Assessing the Influence of Hyaluronan Dressing on Wound Healing on Split-Thickness Skin Graft Donor Sites Using a Three-Dimensional Scanner
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Wounds and Wound Dressing
2.3. Three-Dimensional Imaging
2.4. Evaluation Scales
2.5. Wound Healing Process Analysis
2.6. Statistical Analysis
3. Results
3.1. Participants
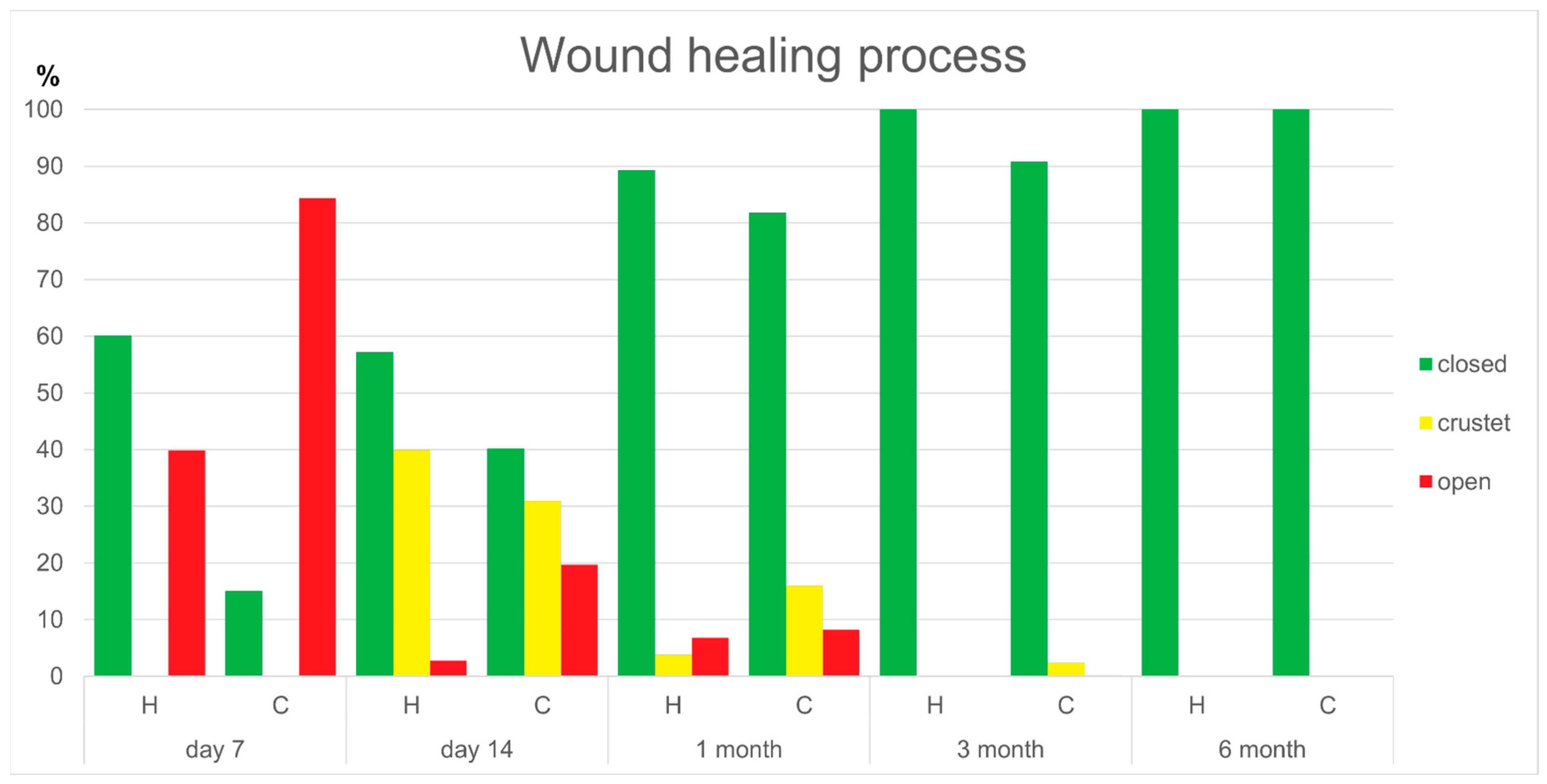
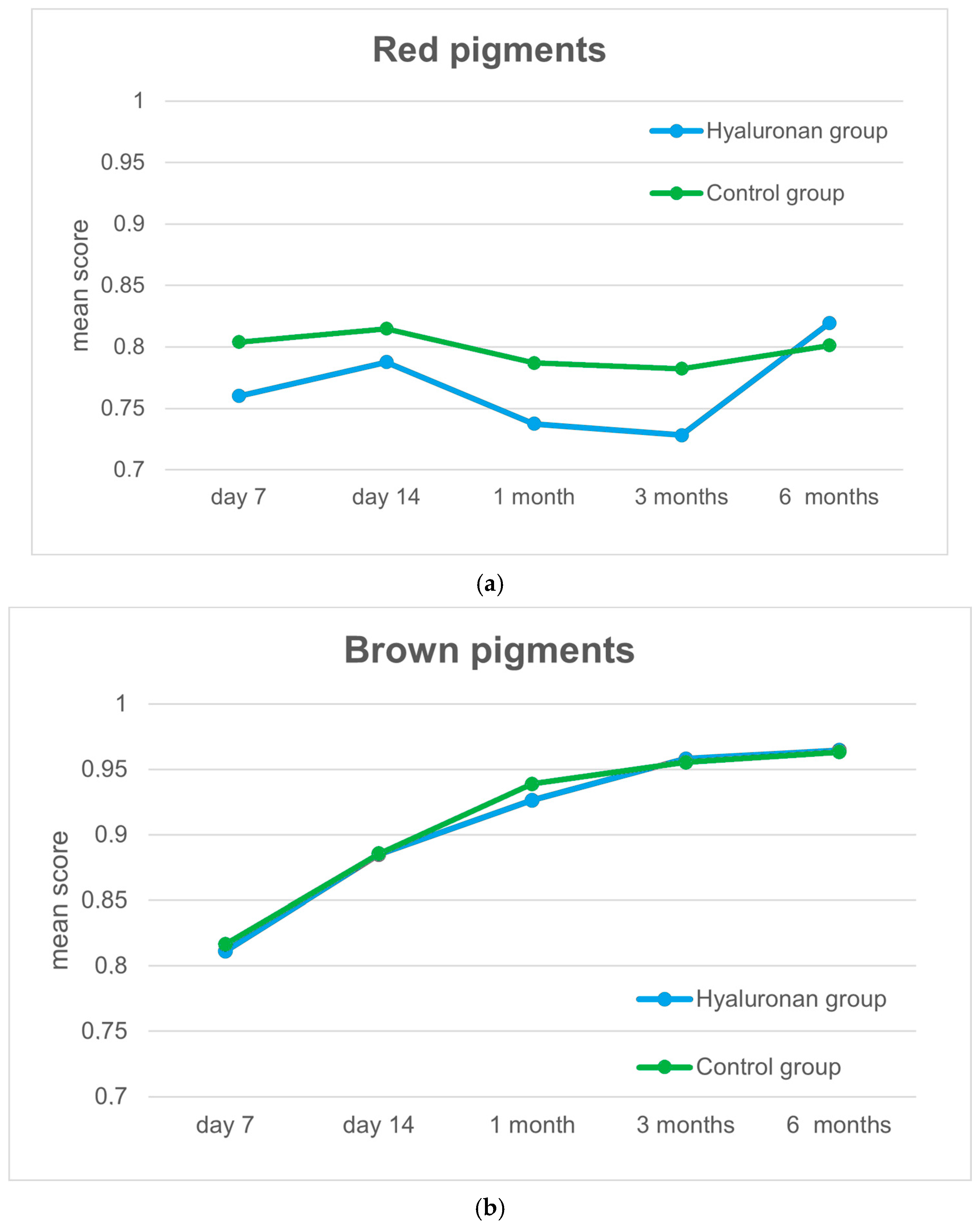
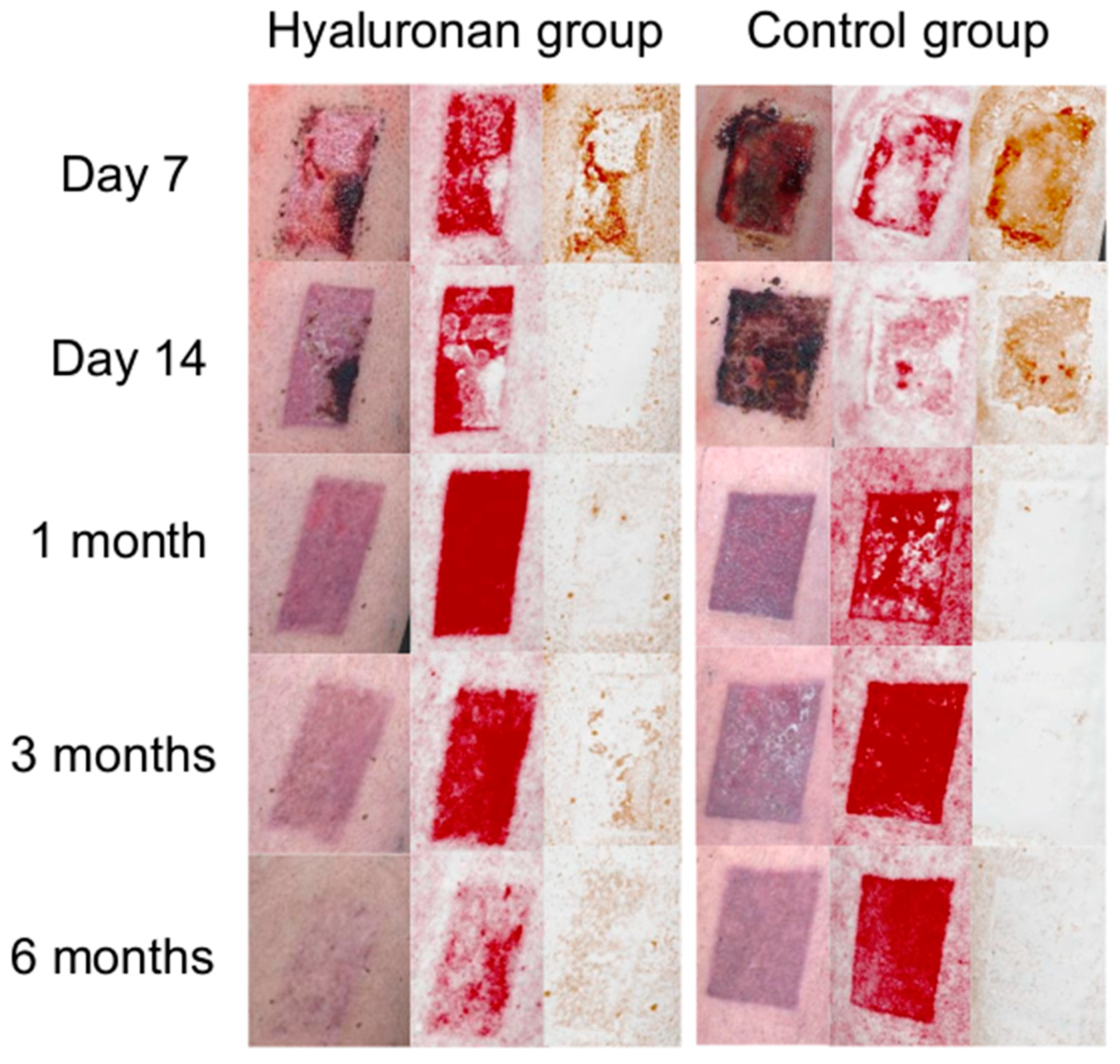
3.2. Wound Healing Process
3.3. Patients’ Scar Outcome Assessments After 6 Months
3.4. Examiner’s Scar Outcome Assessment after 6 Months
3.5. Comparison of the Patients’ and Experts’ Evaluations 6 Months Post-Operatively
4. Discussion
4.1. Wound Healing Process
4.2. Scar Outcomes
4.3. Experts’ Assessments
4.4. Three-Dimensional Scanner
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frenkel, J.S. The role of hyaluronan in wound healing. Int. Wound J. 2014, 11, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Gurtner, G.C. Tissue engineering for the management of chronic wounds: Current concepts and future perspectives. Exp. Dermatol. 2012, 21, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Akhundov, K.; Pietramaggiori, D.S.; Waselle, S.; Darwiche, S.; Guerid, S.; Scaletta, C.; Hirt-Burri, N.; Applegate, L.A.; Raffoul, W.V. Development of a cost-effective method for platelet-rich plasma (PRP) preparation for topical wound healing. Ann. Burn. Fire Disasters 2012, 25, 207–2013. [Google Scholar]
- Hoppe, H.D.; Lobmann, R. Hyaluronsäure-Ihre Bedeutung für die Wundheilung“ Medizin &Praxis. Dent. Spieg. 2008, 77, 82. [Google Scholar]
- Keen, M.A. Hyaluronic Acid in Dermatology. Skinmed 2017, 15, 441–448. [Google Scholar]
- Chen, W.Y.; Abatangelo, G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999, 7, 79–89. [Google Scholar] [CrossRef]
- Mineo, A.; Suzuki, R.; Kuroyanagi, Y. Development of an artificial dermis composed of hyaluronic acid and collagen. J. Biomater. Sci. Polym. 2013, 24, 726–740. [Google Scholar] [CrossRef]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds 2016, 28, 78–88. [Google Scholar]
- Eskandarinia, A.; Kefayat, A.; Gharakhloo, M.; Agheb, M.; Khodabakhshi, D.; Khorshidi, M.; Sheikhmoradi, V.; Rafienia, M.; Salehi, H. A propolis enriched polyurethane-hyaluronic acid nanofibrous wound dressing with remarkable antibacterial and wound healing activities. Int. J. Biol. Macromol. 2020, 149, 467–476. [Google Scholar] [CrossRef]
- Gao, Z.; Golland, B.; Tronci, G.; Thornton, P.D. A redox-responsive hyaluronic acid-based hydrogel for chronic wound management. J. Mater. Chem. B. 2019, 7, 7494–7501. [Google Scholar] [CrossRef]
- Kim, Y.; Moon, C.H.; Kim, B.Y.; Jang, S.Y. Oral Hyaluronic Acid Supplementation for the Treatment of Dry Eye Disease: A Pilot Study. J. Ophthalmol. 2019, 2019, 5491626. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, R.; Guner, A.; Cekic, A.B.; Usta, M.A.; Ulusahin, M.; Turkyilmaz, S. Outcomes of the Use of Hyaluronic Acid-Based Wound Dressings for the Treatment of Partial-Thickness Facial Burns. J. Burn Care Res. 2019, 44, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Suchánek, J.; Ivančaková, R.K.; Mottl, R.; Browne, K.Z.; Pilneyová, K.C.; Pilbauerová, N.; Schmidt, J.; Suchánková Kleplová, T. Hyaluronic Acid-Based Medical Device for Treatment of Alveolar Osteitis-Clinical Study. Int. J. Environ. Res. Public Health 2019, 16, 3698. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, B.; D’Autilio, M.F.L.M.; Orlandi, F.; Pepe, G.; Garcovich, S.; Scioli, M.G.; Orlandi, A.; Cervelli, V.; Gentile, P. Wound Healing: In Vitro and In Vivo Evaluation of a Bio-Functionalized Scaffold Based on Hyaluronic Acid and Platelet-Rich Plasma in Chronic Ulcers. J. Clin. Med. 2019, 8, 1486. [Google Scholar] [CrossRef] [PubMed]
- Çankaya, Z.T.; Gürbüz, S.; Bakirarar, B.; Kurtiş, B. Evaluation of the Effect of Hyaluronic Acid Application on the Vascularization of Free Gingival Graft for Both Donor and Recipient Sites with Laser Doppler Flowmetry: A Randomized, Examiner-Blinded, Controlled Clinical Trial. Int. J. Periodontics Restor. Dent. 2020, 40, 233–243. [Google Scholar] [CrossRef]
- Su, Z.; Ma, H.; Wu, Z.; Zeng, H.; Li, Z.; Wang, Y.; Liu, G.; Xu, B.; Lin, Y.; Zhang, P.; et al. Enhancement of skin wound healing with decellularized scaffolds loaded with hyaluronic acid and epidermal growth factor. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Dereure, O.; Czubek, M.; Combemale, P. Efficacy and safety of hyaluronic acid in treatment of leg ulcers: A double-blind RCT. J. Wound Care 2012, 21, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Prohaska, J.; Cook, C. Skin Grafting. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532874/ (accessed on 26 October 2024).
- Jauch, K.W. Chirurgie Basisweiterbildung, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Petres, J.; Rompel, R. Operative Dermatologie: Lehrbuch und Atlas, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Eder, S. Die Sinnvolle Lokale Wundtherapie einer Spalthautentnahmestelle am Oberschenkel, Gefässchirurgie; Springer Medizin Verlag: Berlin/Heidelberg, Germany, 2017; Volume 22, pp. 568–571. [Google Scholar]
- Brown, J.E.; Holloway, S.L. An evidence-based review of split-thickness skin graft donor site dressings. Int. Wound J. 2018, 15, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.L. Which dressing for split-thickness skin graft donor sites? Ann. Plast. Surg. 1991, 27, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Voineskos, S.H.; Ayeni, O.A.; McKnight, L.; Thoma, A. Systematic review of skin graft donor-site dressings. Plast. Reconstr. Surg. 2009, 124, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Birchall, M.A.; Varma, S.; Milward, T.M. The Moriarty sign: An appraisal. Br. J. Plast. Surg. 1991, 44, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Zillmer, R.; Agren, M.S.; Gottrup, F.; Karlsmark, T. Biophysical effects of repetitive removal of adhesive dressings on peri-ulcer skin. J. Wound Care 2006, 15, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Gee Kee, E.L.; Kimble, R.M.; Cuttle, L.; Khan, A.; Stockton, K.A. Randomized controlled trial of three burns dressings for partial thickness burns in children. Burns 2015, 41, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Bugmann, P.; Taylor, S.; Gyger, D.; Lironi, A.; Genin, B.; Vunda, A.; La Scala, G.; Birraux, J.; Le Coultre, C. A silicone-coated nylon dressing reduces healing time in burned paediatric patients in comparison with standard sulfadiazine treatment: A prospective randomized trial. Burns 1998, 24, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Atkin, L.; Nierenberg, N.; Wild, T. Case series: ALLEVYN LIFE™ non-bordered foam dressing for managing moderate to heavily exuding wounds. Wounds Int. 2018, 9, 38–42. [Google Scholar]
- Bock, A.; Suschek, C.V.; Opländer, C.; Hölzle, F.; Modabber, A.; Pallua, N. Evaluation of facial blood flow using three-dimensional scanning. Br. J. Oral Maxillofac. Surg. 2017, 55, 974–976. [Google Scholar] [CrossRef] [PubMed]
- Fearmonti, R.; Bond, J.; Erdmann, D.; Levinson, H. A review of scar scales and scar measuring devices. Eplasty 2010, 10, e43. [Google Scholar] [PubMed] [PubMed Central]
- Beausang, E.; Floyd, H.; Dunn, K.W.; Orton, C.I.; Ferguson, M.W. A new quantitative scale for clinical scar assessment. Plast. Reconstr. Surg. 1998, 102, 1954–1961. [Google Scholar] [CrossRef]
- Draaijers, L.J.; Tempelman, F.R.; Botman, Y.A.; Tuinebreijer, W.E.; Middelkoop, E.; Kreis, R.W.; van Zuijlen, P.P. The patient and observer scar assessment scale: A reliable and feasible tool for scar evaluation. Plast. Reconstr. Surg. 2004, 113, 1960–1965; discussion 1966–1967. [Google Scholar] [CrossRef] [PubMed]
- Aya, K.L.; Stern, R. Hyaluronan in wound healing: Rediscovering a major player. Wound Repair Regen. 2014, 22, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Kazanavičius, M.; Cepas, A.; Kolaityte, V.; Simoliuniene, R.; Rimdeika, R. The use of modern dressings in managing split-thickness skin graft donor sites: A single-centre randomised controlled trial. J. Wound Care 2017, 26, 281–291. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, F.; Saparov, A.; Riccio, M. Hyaluronic acid accelerates re-epithelialization and healing of acute cutaneous wounds. Eur. Rev. Med. Pharmacol. Sci. 2023, 27 (Suppl. S3), 37–45. [Google Scholar] [CrossRef] [PubMed]
- Juhaščik, M.; Kováčik, A.; Huerta-Ángeles, G. Recent Advances of Hyaluronan for Skin Delivery: From Structure to Fabrication Strategies and Applications. Polymers 2022, 14, 4833. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Davis, S.C.; Cazzaniga, A.L.; Ricotti, C.; Zalesky, P.; Hsu, L.C.; Creech, J.; Eaglstein, W.H.; Mertz, P.M. Topical oxygen emulsion: A novel wound therapy. Arch. Dermatol. 2007, 143, 1252–1256. [Google Scholar] [CrossRef] [PubMed]
- Kaartinen, I.S.; Kuokkanen, H.O. Suprathel(®) causes less bleeding and scarring than Mepilex(®) Transfer in the treatment of donor sites of split-thickness skin grafts. J. Plast. Surg. Hand Surg. 2011, 45, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Barrit, D.; Birke-Sorensen, H. Dressings for split thickness skin graft donor sites: A comparison of three dressings. EWMA J. 2014, 14, 15–20. [Google Scholar]
- Healy, C.; Greig, A.V.H.; Murphy, A.D.; Powell, C.; Pinder, R.J.; Saour, S.; Abela, C.; Knight, W.; Geh, J.L.C. Prospective randomized controlled trial: Fibrin sealant reduces split skin graft donor-site pain. Plast. Reconstr. Surg. 2013, 132, 139e–146e. [Google Scholar] [CrossRef] [PubMed]
- Asuku, M.; Yu, T.C.; Yan, Q.; Böing, E.; Hahn, H.; Hovland, S.; Donelan, M.B. Split-thickness skin graft donor-site morbidity: A systematic literature review. Burns 2021, 47, 1525–1546. [Google Scholar] [CrossRef] [PubMed]
- Rippa, A.L.; Kalabusheva, E.P.; Vorotelyak, E.A. Regeneration of Dermis: Scarring and Cells Involved. Cells 2019, 8, 607. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spanholtz, T.A.; Leitsch, S.; Holzbach, T.; Volkmer, E.; Engelhardt, T.; Giunta, R.E. 3-dimensionale Bilderfassung: Erste Erfahrungen in der Planung und Dokumentation plastisch-chirurgischer Operationen [3-dimensional imaging systems: First experience in planning and documentation of plastic surgery procedures]. Handchir. Mikrochir. Plast. Chir. 2012, 44, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Blunck, D.; Schöffski, O. Hyaluronic acid treatment versus standard of care in chronic wounds in a German setting: Cost-effectiveness analysis. Health Sci. Rep. 2022, 6, e969. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dissemond, J. Blickdiagnose Chronischer Wunden; Viavital Verlag GmbH: Köln, Germany, 2009; ISBN 978-3-934371-43-9. [Google Scholar]
- Garg, S.P.; Williams, T.; Taritsa, I.C.; Wan, R.; Goel, C.; Harris, R.; Huffman, K.; Galiano, R.D. Evaluating skin colour diversity in the validation of scar assessment tools. Wound Repair Regen. 2023, 31, 731–737. [Google Scholar] [CrossRef] [PubMed]


| Variable | Intervention | Control | p Value |
|---|---|---|---|
| Age (years) | 60.0 (15) | 59.0 (14) | 0.689 |
| Body mass index (kg/m2) | 24.7 (7.6) | 27.5 (5.9) | 0.133 |
| Sex (n) | |||
| Male | 13 | 11 | 0.783 |
| Female | 10 | 10 | |
| ASA (n) | |||
| ≤2 | 15 | 11 | 0.387 |
| >2 | 8 | 10 |
| Variable | Intervention | Control | p Value |
|---|---|---|---|
| AW7D wound closed | 0.597 (0.5) | 0.128 (0.4) | 0.003 |
| AW14D wound closed | 0.603 (0.3) | 0.377 (0.5) | 0.181 |
| AW14D wound crusted | 0.364 (0.3) | 0.297 (0.6) | 0.545 |
| AW14D wound open | 0.000 (0.0) | 0.037 (0.3) | 0.024 |
| AW1M wound closed | 1.000 (0.2) | 0.814 (0.3) | 0.015 |
| AW1M wound crusted | 0.000 (0.0) | 0.105 (0.2) | 0.032 |
| AW1M wound open | 0.000 (0.0) | 0.015 (0.2) | 0.016 |
| AW3M wound closed | 1.000 (0.0) | 1.000 (0.0) | 0.250 |
| AW3M wound crusted | 0.000 (0.0) | 0.000 (0.0) | 0.250 |
| AW3M wound open | 0.000 (0.0) | 0.000 (0.0) | 1.000 |
| AW6M wound closed | 1.000 (0.0) | 1.000 (0.0) | n.a. |
| AW6M wound crusted | 0.000 (0.0) | 0.000 (0.0) | n.a. |
| AW6M wound open | 0.000 (0.0) | 0.000 (0.0) | n.a. |
| Variable | Intervention | Control | p Value |
|---|---|---|---|
| AW7 VAS | 8.0 (1.0) | 10.0 (1.0) | <0.001 |
| AW7 Color | 4.0 (0.5) | 4.0 (0.0) | 0.022 |
| AW7 Surface | 2.0 (0.0) | 2.0 (0.0) | 0.792 |
| AW7 Vascularization | 7.5 (1.0) | 8.0 (0.9) | 0.002 |
| AW7 Pigmentation | 8.0 (0.0) | 8.5 (1.0) | <0.001 |
| AW14 VAS | 7.0 (2.0) | 8.0 (1.5) | <0.001 |
| AW14 Color | 3.5 (0.0) | 3.5 (0.5) | 0.040 |
| AW14 Surface | 1.0 (1.0) | 1.0 (1.0) | 0.837 |
| AW14 Vascularization | 7.0 (1.5) | 7.5 (1.0) | 0.040 |
| AW14 Pigmentation | 7.0 (1.0) | 7.5 (1.5) | 0.391 |
| AW1M VAS | 6.75 (1.4) | 7.75 (1.5) | 0.001 |
| AW1M Color | 3.5 (0.5) | 3.5 (0.0) | 0.382 |
| AW1M Surface | 1.0 (0.5) | 1.5 (0.5) | 0.445 |
| AW1M Vascularization | 7.5 (0.9) | 7.5 (1.4) | 0.724 |
| AW1M Pigmentation | 5.25 (1.4) | 6.0 (0.5) | 0.007 |
| AW3M VAS | 5.0 (1.5) | 6.75 (1.9) | <0.001 |
| AW3M Color | 2.5 (0.0) | 3.5 (0.9) | 0.006 |
| AW3M Surface | 1.0 (0.0) | 1.5 (0.5) | 0.002 |
| AW3M Vascularization | 6.0 (1.0) | 6.5 (2.0) | 0.423 |
| AW3M Pigmentation | 4.0 (0.5) | 5.5 (0.9) | <0.001 |
| AW6M VAS | 2.5 (1.5) | 5.0 (2.5) | <0.001 |
| AW6M Color | 1.5 (1.0) | 2.5 (0.5) | 0.002 |
| AW6M Surface | 1.0 (0.0) | 1.5 (0.5) | <0.001 |
| AW6M Vascularization | 3.5 (2.0) | 5.0 (3.5) | 0.037 |
| AW6M Pigmentation | 3.0 (2.0) | 4.0 (1.0) | 0.103 |
| Variable | Intervention | Control | p Value |
|---|---|---|---|
| MSS VAS | 2.0 (1.5) | 4.0 (2.5) | <0.001 |
| MSS Color | 2.0 (2.0) | 3.0 (1.0) | <0.001 |
| MSS Surface | 1.0 (0.0) | 2.0 (1.0) | 0.003 |
| MSS Contour | 1.0 (0.0) | 1.0 (0.0) | 0.591 |
| MSS Distortion | 1.0 (0.0) | 1.0 (0.0) | 0.345 |
| MSS Texture | 1.0 (0.0) | 1.0 (1.0) | 0.121 |
| POSAS Painful | 1.0 (0.0) | 1.0 (0.0) | 0.974 |
| POSAS Itching | 1.0 (0.0) | 1.0 (0.0) | 0.334 |
| POSAS Color different | 4.0 (6.0) | 5.0 (5.5) | 0.293 |
| POSAS Stiffness | 1.0 (1.0) | 2.0 (2.5) | 0.304 |
| POSAS Thickness | 1.0 (1.0) | 1.0 (1.0) | 0.482 |
| POSAS Irregular | 1.0 (1.0) | 4.0 (5.5) | 0.002 |
| Variable | Intervention | Control | p Value |
|---|---|---|---|
| MSS VAS | 3.0 (2.0) | 7.0 (4.0) | <0.001 |
| MSS Color | 2.0 (1.0) | 4.0 (1.0) | <0.001 |
| MSS Surface | 1.0 (0.0) | 2.0 (0.5) | <0.001 |
| MSS Contour | 1.0 (0.0) | 1.0 (0.0) | 0.323 |
| MSS Distortion | 1.0 (0.0) | 1.0 (0.0) | 0.888 |
| MSS Texture | 1.0 (1.0) | 1.0 (1.0) | 0.941 |
| POSAS Vascularization | 2.0 (4.0) | 8.0 (4.5) | <0.001 |
| POSAS Pigmentation | 3.0 (2.0) | 5.0 (4.0) | <0.001 |
| POSAS Thickness | 1.0 (0.0) | 1.0 (0.0) | 0.753 |
| POSAS Relief | 1.0 (1.0) | 1.0 (0.5) | 0.410 |
| POSAS Pliability | 1.0 (1.0) | 1.0 (0.0) | 0.577 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bock, A.; Peters, F.; Heitzer, M.; Winnand, P.; Kniha, K.; Katz, M.S.; Hölzle, F.; Modabber, A. Assessing the Influence of Hyaluronan Dressing on Wound Healing on Split-Thickness Skin Graft Donor Sites Using a Three-Dimensional Scanner. J. Clin. Med. 2024, 13, 6433. https://doi.org/10.3390/jcm13216433
Bock A, Peters F, Heitzer M, Winnand P, Kniha K, Katz MS, Hölzle F, Modabber A. Assessing the Influence of Hyaluronan Dressing on Wound Healing on Split-Thickness Skin Graft Donor Sites Using a Three-Dimensional Scanner. Journal of Clinical Medicine. 2024; 13(21):6433. https://doi.org/10.3390/jcm13216433
Chicago/Turabian StyleBock, Anna, Florian Peters, Marius Heitzer, Philipp Winnand, Kristian Kniha, Marie Sophie Katz, Frank Hölzle, and Ali Modabber. 2024. "Assessing the Influence of Hyaluronan Dressing on Wound Healing on Split-Thickness Skin Graft Donor Sites Using a Three-Dimensional Scanner" Journal of Clinical Medicine 13, no. 21: 6433. https://doi.org/10.3390/jcm13216433
APA StyleBock, A., Peters, F., Heitzer, M., Winnand, P., Kniha, K., Katz, M. S., Hölzle, F., & Modabber, A. (2024). Assessing the Influence of Hyaluronan Dressing on Wound Healing on Split-Thickness Skin Graft Donor Sites Using a Three-Dimensional Scanner. Journal of Clinical Medicine, 13(21), 6433. https://doi.org/10.3390/jcm13216433






