Scholar] [CrossRef] [PubMed]
- Nava, M.B.; Catanuto, G.; Pennati, A.; Garganese, G.; Spano, A. Conservative Mastectomies. Aesthetic Plast. Surg. 2009, 33, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Rancati, A.; Angrigiani, C.; Hammond, D.; Nava, M.; Gonzalez, E.; Rostagno, R.; Gercovich, G. Preoperative digital mammography imaging in conservative mastectomy and immediate reconstruction. Gland Surg. 2016, 5, 9–14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J.; Han, M.W.; Hong, K.Y. Prospective Clinical Trial for Predicting Mastectomy Skin Flap Necrosis with Indocyanine Green Angiography in Implant-Based Prepectoral Breast Reconstruction. Aesthetic Plast. Surg. 2024; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Barellini, L.; Marcasciano, M.; Torto, F.L.; Fausto, A.; Ribuffo, D.; Casella, D. Intraoperative Ultrasound and Oncoplastic Combined Approach: An Additional Tool for the Oncoplastic Surgeon to Obtain Tumor-Free Margins in Breast Conservative Surgery—A 2-Year Single-Center Prospective Study. Clin. Breast Cancer 2020, 20, e290–e294. [Google Scholar] [CrossRef] [PubMed]
- Ribuffo, D.; Berna, G.; De Vita, R.; Di Benedetto, G.; Cigna, E.; Greco, M.; Valdatta, L.; Onesti, M.G.; Torto, F.L.; Marcasciano, M.; et al. Dual-Plane Retro-pectoral Versus Pre-pectoral DTI Breast Reconstruction: An Italian Multicenter Experience. Aesthetic Plast. Surg. 2021, 45, 51–60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roostaeian, J.; Sanchez, I.; Vardanian, A.; Herrera, F.; Galanis, C.; Da Lio, A.; Festekjian, J.; Crisera, C.A. Comparison of immediate implant placement versus the staged tissue expander technique in breast reconstruction. Plast. Reconstr. Surg. 2012, 129, 909e–918e. [Google Scholar] [CrossRef] [PubMed]
- Casella, D.; Di Taranto, G.; Marcasciano, M.; Torto, F.L.; Barellini, L.; Sordi, S.; Gaggelli, I.; Roncella, M.; Calabrese, C.; Ribuffo, D. Subcutaneous expanders and synthetic mesh for breast reconstruction: Long-term and patient-reported BREAST-Q outcomes of a single-center prospective study. J. Plast. Reconstr. Aesthetic Surg. 2019, 72, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Sigalove, S.; Maxwell, G.P.; Sigalove, N.M.; Storm-Dickerson, T.L.; Pope, N.; Rice, J.; Gabriel, A. Prepectoral Implant-Based Breast Reconstruction: Rationale, Indications, and Preliminary Results. Plast. Reconstr. Surg. 2017, 139, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Casella, D.M.; Kaciulyte, J.; Torto, F.L.; Mori, F.L.R.; Barellini, L.; Fausto, A.; Fanelli, B.; Greco, M.; Ribuffo, D.M.; Marcasciano, M.M. “To Pre or Not to Pre”: Introduction of a Prepectoral Breast Reconstruction Assessment Score to Help Surgeons Solving the Decision-Making Dilemma. Retrospective Results of a Multicenter Experience. Plast. Reconstr. Surg. 2021, 147, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Casella, D.M.; Di Taranto, G.; Torto, F.L.; Marcasciano, M.; Kaciulyte, J.; Greco, M.; Onesti, M.G.; Ribuffo, D. Body mass index can predict outcomes in direct-to-implant prepectoral breast reconstruction. Plast. Reconstr. Surg. 2020, 145, 867e–868e. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.J.; Park, J.G.; Maus, J.C.; Motamedi, V.B.; Rebowe, R.E.; Runyan, C.M.; Tucker, S.L. Prepectoral Versus Subpectoral Breast Reconstruction in High–Body Mass Index Patients. Ann. Plast. Surg. 2021, 87, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, A.; Sigalove, S.; Sigalove, N.M.; Storm-Dickerson, T.L.; Pope, N.; Rice, J.; Maxwell, G.P. Effect of Body Mass Index on Outcomes after Prepectoral Breast Reconstruction. Plast. Reconstr. Surg. 2019, 144, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Nava, M.B.; Ottolenghi, J.; Pennati, A.; Spano, A.; Bruno, N.; Catanuto, G.; Boliglowa, D.; Visintini, V.; Santoro, S.; Folli, S. Skin/nipple sparing mastectomies and implant-based breast reconstruction in patients with large and ptotic breast: Oncological and reconstructive results. Breast 2012, 21, 267–271. [Google Scholar] [CrossRef]
- Maruccia, M.; Elia, R.; Nacchiero, E.; Giudice, G. Skin Reducing Mastectomy and Prepectoral Breast Reconstruction in Large Ptotic Breasts. Aesthetic Plast. Surg. 2021, 45, 1357–1358. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.; Ho, K.; Ike, C.; Wood, S.H.; Thiruchelvam, P.T.R.; Khan, A.; Leff, D.R. Pre-operative chemoradiotherapy followed by mastectomy and breast reconstruction—A systematic review of clinical, oncological, reconstructive and aesthetic outcomes. J. Plast. Reconstr. Aesthetic Surg. 2024, 96, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Ostapenko, E.; Nixdorf, L.; Devyatko, Y.; Exner, R.; Wimmer, K.; Fitzal, F. The Impact of Adjuvant Radiotherapy on Immediate Prepectoral Implant-Based Breast Reconstruction. Aesthetic Plast. Surg. 2024, 48, 2432–2438. [Google Scholar] [CrossRef] [PubMed]
- Nava, M.B.; Catanuto, G.; Pennati, A.; Cividin, V.V.; Spano, A. Expander-implants breast reconstruction. In Plastic Surgery, 3rd ed.; Neligan, P.C., Ed.; Elsevier Ltd.: New York, NY, USA, 2013; pp. 336–369. [Google Scholar]
- Spear, S.L.; Boehmler, J.H.; Bogue, D.P.; Mafi, A.A. Options in reconstructing the irradiated breast. Plast. Reconstr. Surg. 2008, 122, 379–388. [Google Scholar] [CrossRef]
- Steven, J.; Kronowitz, M.D.; Geoffrey, L.; Robb, M.D. Radiation therapy and breast reconstruction: A critical review of the literature. Plast. Reconstr. Surg. 2009, 124, 395–408. [Google Scholar]
- Lo Torto, F.; Vaia, N.; Ribuffo, D. Postmastectomy Radiation Therapy and Two-Stage Implant-Based Breast Reconstruction: Is There a Better Time to Irradiate? Plast. Reconstr. Surg. 2017, 139, 1364e–1365e. [Google Scholar] [CrossRef] [PubMed]
- Ribuffo, D.; Torto, F.L.; Atzeni, M.; Serratore, F. The effects of postmastectomy adjuvant radiotherapy on immediate two-stage prosthetic breast reconstruction: A systematic review. Plast. Reconstr. Surg. 2015, 135, 445e. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, N.; Secanho, M.; Kokosis, G.; Malgor, R.D.; Winocour, J.; Yu, J.W.; Mathes, D.W.; Kaoutzanis, C. Capsular contracture in breast reconstruction: A systematic review and meta-analysis. J. Plast. Reconstr. Aesthetic Surg. 2024, 98, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.T.; Lanier, S.T.; Conkling, N. Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: Results of a prospective trial. Plast. Reconstr. Surg. 2012, 129, 778e–788e. [Google Scholar] [CrossRef] [PubMed]
- Singer, R.; Lewis, C.M.; Franklin, J.D.; Lynch, J.B. Fluorescein test for prediction of flap viability during breast reconstructions. Plast. Reconstr. Surg. 1978, 61, 371–375. [Google Scholar] [CrossRef]
- Losken, A.; Styblo, T.M.; Schaefer, T.G.; Carlson, G.W. The use of fluorescein dye as a predictor of mastectomy skin flap viability following autologous tissue reconstruction. Ann. Plast. Surg. 2008, 61, 24–29. [Google Scholar] [CrossRef]
- Komorowska-Timek, E.; Gurtner, G.C. Intraoperative perfusion mapping with laser-assisted indocyanine green imaging can predict and prevent complications in immediate breast re- construction. Plast. Reconstr. Surg. 2010, 125, 1065–1073. [Google Scholar] [CrossRef]
- Pagliara, D.; Montella, R.A.; Garganese, G.; Bove, S.; Costantini, M.; Rinaldi, P.M.; Pino, V.; Grieco, F.; Rubino, C.; Salgarello, M. Improving Decision-making in Prepectoral Direct-to-implant Reconstruction After Nipple Sparing Mastectomy: The Key Role of Flap Thickness Ratio. Clin. Breast Cancer 2023, 23, e37–e44. [Google Scholar] [CrossRef] [PubMed]
- Safran, T.; Al-Halabi, B.M.; Viezel-Mathieu, A.M.; Hazan, J.; Dionisopoulos, T.M. Direct-to-Implant Prepectoral Breast Reconstruction: Patient-Reported Outcomes. Plast. Reconstr. Surg. 2021, 148, 882e–890e. [Google Scholar] [CrossRef] [PubMed]
- Storm-Dickerson, T.; Sigalove, N. Prepectoral Breast Reconstruction: The Breast Surgeon’s Perspective. Plast. Reconstr. Surg. 2017, 140, 43S–48S. [Google Scholar] [CrossRef] [PubMed]
- Graziano, F.D.; Henderson, P.W.; Jacobs, J.; Salzberg, C.A.; Sbitany, H. How to Optimize Prepectoral Breast Reconstruction. Aesthetic Surg. J. 2020, 40 (Suppl. S2), S22–S28. [Google Scholar] [CrossRef] [PubMed]
- Kraenzlin, F.; Chopra, K.; Kokosis, G.; Venturi, M.L.; Mesbahi, A.; Nahabedian, M.Y. Revision Breast Reconstruction with Prepectoral Pocket Conversion of Submuscular Breast Implants. Plast. Reconstr. Surg. 2021, 147, 743e–748e. [Google Scholar] [CrossRef] [PubMed]
- Marcasciano, M.; Frattaroli, J.; Mori, F.L.R.; Torto, F.L.; Fioramonti, P.; Cavalieri, E.; Kaciulyte, J.; Greco, M.; Casella, D.; Ribuffo, D. The New Trend of Pre-pectoral Breast Reconstruction: An Objective Evaluation of the Quality of Online Information for Patients Undergoing Breast Reconstruction. Aesthetic Plast. Surg. 2019, 43, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.; Fregosi, N. The Impact of Animation Deformity on Quality of Life in Post-Mastectomy Reconstruction Patients. Aesthetic Surg. J. 2017, 37, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Sisti, A.; Sadeghi, P.; Cuomo, R.; Alvarez, S.M. Pre-Pectoral One-Stage Breast Reconstruction with Anterior Coverage Using Superior Anterior Biological Acellular Dermal Matrix (ADM) and Inferior Anterior Dermal Sling Support. Medicina 2022, 58, 992. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-E. Prepectoral breast reconstruction. Yeungnam Univ. J. Med. 2019, 36, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, R. Submuscular and Pre-pectoral ADM Assisted Immediate Breast Reconstruction: A Literature Review. Medicina 2020, 56, 256. [Google Scholar] [CrossRef]
- Chinta, S.; Koh, D.J.; Sobti, N.; Packowski, K.; Rosado, N.; Austen, W.; Jimenez, R.B.; Specht, M.; Liao, E.C. Cost analysis of pre-pectoral implant-based breast reconstruction. Sci. Rep. 2022, 12, 17512. [Google Scholar] [CrossRef]
- Frey, J.D.; Choi, M.; Salibian, A.A.; Karp, N.S. Comparison of Outcomes with Tissue Expander, Immediate Implant, and Autologous Breast Reconstruction in Greater Than 1000 Nipple-Sparing Mastectomies. Plast. Reconstr. Surg. 2017, 139, 1300–1310. [Google Scholar] [CrossRef]
- Lagares-Borrego, A.; Gacto-Sanchez, P.; Infante-Cossio, P.; Barrera-Pulido, F.; Sicilia-Castro, D.; Gomez-Cia, T. A comparison of long-term cost and clinical outcomes between the two-stage sequence expander/prosthesis and autologous deep inferior epigastric flap methods for breast reconstruction in a public hospital. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Mohan, A.T.; Abdelsattar, J.M.; Wang, Z.; Vijayasekaran, A.; Hwang, S.M.; Tran, N.V.; Saint-Cyr, M. Comparison of subcutaneous versus submuscular expander placement in the first stage of immediate breast reconstruction. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, e77–e86. [Google Scholar] [CrossRef]
- Taylor, J.M.B.; Moman, P.D.B.; Chevalier, J.M.B.; Tseng, C.Y.; Festekjian, J.H.; Delong, M.R. Enhanced Recovery after Surgery Protocol Decreases Length of Stay and Postoperative Narcotic Use in Tissue Expander-based Breast Reconstruction. Plast. Reconstr. Surg. Glob. Open 2024, 12, e5879. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kraenzlin, F.; Darrach, H.; Khavanin, N.; Kokosis, G.; Aliu, O.; Broderick, K.; Rosson, G.D.; Manahan, M.A.; Sacks, J.M. Tissue Expander–Based Breast Reconstruction in the Prepectoral Versus Subpectoral Plane: An Analysis of Short-Term Outcomes. Ann. Plast. Surg. 2021, 86, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Holland, M.; Su, P.; Piper, M.; Withers, J.; Harbell, M.W.; Bokoch, M.P.; Sbitany, H. Prepectoral Breast Reconstruction Reduces Opioid Consumption and Pain after Mastectomy: A Head-to-Head Comparison with Submuscular Reconstruction. Ann. Plast. Surg. 2022, 89, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Hudson, D.A.; van Zyl, J.E.; Duminy, F.J.; Silfen, R. Staged TRAM breast reconstruction: Combining the advantages of tissue expansion with surgical delay. Aesthetic Plast. Surg. 2000, 24, 202–205. [Google Scholar] [CrossRef]
- Gurunluoglu, R.; Spanio, S.; Rainer, C.; Ninkovic, M. Skin expansion before breast reconstruction with the superior gluteal artery perforator flap improves aesthetic outcome. Ann. Plast. Surg. 2003, 50, 475–479. [Google Scholar] [CrossRef]
- Kajikawa, A.; Ueda, K.; Tateshita, T.; Katsuragi, Y. Breast reconstruction using tissue expander and TRAM flap with vascular enhancement procedures. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, 1148–1153. [Google Scholar] [CrossRef]
- Schwarz, K.; Tahiri, Y. Subcutaneous pre-expansion of mastectomy flaps before breast reconstruction with deep inferior epigastric perforator flaps: Eliminating the patch-like appearance and improving aesthetic outcomes. Ann. Plast. Surg. 2011, 66, 124–127. [Google Scholar] [CrossRef]
- Bloom, J.A.; Shah, S.A.; Long, E.A.; Chatterjee, A.; Lee, B.T. Post-Mastectomy Tissue Expander Placement Followed by Radiation Therapy: A Cost-Effectiveness Analysis of Staged Autologous Versus Implant-Based Unilateral Reconstruction. Ann. Surg. Oncol. 2023, 30, 1075–1083. [Google Scholar] [CrossRef]
| Risk Factor | Score | Range of Score per Factor | ||
|---|---|---|---|---|
| 0 | 1 | 2 | ||
| Patient’s age, yr | >70 | 50–70 | <50 | 0–2 |
| Diabetes | Yes | No | 0–1 | |
| Smoker | Current smoker | Ex-smoker | Never smoker | 0–2 |
| BMI | Low: <22 | High: >25 | Medium: 22–25 | 0–2 |
| Breast ptosis: indication for skin-reducing mastectomy | Yes | No | 0–1 | |
| Previous breast surgery | Yes | No | 0–1 | |
| Radiotherapy | Yes | No | 0–1 | |
| Mastectomy flap thickness, cm | <1 | 1–2 | >2 | 0–2 |
| Score | Implant-Based Breast Reconstruction |
|---|---|
| 0–4 | No indication for prepectoral reconstruction; submuscular placement of the implant |
| 5–8 | Two-stage reconstruction with prepectoral tissue expander first and subcutaneous definitive prosthesis second |
| 9–12 | Prepectoral direct-to-implant breast reconstruction |
| Patient | Age | Diabetes | Smoke | BMI (kg/m2) | Breast Ptosis | Previous Breast Surgeries | Radiotherapy | Mastectomy Flap Thickness (cm) | Complications | Total Score |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 35 | no | yes | 27.2 | no | yes | no | 1.30 | 7 | |
| 2 | 48 | no | yes | 23 | yes | no | no | 1.2 | 8 | |
| 3 | 55 | yes | no | 26.1 | no | yes | yes | 1.4 | 6 | |
| 4 | 57 | no | ex | 27.3 | no | no | no | 0.9 | 7 | |
| 5 | 46 | yes | ex | 24.6 | yes | no | yes | 0.8 | Superficial skin necrosis | 6 |
| 6 | 63 | yes | yes | 25.7 | no | no | no | 0.9 | Breast expander exposure and removal | 5 |
| 7 | 42 | no | ex | 22.3 | no | no | yes | 0.9 | 8 | |
| 8 | 45 | no | ex | 21.8 | no | yes | no | 1.3 | 7 | |
| 9 | 38 | no | no | 21.7 | no | no | yes | 0.8 | 7 | |
| 10 | 52 | no | ex | 26.3 | no | no | no | 1.7 | 8 | |
| 11 | 56 | yes | no | 28.5 | no | no | no | 1.3 | 8 | |
| 12 | 55 | no | no | 21 | no | yes | no | 1.2 | 7 | |
| 13 | 58 | yes | ex | 24.3 | no | no | no | 0.8 | Seroma | 7 |
| 14 | 49 | no | no | 19 | no | yes | yes | 0.8 | 6 | |
| 15 | 52 | no | ex | 23.2 | no | no | no | 0.7 | 8 | |
| 16 | 51 | no | no | 19.7 | no | yes | no | 0.8 | 6 | |
| 17 | 53 | no | ex | 22 | no | yes | no | 0.9 | 7 | |
| 18 | 61 | yes | ex | 24.1 | no | yes | no | 1.4 | 7 | |
| 19 | 71 | no | yes | 21.9 | no | no | no | 1.1 | 5 | |
| 20 | 50 | yes | ex | 33.2 | yes | no | no | 1.6 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Torto, F.; Turriziani, G.; Carella, S.; Pagnotta, A.; Ribuffo, D. Impact of the Prepectoral Breast Reconstruction Assessment Score on Expander-Based Reconstruction Success. J. Clin. Med. 2024, 13, 6466. https://doi.org/10.3390/jcm13216466
Lo Torto F, Turriziani G, Carella S, Pagnotta A, Ribuffo D. Impact of the Prepectoral Breast Reconstruction Assessment Score on Expander-Based Reconstruction Success. Journal of Clinical Medicine. 2024; 13(21):6466. https://doi.org/10.3390/jcm13216466
Chicago/Turabian StyleLo Torto, Federico, Gianmarco Turriziani, Sara Carella, Alessia Pagnotta, and Diego Ribuffo. 2024. "Impact of the Prepectoral Breast Reconstruction Assessment Score on Expander-Based Reconstruction Success" Journal of Clinical Medicine 13, no. 21: 6466. https://doi.org/10.3390/jcm13216466
APA StyleLo Torto, F., Turriziani, G., Carella, S., Pagnotta, A., & Ribuffo, D. (2024). Impact of the Prepectoral Breast Reconstruction Assessment Score on Expander-Based Reconstruction Success. Journal of Clinical Medicine, 13(21), 6466. https://doi.org/10.3390/jcm13216466







