Abstract
Objectives: There are no studies related to the use of PRF associated with cyanoacrylates in fresh post-extraction sockets. Thus, the aim of this study was to assess the effect of ethyl-cyanoacrylate combined with PRF in fresh sockets of rabbits subjected to anticoagulant therapy. Methods: Twelve adults rabbits were selected and premedicated with heparin 1 week before surgery to induce and simulate anticoagulant therapy. Upper and lower first premolars on the right side were extracted and then were divided into four groups of three animals each, with the groups distributed according to the type of intervention in the sockets (n = 6): (1) clot and suture (control); (2) PRF and suture; (3) clot and ethyl-cyanoacrylate; (4) PRF and ethyl-cyanoacrylate. At 12 weeks, the animals were sacrificed and the sockets were analyzed histologically and quantitatively. Total bone area, inflammation infiltrate, and adhesive remnants were assessed. Results: No remnants of adhesive were found in the samples. Groups 1 and 2 showed the highest bone area (G1 = 37.87% ± 17.86; G2 = 30.31 ± 9.36) with significant differences to those treated with ethyl-cyanoacrylate adhesive (G3 = 26.6% ± 11.82; G4 = 24.29% ± 6.25). Conclusions: The groups that used ethyl-cyanoacrylate as a closure method in sockets exhibited less bone area than the groups that used sutures. Both groups that used PRF as therapy did not show a significant improvement in bone healing at 12 weeks compared with the clot groups.
1. Introduction
Ethyl-cyanoacrylate, one of the shortest-chain cyanoacrylate adhesives, was among the first adhesives tested for clinical use [1] and has shown good biocompatibility in human osteoblast cell cultures [2]. Its excellent adhesive strength, stability, rapid polymerization in a moist environment, and adequate working time promote its use in both medicine and dentistry [3]. The literature reports several advantages of cyanoacrylate adhesives in oral surgery procedures [3], including closure methods for mandibular third molar extraction [4], free gingival graft surgery [5], bone graft fixation [6], and apical surgery [7], among others.
Cyanoacrylate adhesives are a good option for patients who have undergone anticoagulant therapy. Muzumdar and Feng [8] reported their use as a hemostatic therapy on the skin after dermatologic surgery in elderly patients. Borie et al. [3] reported the application of ethyl cyanoacrylate adhesive during a dental implant placement procedure to achieve immediate hemostasis in a patient undergoing anticoagulant therapy. Additionally, the use of cyanoacrylate adhesives as a hemostatic agent has been reported in patients undergoing anticoagulant therapy during multiple exodontia [9].
On the other hand, platelet-rich fibrin (PRF) is a beneficial tool for obtaining concentrated growth factors from the patient themselves, prepared through centrifugation without the need to add any chemicals [10]. PRF significantly improves bone and soft tissue regeneration. Over the last decade, its use has increased consistently, along with the number of published papers related to various clinical applications of PRF, with a high level of scientific evidence [11]. However, the use of PRF in fresh sockets to prevent bone loss and promote bone healing remains uncertain. Some studies [12,13] have found that PRF results in reduced bone volume loss compared to spontaneous healing in alveolar ridge preservation. Nevertheless, other studies [14,15] conclude that PRF does not reduce the rate of ridge resorption in either the vertical or horizontal dimensions of extraction sockets, nor does it induce greater new bone formation.
The association between PRF and cyanoacrylates has been studied in soft tissue management, such as PRF stabilization in free epithelialized mucosal grafts as well as the palatal donor site, observing promising results [16,17,18,19,20]. However, there are no studies related to the use of PRF associated with cyanoacrylates in fresh post-extraction sockets. Thus, the aim of this study was to assess the effect of ethyl-cyanoacrylate combined with PRF in fresh sockets of rabbits subjected to anticoagulant therapy. The hypothesis was that the combination of ethyl cyanoacrylate and PRF in fresh sockets of rabbits undergoing anticoagulant therapy would improve bone regeneration.
2. Materials and Methods
The research was approved by the Universidad de La Frontera Ethics Committee (Nº 126/21).
Twelve 7-month-old, healthy male rabbits (Oryctolagus cuniculus) with an average weight of 2.5 kg (±0.2 kg) were selected [21]. The care and handling of the animals were carried out following the ethical standards of the “Guide for the Care and Use of Laboratory Animals” [22] in the animal surgery room at the Bioterium of the Center of Excellence in Morphological and Surgical Studies (CEMYQ) of the University of the Frontera, Temuco, Chile. All rabbits were kept in the animal room with free access to water and food, at a temperature of 23–25 °C, humidity of 50–60%, and a 12 h light/dark cycle [23]. The animal room was clean, dry, and ventilated.
The rabbits were premedicated before surgery with an injection of heparin 30 IU/kg into the marginal auricular vein [24] once daily for 1 week to induce and simulate anticoagulant therapy. One hour before surgery, amoxicillin (50 mg/kg) was administered as a prophylactic antibiotic via intramuscular route. For sedation of the animals, a dose of Ketamine (40 mg/kg i.m.) supplemented with Xylazine (5 mg/kg i.m.) was used. In addition, as a local anesthetic, 0.4 mL of 2% lidocaine with a dose of 1:100,000 epinephrine (Octocaine-100, Cambridge, ON, Canada) was applied using an infiltrative technique of the upper and lower first premolars on the right side. Tooth extraction was only performed by one dentist with more than five years of experience and previously calibrated to obtain a clean extraction without root fracture, according to Hamad et al.’s [23] technique, where the first premolar is extracted, and once luxated, the alveolus is compressed to destroy the germ and thus prevent the growth of another tooth. To control the risk of infection in the animals, enrofloxacin (5 mg/kg i.m.) was indicated, while meloxicam (0.4 mg/kg i.m.) [25] was used for pain management.
Once the tooth extraction procedure was completed, the rabbits were divided into four groups of three animals each, with the groups distributed according to the type of intervention in the sockets (n = 6), which were as follows: (1) clot and suture (control); (2) PRF and suture; (3) clot and ethyl-cyanoacrylate; (4) PRF and ethyl-cyanoacrylate.
In both groups of sutures, absorbable sutures were used (Vicryl®, Novartis Animal Health US, Inc., Greensboro, NC, USA). In the groups that used ethyl-cyanoacrylate adhesive (Superbonder Loctite®, Düsseldorf, Germany), it was applied directly in an attempt to close the edges and occlude the alveolar bed. In the groups that used platelet-rich fibrin (PRF), it was obtained from a 1.8 mL sample of auricular venous blood in red tubes without biochemical additives. Immediately after obtaining the blood, this sample was centrifuged for 10 min at 3000 rpm in a clinical centrifuge (EBA 200, Kirchlengern, Germany) according to the protocol previously described [26,27].
Animals were euthanized with an overdose of sodium pentobarbital i.m. (60 mg/kg) at 12 weeks [21]. After euthanasia, the experimental sockets were removed with a security margin using an electric saw.
Sample processing and histological analysis were performed according to the protocol described by Ricardo et al. [28]. The alveolar bone samples were immersed in formaldehyde 4% for 24 h, followed by decalcification with EDTA + TRIS 0.5 M, which was changed every 2 days. The samples were dehydrated in a crescent alcohol series, diaphanized in xylol, and embedded in paraffin [28]. There were performed sample sections with 6 μm thickness stained with Masson trichrome for morphological evaluation. The qualitative analysis was performed with a light microscope (DM 4000B, Wetzlar, Germany) equipped with a digital camera (DFC310FX, Wetzlar, Germany).
Morphometric quantification was performed with ImageJ software (1.53e, NIH, Bethesda, MD, USA) in images with X10 magnification, applying a grid-like test system, with a total of 130 points applied to the photomicrographs obtained [28,29]. Total bone area, inflammation infiltrate, and adhesive remnants were analyzed.
The statistical analysis was performed using the “SigmaPlot 15.0” software. The normality of the data was confirmed by the Shapiro–Wilk test. Therefore, to compare the quantitative results, the one-way ANOVA test was applied with the Holm–Sidak post hoc test, allowing the analysis of multiple groups (data, mean ± SD, standard error of the mean).
3. Results
Regarding groups 3 and 4, where ethyl cyanoacrylate adhesive was applied, hemostasis was immediate. In contrast, in groups 3 and 4, which received resorbable sutures, achieving hemostasis took up to 3 min.
Group 1, treated with sutures and blood clots (Figure 1A,B), revealed robust bone formation with apparently more regular structures compared to groups treated with cyanoacrylate (group 3) and cyanoacrylate + PRF (group 4). The newly formed bone in this group revealed a large number of blood vessels in the region of bone formation. In addition, areas of bone formation were observed in which a large number of osteoblasts were observed. In this group, the formation of loose connective tissue with areas suggestive of adipose formation was not so evident.
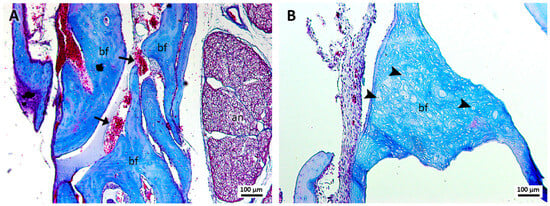
Figure 1.
(A,B). Histological analysis of the sockets at 12 weeks. G1—suture and clot—robust bone formation (bf), presence of blood vessels (arrows), osteoblasts (arrowheads), and the alveolar nerve (an) were observed. Mag.: ×10.
In group 2, treated with PRF and sutures (Figure 2A,B), robust bone formation was revealed with the presence of massive osteocytes and some blood capillaries. The presence of lax tissue similar to adipose cells was evident, as well as erythrocytes and structures suggestive of clots in the region of the dental alveolus.
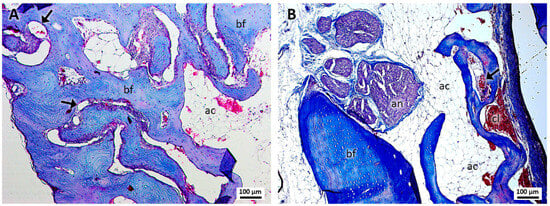
Figure 2.
(A,B). Histological analysis of the sockets at 12 weeks. G2—PRF and suture—robust bone formation (bf), presence of blood capillaries (arrows), lax tissue similar to adipose cells (ac), the alveolar nerve (an), and structures suggestive of a blood clot (cl) were noted. Mag.: ×10.
In group 3, treated with cyanoacrylate and clots (Figure 3A,B), no remnants of this material were observed in the samples, identifying little bone formation with osteocytes and the presence of few and small bone islets. The presence of loose connective tissue with characteristics similar to those of adipocytes was evident, in addition to the presence of formations suggestive of blood clots.
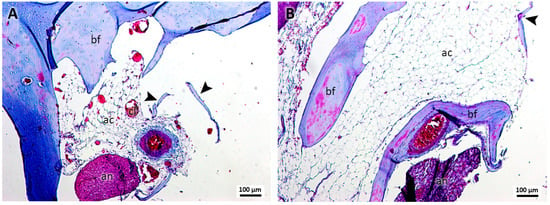
Figure 3.
(A,B). Histological analysis of the sockets at 12 weeks. G3—ethyl-cyanoacrylate and clot—It is possible to observe bone formation (bf), bone islets (arrowhead), structures similar to adipose cells (ac), structures similar to blood clots (cl), and the alveolar nerve (an). Mag.: ×10.
In the samples of group 4, treated with cyanoacrylate and PRF (Figure 4A,B), the presence of PRF and cyanoacrylate was not identified. Bone formation was observed with the presence of osteocytes and a few small bone islets. The bone tissue showed capillaries and few cells suggestive of osteoblasts trapped by the structure. In the region of loose connective tissue, the presence of structures suggestive of clots and formations of adipose tissue was observed.
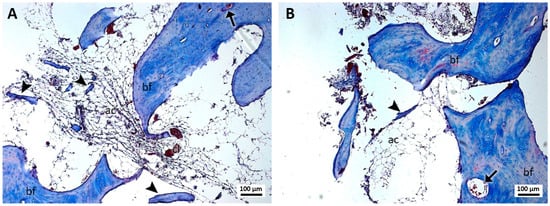
Figure 4.
(A,B). Histological analysis of the sockets at 12 weeks. G4—ethyl-cyanoacrylate and PRF—bone formation (bf), bone islets (arrowhead), blood capillaries (arrow), structures similar to fat cells (ac), and structures similar to blood clots (cl) can be observed. Mag.: ×10.
Considering that neither areas with inflammatory infiltrate nor remains of ethyl cyanoacrylate were observed in the samples from the four groups analyzed, quantification and comparisons only of the area of bone tissue formed were performed.
Regarding the bone tissue area (Table 1), the control group (clot and suture) showed a higher bone area (37.87% ± 17.86) together with the PRF and suture group (30.31 ± 9.36), without observing significant differences between both groups (Figure 5). On the other hand, the groups treated with ethyl-cyanoacrylate adhesive showed the lowest total bone area, without significant differences between them (G3 = 26.6% ± 11.82 vs. G4 = 24.29% ± 6.25); however, the PRF and ethyl-cyanoacrylate group showed significant differences with both the groups treated with resorbable sutures.

Table 1.
Mean, standard deviation (SD), and standard error of the mean (SEM) of total bone area measured in the different groups.
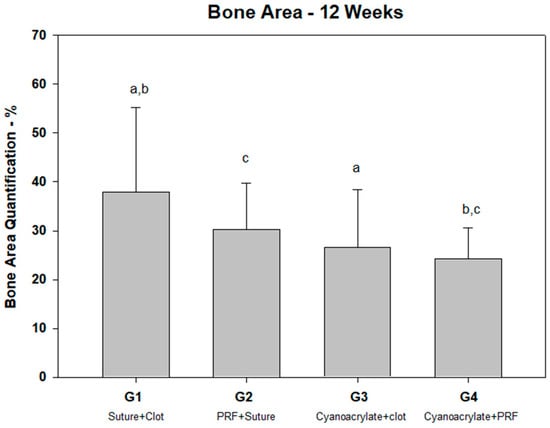
Figure 5.
Percentage of bone area quantification. Note the differences between groups: a (p = 0.018); b (p = 0.01); c (p =0.037).
4. Discussion
The hypothesis was rejected due to the fact that ethyl-cyanoacrylate combined with PRF in fresh sockets of rabbits subjected to anticoagulant therapy did not improve bone regeneration.
Some studies support the use of ethyl-cyanoacrylates in bone healing, concluding that they are biocompatible with osteoblastic cells [2] and do not interfere with the process of bone healing and graft integration [6,30,31]. However, the results observed in our study demonstrated less bone formation in the groups where ethyl-cyanoacrylate was used. In this context, several studies support our findings. A study of ethyl-cyanoacrylate as a bone graft fixation method [32] demonstrated no graft integration during 120 days of follow-up in rabbit calvaria. A similar pilot study [33] involving autologous bone grafts in rabbits demonstrated the compatibility of ethyl-cyanoacrylate but not its effectiveness since there was no total integration of the graft. Other studies on rats showed similar results with cyanoacrylate adhesives, observing no graft fixation, no signs of bone apposition, and the absence of newly formed bone tissue [34,35,36].
In contrast from these last studies, the presence of inflammatory infiltrate and cyanoacrylate remnants were not identified. Some studies [1,30,32,33,34] found remnants of cyanoacrylates in their histological description, with some of them even preventing revascularization [34,36]. It is reported that the degradation of the cyanoacrylate adhesive can take up to 48 weeks [37]. Tzagiollari et al. [38] report that one of the major disadvantages of synthetic adhesives such as cyanoacrylates is related to their biocompatibility and, mainly, their biodegradability. The difference observed in our study may be explained by the nature of the socket, which is an open cavity in constant contact with the tongue, tissues, and food, potentially leading to the dislodgment of the adhesive during the follow-up period.
Also, in both groups using ethyl-cyanoacrylates adhesives as a closure method, the presence of structures suggestive of clots and adipose tissue formation were observed, similar to the reports of Saska et al. [32]. The findings suggest that adipose tissue could be related to mesenchymal precursors and could differentiate into osteoblasts or adipocytes depending on the transcription factors [39]. In this sense, the presence of ethyl-cyanoacrylate could have favored adipogenesis and consequently inhibited osteoblastogenesis.
In the groups in which sutures were used as a closure method, robust areas of bone formation were observed in which a large number of osteoblasts were identified. This result is completely opposed to our initial expectations, as we hypothesized that the ethyl-cyanoacrylate adhesive would allow the stabilization of the blood clot or the PRF, allowing for alveolar bone neoformation due to the presence of growth factors; however, both groups in which ethyl-cyanoacrylate adhesive was used showed significantly less bone neoformation than the clot and suture group, which means it can be inferred that this adhesive negatively influenced the alveolar repair process.
There were no significant differences in the total bone area between the sockets treated with PRF and those treated with clots at 12 weeks. Platelet-rich fibrin seems to be important in the bone healing process only during the early period [40]. Matsumura et al. [41] identified only significant differences in bone healing in dog sockets at 2 weeks. Recent studies [42,43] on rabbit sockets observed differences in terms of the rate and amount of new bone formation compared with the control at 2 weeks, without observing differences at 4 and 8 weeks [43]. However, Damayanti and Rachmawati [44] compared the inhibition of MMP-13 in rabbits 3–7 and 14 days after tooth extraction, reporting no differences between the group treated only with PRF and blood clots.
One of the most clinically notable aspects is the immediate hemostasis achieved in both ethyl-cyanoacrylate groups, which concurs with the findings of Xavier et al. [30], who noted a fast polymerization of the adhesive taking up to 30 s. However, this great feature is not comparable with the poor histological findings in both groups that used ethyl-cyanoacrylate.
Among the limitations of our research are the single evaluation time (12 weeks), the low sample size, and the difficulty of controlling the sockets concerning food and friction; however, the findings are interesting regarding the possible inhibition of bone regeneration in the groups in which ethyl-cyanoacrylate was used.
Although this is an animal study, histological findings suggest that ethyl-cyanoacrylate negatively affects bone regeneration in rabbit sockets. Therefore, its use in patients is not recommended until future studies prove otherwise. However, further studies involving other types of tissue adhesives are necessary to determine whether ethyl-cyanoacrylate specifically interferes with bone regeneration in sockets, or if the entire family of cyanoacrylates, including butyl and octyl-cyanoacrylate, affects it too.
5. Conclusions
The groups that used ethyl-cyanoacrylate as a closure method in sockets exhibited less bone area than the groups that used sutures. Both groups that used PRF as therapy did not show a significant improvement in bone healing at 12 weeks compared with the clot groups.
Author Contributions
Conceptualization, E.B. and F.J.D.; methodology, S.O. and D.P.; validation, E.R., D.P. and J.P.M.I.; formal analysis, D.P.; investigation, E.B. and F.J.D.; resources, E.B.; data curation, S.O.; writing—original draft preparation, E.R., S.O. and D.P.; writing—review and editing, E.B., F.J.D. and J.P.M.I.; supervision, E.B.; project administration, E.B.; funding acquisition, E.B. and F.J.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the DIUFRO project, number DI20-0048.
Institutional Review Board Statement
The animal study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Universidad de la Frontera (protocol code 126/21, Approved 3 May 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors want to acknowledge Carlos Veuthey for their support during animal surgery and aftercare.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Esteves, J.C.; Borrasca, A.G.; Aranega, A.M.; Garcia Junior, I.R.; Magro Filho, O. Histomorphometric analysis of the repair process of autogenous bone grafts fixed at rat calvaria with cyanoacrylate. J. Appl. Oral Sci. 2011, 19, 529–534. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Melo, W.M.; Maximiano, W.M.; Antunes, A.A.; Beloti, M.M.; Rosa, A.L.; de Oliveira, P.T. Cytotoxicity testing of methyl and ethyl 2-cyanoacrylate using direct contact assay on osteoblast cell cultures. J. Oral Maxillofac. Surg. 2013, 71, 35–41. [Google Scholar] [CrossRef]
- Borie, E.; Rosas, E.; Kuramochi, G.; Etcheberry, S.; Olate, S.; Weber, B. Oral Applications of Cyanoacrylate Adhesives: A Literature Review. BioMed Res. Int. 2019, 2019, 8217602. [Google Scholar] [CrossRef] [PubMed]
- Santmarti-Oliver, M.; Bazal-Bonelli, S.; Sanchez-Labrador, L.; Beca-Campoy, T.; Perez-Gonzalez, F.; Cobo-Vazquez, C.M.; Madrigal Martinez-Pereda, C.; Meniz-Garcia, C. Cyanoacrylate versus suture as flap closure methods in mandibular third molar surgery: A split-mouth randomized controlled clinical study. Med. Oral Patol. Oral Cir. Bucal. 2024, 29, e458–e467. [Google Scholar] [CrossRef] [PubMed]
- Tavelli, L.; Asa’ad, F.; Acunzo, R.; Pagni, G.; Consonni, D.; Rasperini, G. Minimizing Patient Morbidity Following Palatal Gingival Harvesting: A Randomized Controlled Clinical Study. Int. J. Periodontics Restor. Dent. 2018, 38, e127–e134. [Google Scholar] [CrossRef]
- Claudino, M.; Almeida Mecca, L.E.; Ferreira Soares, P.B.; Ribeiro, L.; Farago, P.V.; Bernardes, S.R.; Gonzaga, C.C.; de Araujo, M.R. Implant Osseointegration in Grafted Autogenous Bone Blocks Fixed with Screws and Cyanoacrylate-Based Adhesive: Histomorphometric and Micro-CT Analyses. Int. J. Oral Maxillofac. Implant. 2022, 37, 311–319. [Google Scholar] [CrossRef]
- Perez, M.; Fernandez, I.; Marquez, D.; Bretana, R.M. Use of N-butyl-2-cyanoacrylate in oral surgery: Biological and clinical evaluation. Artif. Organs 2000, 24, 241–243. [Google Scholar] [CrossRef]
- Muzumdar, S.; Feng, H. Application of cyanoacrylate to achieve hemostasis in elderly patients with inflamed, friable, and fragile skin receiving anticoagulation therapy after dermatologic surgery. J. Am. Acad. Dermatol. 2020, 83, e97. [Google Scholar] [CrossRef]
- Al-Belasy, F.A.; Amer, M.Z. Hemostatic effect of n-butyl-2-cyanoacrylate (histoacryl) glue in warfarin-treated patients undergoing oral surgery. J. Oral Maxillofac. Surg. 2003, 61, 1405–1409. [Google Scholar] [CrossRef]
- Borie, E.; Olivi, D.G.; Orsi, I.A.; Garlet, K.; Weber, B.; Beltran, V.; Fuentes, R. Platelet-rich fibrin application in dentistry: A literature review. Int. J. Clin. Exp. Med. 2015, 8, 7922–7929. [Google Scholar]
- Ghanaati, S.; Herrera-Vizcaino, C.; Al-Maawi, S.; Lorenz, J.; Miron, R.J.; Nelson, K.; Schwarz, F.; Choukroun, J.; Sader, R. Fifteen Years of Platelet Rich Fibrin in Dentistry and Oromaxillofacial Surgery: How High is the Level of Scientific Evidence? J. Oral Implantol. 2018, 44, 471–492. [Google Scholar] [CrossRef] [PubMed]
- Khaddour, A.S.; Ghita, R.E.; Ionescu, M.; Rica, R.G.; Mercut, V.; Manolea, H.O.; Camen, A.; Draghici, E.C.; Radu, A.; Popescu, S.M. Healing of Extraction Sites after Alveolar Ridge Preservation Using Advanced Platelet-Rich Fibrin: A Retrospective Study. Bioengineering 2024, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Lahham, C.; Ta’a, M.A.; Lahham, E.; Michael, S.; Zarif, W. The effect of recurrent application of concentrated platelet-rich fibrin inside the extraction socket on the hard and soft tissues. a randomized controlled trial. BMC Oral Health 2023, 23, 677. [Google Scholar] [CrossRef]
- Mousavi, Y.; Paknejad, M.; Taheri, M.; Aslroosta, H.; Aminishakib, P.; Panjnoush, M.; Shamshiri, A. Comparison of histologic and radiographic changes of sockets grafted with LPRF and sockets without intervention after tooth extraction. Oral Maxillofac. Surg. 2024, 28, 667–677. [Google Scholar] [CrossRef]
- Zhang, Y.; Ruan, Z.; Shen, M.; Tan, L.; Huang, W.; Wang, L.; Huang, Y. Clinical effect of platelet-rich fibrin on the preservation of the alveolar ridge following tooth extraction. Exp. Ther. Med. 2018, 15, 2277–2286. [Google Scholar] [CrossRef]
- Ozcan, M.; Ucak, O.; Alkaya, B.; Keceli, S.; Seydaoglu, G.; Haytac, M.C. Effects of Platelet-Rich Fibrin on Palatal Wound Healing After Free Gingival Graft Harvesting: A Comparative Randomized Controlled Clinical Trial. Int. J. Periodontics Restor. Dent. 2017, 37, e270–e278. [Google Scholar] [CrossRef] [PubMed]
- Ibraheem, W. Effect of Platelet-rich Fibrin and Free Gingival Graft in the Treatment of Soft Tissue Defect preceding Implant Placement. J. Contemp. Dent. Pract. 2018, 19, 895–899. [Google Scholar] [CrossRef]
- Basma, H.S.; Saleh, M.H.A.; Abou-Arraj, R.V.; Imbrogno, M.; Ravida, A.; Wang, H.L.; Li, P.; Geurs, N. Patient-reported outcomes of palatal donor site healing using four different wound dressing modalities following free epithelialized mucosal grafts: A four-arm randomized controlled clinical trial. J. Periodontol. 2023, 94, 88–97. [Google Scholar] [CrossRef]
- Silva, A.L.M.; de Souza, J.A.C.; Nogueira, T.E. Postoperative local interventions for the palate as a gingival graft donor area: A scoping review. Clin. Oral Investig. 2023, 27, 6971–7006. [Google Scholar] [CrossRef]
- de Almeida, M.C.L.; Rocha, R.G.G.; Magno, M.B.; Lima, R.R.; Saito, M.T. Performance of multiple therapeutic approaches for palatal wound healing after soft tissue graft removal—An overview of systematic reviews. Clin. Oral Investig. 2024, 28, 347. [Google Scholar] [CrossRef]
- Borie, E.; Fuentes, R.; Del Sol, M.; Oporto, G.; Engelke, W. The influence of FDBA and autogenous bone particles on regeneration of calvaria defects in the rabbit: A pilot study. Ann. Anat. 2011, 193, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals. In Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011.
- Hamad, S.A.; Naif, J.S.; Abdullah, M.A. Effect of Diode Laser on Healing of Tooth Extraction Socket: An Experimental Study in Rabbits. J. Maxillofac. Oral Surg. 2016, 15, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Pekow, C.A. Basic Experimental Methods in the Rabbit. In American College of Laboratory Animal Medicine, The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents, 1st ed.; Suckow, M.A., Stevens, K.A., Wilson, R.P., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 243–258. [Google Scholar]
- Turner, P.V.; Chen, H.C.; Taylor, W.M. Pharmacokinetics of meloxicam in rabbits after single and repeat oral dosing. Comp. Med. 2006, 56, 63–67. [Google Scholar]
- Bacevic, M.; Brkovic, B.; Lambert, F.; Djukic, L.; Petrovic, N.; Roganovic, J. Leukocyte- and platelet-rich fibrin as graft material improves microRNA-21 expression and decreases oxidative stress in the calvarial defects of diabetic rabbits. Arch. Oral Biol. 2019, 102, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Bayram, B.; Akdeniz, S.S.; Diker, N.; Helvacioglu, F.; Erdem, S.R. Effects of Platelet-Rich Fibrin Membrane on Sciatic Nerve Regeneration. J. Craniofac. Surg. 2018, 29, e239–e243. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, V.; Sousa, L.G.; Regalo, I.H.; Pitol, D.L.; Bombonato-Prado, K.F.; Regalo, S.C.H.; Siessere, S. Lycopene enhances bone neoformation in calvaria bone defects of ovariectomized rats. Braz. Dent. J. 2023, 34, 50–56. [Google Scholar] [CrossRef]
- Rosa, A.P.; de Sousa, L.G.; Regalo, S.C.; Issa, J.P.; Barbosa, A.P.; Pitol, D.L.; de Oliveira, R.H.; de Vasconcelos, P.B.; Dias, F.J.; Chimello, D.T.; et al. Effects of the combination of low-level laser irradiation and recombinant human bone morphogenetic protein-2 in bone repair. Lasers Med. Sci. 2012, 27, 971–977. [Google Scholar] [CrossRef]
- Xavier, M.S.; Leite, V.M. The Effect of 2-Butyl-Cyanoacrylate Adhesive in Osteotomies and Bone Grafts in Rabbits: Macroscopic and Radiographic Characteristics. Rev. Bras. Ortop. 2012, 47, 638–645. [Google Scholar] [CrossRef]
- Lee, J.S.; Ko, S.H.; Kim, Y.T.; Jung, U.W.; Choi, S.H. Guided bone regeneration using cyanoacrylate-combined calcium phosphate in a dehiscence defect: A histologic study in dogs. J. Oral Maxillofac. Surg. 2012, 70, 2070–2079. [Google Scholar] [CrossRef]
- Saska, S.; Hochuli-Vieira, E.; Minarelli-Gaspar, A.M.; Gabrielli, M.F.; Capela, M.V.; Gabrielli, M.A. Fixation of autogenous bone grafts with ethyl-cyanoacrylate glue or titanium screws in the calvaria of rabbits. Int. J. Oral Maxillofac. Surg. 2009, 38, 180–186. [Google Scholar] [CrossRef]
- Hochuli-Vieira, E.; Engler Pinto, A.C.B.; Pereira-Filho, V.A.; Saska, S.; Monnazzi, M.S. Adhesives based on butyl-cyanoacrylate for fixation of autologous bone graft: Pilot study in rabbits. Dent. Traumatol. 2017, 33, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Esteves, J.C.; Monteiro, J.M.; Aranega, A.M.; Betoni Junior, W.; Sonoda, C.K. Utilization of ethyl cyanoacrylate and 2-octyl cyanoacrylate adhesives for autogenous bone graft fixation: Histomorphometric study in rats. J. Oral Implantol. 2014, 40, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.J.; Gruber, T.M.; Zahorsky-Reeves, J.L.; Lawson, G.W. Comparison of 2-Ethyl-Cyanoacrylate and 2-Butyl-Cyanoacrylate for Use on the Calvaria of CD1 Mice. J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 199–203. [Google Scholar] [PubMed]
- Gomez, V.; Cairns, M.; Weinhold, P.; Jeffs, A.D.; Bortner, B.; Paterno, A.V.; Dahners, L.; Draeger, R.W. 2-Octyl Cyanoacrylate (Dermabond(R)) Inhibits Bridging Bone Formation of Articular Fractures in a Rat Model. Cureus 2021, 13, e16758. [Google Scholar] [CrossRef] [PubMed]
- Toriumi, D.M.; Raslan, W.F.; Friedman, M.; Tardy, M.E. Histotoxicity of cyanoacrylate tissue adhesives. A comparative study. Arch. Otolaryngol. Head Neck Surg. 1990, 116, 546–550. [Google Scholar] [CrossRef]
- Tzagiollari, A.; McCarthy, H.O.; Levingstone, T.J.; Dunne, N.J. Biodegradable and Biocompatible Adhesives for the Effective Stabilisation, Repair and Regeneration of Bone. Bioengineering 2022, 9, 250. [Google Scholar] [CrossRef]
- Pierce, J.L.; Begun, D.L.; Westendorf, J.J.; McGee-Lawrence, M.E. Defining osteoblast and adipocyte lineages in the bone marrow. Bone 2019, 118, 2–7. [Google Scholar] [CrossRef]
- Al-Maawi, S.; Becker, K.; Schwarz, F.; Sader, R.; Ghanaati, S. Efficacy of platelet-rich fibrin in promoting the healing of extraction sockets: A systematic review. Int. J. Implant. Dent. 2021, 7, 117. [Google Scholar] [CrossRef]
- Matsumura-Matsuo, M.; To, M.; Okudera, T.; Matsuo, M. Regeneration processes of alveolar bone and microvascular changes after the application of platelet-rich fibrin. J. Oral Biosci. 2023, 65, 218–225. [Google Scholar] [CrossRef]
- Oncu, E.; Bayram, B.; Kantarci, A.; Gulsever, S.; Alaaddinoglu, E.E. Positive effect of platelet rich fibrin on osseointegration. Med. Oral Patol. Oral Cir. Bucal. 2016, 21, e601–e607. [Google Scholar] [CrossRef]
- Li, S.; Yang, H.; Duan, Q.; Bao, H.; Li, A.; Li, W.; Chen, J.; He, Y. A comparative study of the effects of platelet-rich fibrin, concentrated growth factor and platelet-poor plasma on the healing of tooth extraction sockets in rabbits. BMC Oral Health 2022, 22, 87. [Google Scholar] [CrossRef] [PubMed]
- Damayanti, M.M.; Rachmawati, M. Pre-Clinical Study: Immunohistochemical evaluation of matrix metalloproteinase-13 on rabbit (Oryctolagus cuniculus) socket healing after application of platelet-rich fibrin with and without hydroxyapatite. F1000Research 2022, 11, 29. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).