A Scoping Review of GLP-1 Receptor Agonists: Are They Associated with Increased Gastric Contents, Regurgitation, and Aspiration Events?
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Abstraction
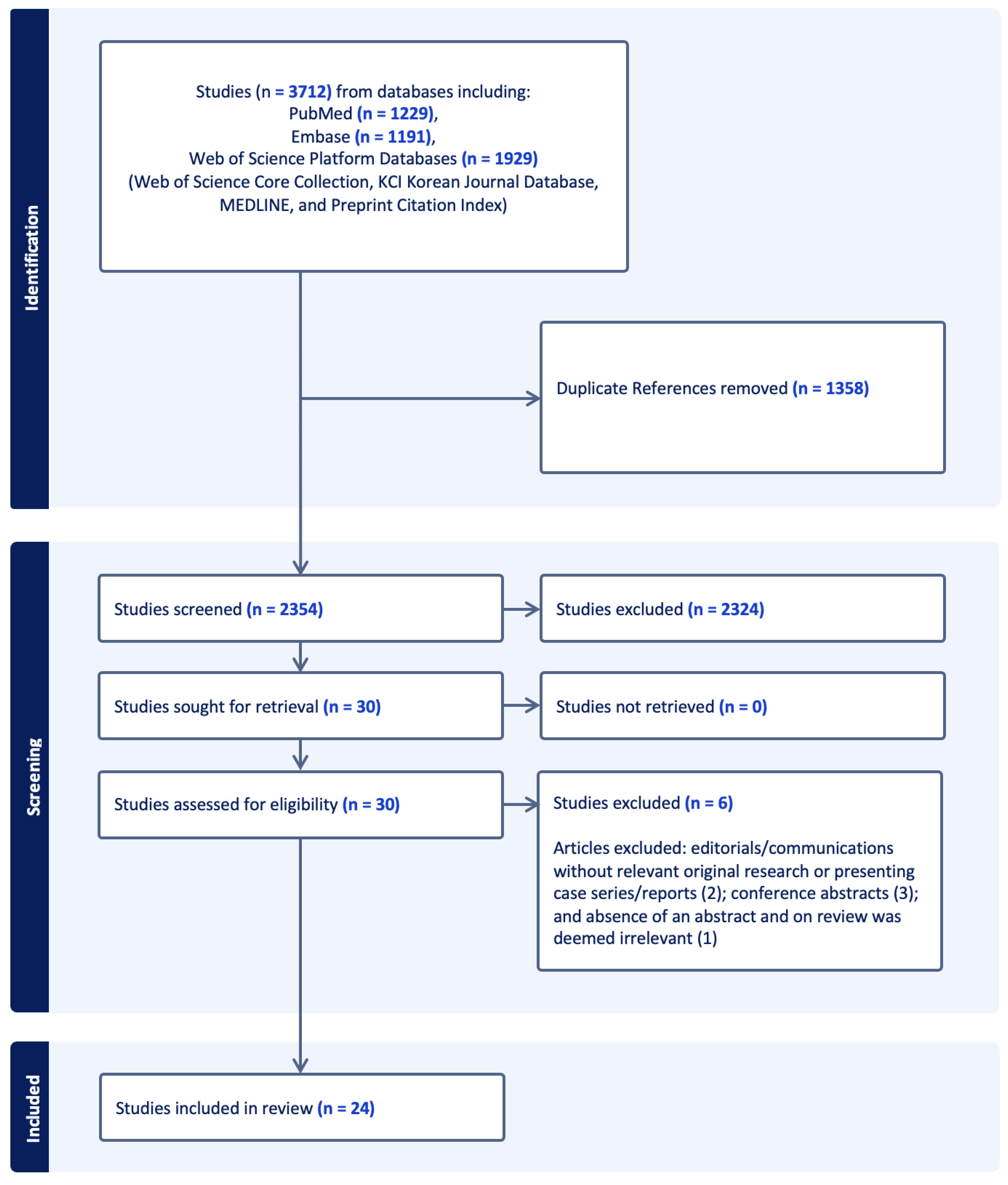
3. Results
3.1. Types and Scale of Studies
3.2. Confounding Factors
3.3. Holding GLP-1 Agonist Duration
3.4. GI Symptoms on Presentation
3.5. Endoscopic, Ultrasound, and Gastric Volume Findings
3.6. Aspiration or Regurgitant Events and Outcomes
4. Discussion
4.1. Variable Association of GLP-1 RAs with Retained Gastric Contents
4.2. Variable Association of GLP-1 RAs with Aspiration and Regurgitant Events
4.3. Potential Impact of Diabetes and the Severity of Disease
4.4. Gastrointestinal Symptoms and Risk for Retained Gastric Contents
4.5. Holding of Medications Prior to Procedure
4.6. Prolonged Fasting to Reduce Retained Gastric Food and Residuals
4.7. Gastric Ultrasound Studies to Assess Aspiration Risk
4.8. Type, Dosing, and Duration of GLP-1 RA Use
4.9. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Honigberg, M.C.; Chang, L.S.; McGuire, D.K.; Plutzky, J.; Aroda, V.R.; Vaduganathan, M. Use of Glucagon-Like Peptide-1 Receptor Agonists in Patients with Type 2 Diabetes and Cardiovascular Disease: A Review. JAMA Cardiol. 2020, 5, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.P.; Sharma, A.; Butler, J.; Packer, M.; Zannad, F.; Vasques-Nóvoa, F.; Leite-Moreira, A.; Neves, J.S. Glucagon-Like Peptide-1 Receptor Agonists Across the Spectrum of Heart Failure. J. Clin. Endocrinol. Metab. 2023, 109, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Ussher, J.R.; Drucker, D.J. Glucagon-like peptide 1 receptor agonists: Cardiovascular benefits and mechanisms of action. Nat. Rev. Cardiol. 2023, 20, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Popoviciu, M.S.; Paduraru, L.; Yahya, G.; Metwally, K.; Cavalu, S. Emerging Role of GLP-1 Agonists in Obesity: A Comprehensive Review of Randomised Controlled Trials. Int. J. Mol. Sci. 2023, 24, 10449. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- O’Neil, P.M.; Birkenfeld, A.L.; McGowan, B.; Mosenzon, O.; Pedersen, S.D.; Wharton, S.; Carson, C.G.; Jepsen, C.H.; Kabisch, M.; Wilding, J.P.H. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: A randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet 2018, 392, 637–649. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Abildstrøm, S.Z.; Borlaug, B.A.; Butler, J.; Rasmussen, S.; Davies, M.; Hovingh, G.K.; Kitzman, D.W.; Lindegaard, M.L.; Møller, D.V.; et al. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity. N. Engl. J. Med. 2023, 389, 1069–1084. [Google Scholar] [CrossRef]
- Ferhatbegovic, L.; Mrsic, D.; Macic-Dzankovic, A. The benefits of GLP1 receptors in cardiovascular diseases. Front. Clin. Diabetes Healthc. 2023, 4, 1293926. [Google Scholar] [CrossRef]
- Jones, P.M.; Hobai, I.A.; Murphy, P.M. Anesthesia and glucagon-like peptide-1 receptor agonists: Proceed with caution! Can. J. Anesth. 2023, 70, 1281–1286. [Google Scholar] [CrossRef]
- Marroquin-Harris, M.; Olesnicky, B. Aspiration risk with glucagon-like peptide 1 (GLP-1) agonists. Anaesthesia 2023, 78, 1524. [Google Scholar] [CrossRef]
- Sun, J.; Wei, G.; Hu, L.; Liu, C.; Ding, Z. Perioperative pulmonary aspiration and regurgitation without aspiration in adults: A retrospective observational study of 166,491 anesthesia records. Ann. Palliat. Med. 2021, 10, 4037–4046. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.; Davidson, A. Aspiration under anaesthesia: Risk assessment and decision-making. Contin. Educ. Anaesth. Crit. Care Pain 2013, 14, 171–175. [Google Scholar] [CrossRef]
- Warner, M.A.; Meyerhoff, K.L.; Warner, M.E.; Posner, K.L.; Stephens, L.; Domino, K.B. Pulmonary Aspiration of Gastric Contents: A Closed Claims Analysis. Anesthesiology 2021, 135, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.G.; Bittner, E.A. Comparison of societal guidance on perioperative management of glucagon-like peptide-1 receptor agonists: Implications for clinical practice and future investigations. Can. J. Anaesth. 2024, 71, 1302–1315. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice, C. 16. Diabetes Care in the Hospital: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45 (Suppl. S1), S244–S253. [Google Scholar] [CrossRef]
- Australian and New Zealand College of Anaesthetists. Surgery Warning on Use of Popular Weight Loss Drugs. Available online: https://www.anzca.edu.au/resources/media-releases/2023-media-releases/gastric-emptying-mr.pdf (accessed on 5 January 2024).
- Australian Diabetes Society (ADS) and the Australian and New Zealand College of Anaesthetists & Faculty of Pain Medicine (ANZCA). ADS-ANZCA Perioperative Diabetes and Hyperglycaemia Guidelines (Adults). Updated November 2022. Available online: https://www.diabetessociety.com.au/wp-content/uploads/2023/03/ADS-ANZCA-Perioperative-Diabetes-and-Hyperglycaemia-Guidelines-Adults-November-2022-v2-Final.pdf (accessed on 5 January 2024).
- Blonde, L.; Umpierrez, G.E.; Reddy, S.S.; McGill, J.B.; Berga, S.L.; Bush, M.; Chandrasekaran, S.; DeFronzo, R.A.; Einhorn, D.; Galindo, R.J.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline: Developing a Diabetes Mellitus Comprehensive Care Plan-2022 Update. Endocr. Pract. 2022, 28, 923–1049. [Google Scholar] [CrossRef]
- Canadian Anesthesiolgists’ Society. Canadian Anesthesiologists’ Society Medication Safety Bulletin. Available online: https://www.cas.ca/CASAssets/Documents/Advocacy/Semaglutide-bulletin_final.pdf (accessed on 5 January 2024).
- Centre for Perioperative Care. Guideline for Perioperative Care for People with Diabetes Mellitus Undergoing Elective and Emergency Surgery. Available online: https://www.cpoc.org.uk/sites/cpoc/files/documents/2021-03/CPOC-Guideline%20for%20Perioperative%20Care%20for%20People%20with%20Diabetes%20Mellitus%20Undergoing%20Elective%20and%20Emergency%20Surgery.pdf (accessed on 5 January 2024).
- Dhatariya, K.; Levy, N.; Kilvert, A.; Watson, B.; Cousins, D.; Flanagan, D.; Hilton, L.; Jairam, C.; Leyden, K.; Lipp, A.; et al. NHS Diabetes guideline for the perioperative management of the adult patient with diabetes. Diabet. Med. 2012, 29, 420–433. [Google Scholar] [CrossRef]
- Hashash, J.G.; Thompson, C.C.; Wang, A.Y. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin. Gastroenterol. Hepatol. 2023, 22, 705–707. [Google Scholar] [CrossRef]
- Joshi, G.P.; Abdelmalak, B.B.; Weigel, W.A.; Soriano, S.G.; Harbell, M.W.; Kuo, K.I.; Stricker, P.A.; Domino, K.B.; American Society of Anesthesiologists (ASA) Task Force on Preoperative Fasting. American Society of Anesthesiologists Consensus-Based Guidance on Preoperative Management of Patients (Adults and Children) on Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists. Available online: https://www.asahq.org/about-asa/newsroom/news-releases/2023/06/american-society-of-anesthesiologists-consensus-based-guidance-on-preoperative (accessed on 5 January 2024).
- Membership of the Working Party; Barker, P.; Creasey, P.E.; Dhatariya, K.; Levy, N.; Lipp, A.; Nathanson, M.H.; Penfold, N.; Watson, B.; Woodcock, T. Peri-operative management of the surgical patient with diabetes 2015: Association of Anaesthetists of Great Britain and Ireland. Anaesthesia 2015, 70, 1427–1440. [Google Scholar] [CrossRef]
- Nathanson, M.H.; Dhatariya, K. Correction. Anaesthesia 2019, 74, 810. [Google Scholar] [CrossRef]
- Olesnicky, B. GLP-1 Agonists and Gastric Emptying. Bulletin. Australian and New Zealand College of Anaesthetists (ANZCA) & Faculty of Pain Medicine (FPM); Winter 2023:20. Available online: https://www.anzca.edu.au/getattachment/7a78cb16-7867-4100-8464-265108b091e7/ANZCA-Bulletin-Winter-2023 (accessed on 5 January 2024).
- Pfeifer, K.J.; Selzer, A.; Mendez, C.E.; Whinney, C.M.; Rogers, B.; Simha, V.; Regan, D.; Urman, R.D.; Mauck, K. Preoperative Management of Endocrine, Hormonal, and Urologic Medications: Society for Perioperative Assessment and Quality Improvement (SPAQI) Consensus Statement. Mayo Clin. Proc. 2021, 96, 1655–1669. [Google Scholar] [CrossRef]
- American Association of Nurse Anesthesiology. Anesthesia Care of the Patient on a GLP-1 Receptor Agonist. Available online: https://issuu.com/aanapublishing/docs/anesthesia_care_of_the_patient_on_a_glp-1_receptor?fr=sNzJkODcwOTA0NDQ (accessed on 28 March 2024).
- Institute for Safe Medication Practices Canada. Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists: Risk of Aspiration during Anesthesia. ISMP Canada Safety Bulletin. 27 September 2023. Volume 23. pp. 1–7. Available online: https://ismpcanada.ca/wp-content/uploads/ISMPCSB2023-i9-GLP-1.pdf (accessed on 28 March 2024).
- Rincón-Valenzuela, D.A.; Escobar, B. Evidence-based clinical practice manual: Patient preparation for surgery and transfer to the operating room. Colomb. J. Anesthesiol. 2015, 43, 32–50. [Google Scholar]
- Grant, M.J.; Booth, A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Inf. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, Y.; Maeda, M.; Matsumura, M.; Shimizu, R.; Banba, N.; Aso, Y.; Yasu, T.; Harasawa, H. Effect of GLP-1 receptor agonist on gastrointestinal tract motility and residue rates as evaluated by capsule endoscopy. Diabetes Metab. 2017, 43, 430–437. [Google Scholar] [CrossRef]
- Quast, D.R.; Schenker, N.; Menge, B.A.; Nauck, M.A.; Kapitza, C.; Meier, J.J. Effects of Lixisenatide Versus Liraglutide (Short- and Long-Acting GLP-1 Receptor Agonists) on Esophageal and Gastric Function in Patients with Type 2 Diabetes. Diabetes Care 2020, 43, 2137–2145. [Google Scholar] [CrossRef]
- Sen, S.; Potnuru, P.P.; Hernandez, N.; Goehl, C.; Praestholm, C.; Sridhar, S.; Nwokolo, O.O. Glucagon-Like Peptide-1 Receptor Agonist Use and Residual Gastric Content Before Anesthesia. JAMA Surg. 2024, 159, 660–667. [Google Scholar] [CrossRef]
- Sherwin, M.; Hamburger, J.; Katz, D.; DeMaria, S., Jr. Influence of semaglutide use on the presence of residual gastric solids on gastric ultrasound: A prospective observational study in volunteers without obesity recently started on semaglutide. Can. J. Anaesth. 2023, 70, 1300–1306. [Google Scholar] [CrossRef]
- Anazco, D.; Fansa, S.; Hurtado, M.D.; Camilleri, M.; Acosta, A. Low Incidence of Pulmonary Aspiration During Upper Endoscopy in Patients Prescribed a Glucagon-Like Peptide 1 Receptor Agonist. Clin. Gastroenterol. Hepatol. 2024, 22, 1333–1335.e2. [Google Scholar] [CrossRef]
- Bi, D.; Choi, C.; League, J.; Camilleri, M.; Prichard, D.O. Food Residue During Esophagogastroduodenoscopy Is Commonly Encountered and Is Not Pathognomonic of Delayed Gastric Emptying. Dig. Dis. Sci. 2021, 66, 3951–3959. [Google Scholar] [CrossRef]
- Kobori, T.; Onishi, Y.; Yoshida, Y.; Tahara, T.; Kikuchi, T.; Kubota, T.; Iwamoto, M.; Sawada, T.; Kobayashi, R.; Fujiwara, H.; et al. Association of glucagon-like peptide-1 receptor agonist treatment with gastric residue in an esophagogastroduodenoscopy. J. Diabetes Investig. 2023, 14, 767–773. [Google Scholar] [CrossRef]
- Silveira, S.Q.; da Silva, L.M.; de Campos Vieira Abib, A.; de Moura, D.T.H.; de Moura, E.G.H.; Santos, L.B.; Ho, A.M.-H.; Nersessian, R.S.F.; Lima, F.L.M.; Silva, M.V.; et al. Relationship between perioperative semaglutide use and residual gastric content: A retrospective analysis of patients undergoing elective upper endoscopy. J. Clin. Anesth. 2023, 87, 111091. [Google Scholar] [CrossRef]
- Stark, J.E.; Cole, J.L.; Ghazarian, R.N.; Klass, M.J. Impact of Glucagon-Like Peptide-1 Receptor Agonists (GLP-1RA) on Food Content During Esophagogastroduodenoscopy (EGD). Ann. Pharmacother. 2022, 56, 922–926. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Smith, M.R.; Mueller, A.L.; Klapman, S.A.; Everett, L.L.; Houle, T.; Kuo, B.; Hobai, I.A. Association of glucagon-like peptide receptor 1 agonist therapy with the presence of gastric contents in fasting patients undergoing endoscopy under anesthesia care: A historical cohort study. Can. J. Anaesth. 2024, 71, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Avraham, S.A.; Hossein, J.; Somri, F.; Hawash, N.; Hochman, O. Pulmonary aspiration of gastric contents in two patients taking semaglutide for weight loss. Anaesth. Rep. 2024, 12, e12278. [Google Scholar] [CrossRef] [PubMed]
- Kalas, M.A.; Galura, G.M.; McCallum, R.W. Medication-Induced Gastroparesis: A Case Report. J. Investig. Med. High Impact Case Rep. 2021, 9, 23247096211051919. [Google Scholar] [CrossRef]
- Kittner, S.L.; Talbott, A.L.; Vishneski, S.R.; Narbaiza, J.; Shields, J.S. Retained Gastric Contents After Adequate Fasting Associated with GLP-1 Receptor Agonist Use: A Report of 3 Cases. JBJS Case Connect. 2023, 13, e23.00506. [Google Scholar] [CrossRef]
- Raven, L.M.; Stoita, A.; Feller, R.B.; Brown, C.; Greenfield, J.R. Delayed Gastric Emptying with Perioperative Use of Glucagon-like Peptide-1 Receptor Agonists. Am. J. Med. 2023, 136, e233–e234. [Google Scholar] [CrossRef]
- Wilson, P.R.; Bridges, K.H.; Wilson, S.H. Particulate Gastric Contents in Patients Prescribed Glucagon-Like Peptide 1 Receptor Agonists After Appropriate Perioperative Fasting: A Report of 2 Cases. A A Pract. 2023, 17, e01712. [Google Scholar] [CrossRef]
- Almustanyir, S.; Alhabeeb, H.; AlHusseini, N.; Al Thow, M. Gastroparesis with the Initiation of Liraglutide: A Case Report. Cureus 2020, 12, e11735. [Google Scholar] [CrossRef]
- Espinoza, R.T.; Antongiorgi, Z. Glucagon-Like Peptide-1 Receptor Agonists During Electroconvulsive Therapy: Case Report With Evolving Concerns and Management Considerations. J. ECT 2024, 40, 207–212. [Google Scholar] [CrossRef]
- Fujino, E.; Cobb, K.W.; Schoenherr, J.; Gouker, L.; Lund, E. Anesthesia Considerations for a Patient on Semaglutide and Delayed Gastric Emptying. Cureus 2023, 15, e42153. [Google Scholar] [CrossRef]
- Giron-Arango, L.; Perlas, A. Point-of-Care Gastric Ultrasound to Identify a Full Stomach on a Diabetic Patient Taking a Glucagon-Like Peptide 1 Receptor Agonist. A A Pract. 2024, 18, e01751. [Google Scholar] [CrossRef]
- Gulak, M.A.; Murphy, P. Regurgitation under anesthesia in a fasted patient prescribed semaglutide for weight loss: A case report. Can. J. Anaesth. 2023, 70, 1397–1400. [Google Scholar] [CrossRef]
- Ishihara, Y.; Nishiguchi, S.; Branch, J.; Tanaka, E. Suspected Gastroparesis with Concurrent Gastroesophageal Reflux Disease Induced by Low-Dose Liraglutide. Cureus 2022, 14, e26916. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.R.; Hobai, I.A. Semaglutide, delayed gastric emptying, and intraoperative pulmonary aspiration: A case report. Can. J. Anaesth. 2023, 70, 1394–1396. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Madi, M.Y.; Dickstein, A. Liraglutide-induced Acute Gastroparesis. Cureus 2018, 10, e3791. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Siddarthan, I.; Mack, P.F. Clinically significant emesis in a patient taking a long-acting GLP-1 receptor agonist for weight loss. Br. J. Anaesth. 2023, 131, e37–e39. [Google Scholar] [CrossRef]
- Bohman, J.K.; Jacob, A.K.; Nelsen, K.A.; Diedrich, D.A.; Smischney, N.; Olatoye, O.; Molitor, R.; Oblizajek, N.R.; Hanson, A.C.; Buttar, N.S. Incidence of Gastric-to-Pulmonary Aspiration in Patients Undergoing Elective Upper Gastrointestinal Endoscopy. Clin. Gastroenterol. Hepatol. 2018, 16, 1163–1164. [Google Scholar] [CrossRef]
- Davidson, M.B.; Bate, G.; Kirkpatrick, P. Exenatide. Nat. Rev. Drug Discov. 2005, 4, 713–714. [Google Scholar] [CrossRef]
- Montejo, J.C.; Miñambres, E.; Bordejé, L.; Mesejo, A.; Acosta, J.; Heras, A.; Ferré, M.; Fernandez-Ortega, F.; Vaquerizo, C.I.; Manzanedo, R. Gastric residual volume during enteral nutrition in ICU patients: The REGANE study. Intensive Care Med. 2010, 36, 1386–1393. [Google Scholar] [CrossRef]
- Chen, H.; Wu, Z.; Chen, D.; Huang, F. Correlation between blood glucose level and poor wound healing after posterior lumbar interbody fusion in patients with type 2 diabetes. Int. Wound J. 2024, 21, e14340. [Google Scholar] [CrossRef]
- Ata, A.; Lee, J.; Bestle, S.L.; Desemone, J.; Stain, S.C. Postoperative hyperglycemia and surgical site infection in general surgery patients. Arch. Surg. 2010, 145, 858–864. [Google Scholar] [CrossRef]
- Sreedharan, R.; Khanna, S.; Shaw, A. Perioperative glycemic management in adults presenting for elective cardiac and non-cardiac surgery. Perioper. Med. 2023, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Kemmeries, G.; Holst, J.J.; Meier, J.J. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes 2011, 60, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Umapathysivam, M.M.; Lee, M.Y.; Jones, K.L.; Annink, C.E.; Cousins, C.E.; Trahair, L.G.; Rayner, C.K.; Chapman, M.J.; Nauck, M.A.; Horowitz, M.; et al. Comparative effects of prolonged and intermittent stimulation of the glucagon-like peptide 1 receptor on gastric emptying and glycemia. Diabetes 2014, 63, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Little, T.J.; Pilichiewicz, A.N.; Russo, A.; Phillips, L.; Jones, K.L.; Nauck, M.A.; Wishart, J.; Horowitz, M.; Feinle-Bisset, C. Effects of intravenous glucagon-like peptide-1 on gastric emptying and intragastric distribution in healthy subjects: Relationships with postprandial glycemic and insulinemic responses. J. Clin. Endocrinol. Metab. 2006, 91, 1916–1923. [Google Scholar] [CrossRef]
- Meier, J.J.; Gallwitz, B.; Salmen, S.; Goetze, O.; Holst, J.J.; Schmidt, W.E.; Nauck, M.A. Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2003, 88, 2719–2725. [Google Scholar] [CrossRef]
| Author, Year, and Journal | Type of Study | How Many Patients, Age | BMI | Confounding Factors and Exclusion Criteria | Medication Dosing, Frequency, and Duration of Use | Indication for Medication | Fasting Time Prior to Procedure | Holding GLP-1 Agonist Duration | Symptoms on Presentation | EGD, Capsule, Ultrasound, or Gastric Volume Findings, and Other Notable Imaging Findings and Outcomes | Aspiration or Regurgitant Event and Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nakatani et al. Diabetes Metab. 2017 [33] | Prospective observational study | 15 patients (mean age 60.0 ± 13.6 and 71% male) | BMI 26.9 ± 3.8 | Type 2 diabetes Exclusion criteria: Type 1 diabetes, Type 2 diabetes on insulin, history of arrhythmias and treatment, marked dysautonomia, history of pancreatitis, ileus, or abdominal surgery, and contraindication to capsule endoscopy | Liraglutide 0.3 mg initially and then titrated up by 0.3 mg weekly to a final dose of 0.9 mg weekly, total duration of drug for 1 month prior to capsule endoscopy | Diabetes | 11 h | Taken day of capsule endoscopy | Symptoms not mentioned for day of capsule endoscopy procedure. Reported that the digestive symptoms induced by liraglutide, such as nausea and diarrhea, were not severe, and did not require the drug to be discontinued in any patients. | Capsule endoscopy was performed to study the effects of liraglutide on GI motility in diabetic patients, comparing those with and without diabetic neuropathy (DN). Capsule endoscopy was performed both prior to start liraglutide and after one month of liraglutide administration. In diabetic neuropathy (DN) patients, there was no significant difference in the gastric time after compared to before starting liraglutide (0:48:40 ± 0:32:52 h vs. 1:12:36 ± 1:04:30 h, p = 0.19). In non-DN patients, gastric time was significantly increased after liraglutide administration compared to before starting liraglutide (2:33:29 ± 1:37:24 h vs. 1:01:30 ± 0:52:59 h, p = 0.03). Duodenal and small intestine transit times also increased significantly in only the non-DN group (6:45:31 ± 2:41:36 vs. 3:51:03 ± 0:53:47 h, p = 0.03) and not the DN (6:38:42 ± 3:52:42 h vs. 4:10:34 ± 0:25:54 h, p = 0.09) group. GI residue rates significantly increased post-liraglutide compared to prior to starting liraglutide in both the DN (90.0 ± 9.1% vs. 32.1 ± 24%, p < 0.001) and non-DN (78.3 ± 23.9% vs. 32.1 ± 35.3%, p < 0.001) groups. The findings suggest liraglutide slows gastric emptying and GI motility, with effects less pronounced in DN patients, likely due to dysautonomia. | Not applicable |
| Quast et al. Diabetes Care. 2020 [34] | Randomized, bicentric, investigator-blinded, parallel group study | 57 patients (mean age 60.7 yo ± 7.6 and 41.7% female in lixisenatide group, 60.2 yo ± 7.3 and 30.8% female in liraglutide group) | BMI 18–40, mean baseline BMI of 32 and 31.4 in lixisenatide and liraglutide treatment groups, respectively | Type 2 diabetes, DPP-IV inhibitor medications part of some patients’ medication regimen. Exclusion criteria included patients with decompensated diabetes (A1c > 10), preexisting concomitant diseases not typically associated with diabetes (i.e., liver, renal, and hepatic disease), contraindications to GLP-1 RAs and DDP-IV inhibitors, use of medications that impact gastrointestinal motility and secretions, history of GERD or overt gastroparesis, smokers. | Lixisenatide 10 mcg once daily for 1 week, followed by 20 mcg once daily for remainder of the trial; total duration 10 weeks Liraglutide 0.6 mg once daily for week 1, followed by 1.2 mg once daily for week 2, followed by 1.8 mg once daily for the remainder of the trial; total duration 10 weeks | Type 2 diabetes | Duration not specified; mentions that fasting started 8 pm day prior to tests | Not mentioned | Heartburn, nausea, vomiting, diarrhea or loose stools and hypoglycemic events were reported for patients treated with lixisenatide and liraglutide, but no difference between the two groups | Not specifically mentioned related to anesthesia procedures. Esophageal pH and manometry revealed no significant difference in reflux episode frequency and severity, as well as esophageal motility and lower esophageal sphincter functionality compared to baseline. Gastric acidity was significantly reduced by 20.7% (−40.6, −0.8) (p = 0.041) with GLP-1 RAs. The octanoate acid breath test revealed lixisenatide significantly increased the gastric emptying half-time by 52 min (95% CI 16 to 88, p = 0.0065) and liraglutide by 25 min (CI 3 to 46, p = 0.025). | |
| Sherwin et al. Can J Anesth. 2023 [36] | Prospective study | 20 volunteer participants (10 taking semaglutide, 10 controls) (median age 41.5 yo and 70% male in semaglutide group, 31.5 yo and 50% male in control group) | BMI all < 30, higher in semaglutide group compared to control group (median, 26.9 vs. 20.5, p < 0.001) | All but one participant taking semaglutide for weight loss; one was taking semaglutide for diabetes. No patients in control group had diabetes. Other medical conditions associated with delayed gastric emptying were not excluded. Included in the study were patients with diabetes, Parkinson’s disease, multiple sclerosis, amyloidosis, and scleroderma. Exclusion criteria included past history of upper abdominal surgery, hiatal hernia, and currently taking medications associated with delayed gastric emptying (i.e., opioids, proton pump inhibitors, tricyclic antidepressants, calcium channel blockers, antipsychotic drugs, or lithium). | Semaglutide; dose 0.25 mg (50%), 0.5 (40%), and 0.75 (10%); frequency daily (10%) and weekly (90%); duration on therapy 1–2 weeks (40%), 3–4 weeks (40%), 5–8 weeks (!0%), >8 (10%) Majority on <4 weeks of therapy at time of study enrollment | Indication for all patients was weight loss, except one patient was taking for diabetes. | >10 h for all participants | Not mentioned | Gastrointestinal symptoms not evaluated | This prospective study evaluated the effect of GLP-1 receptor agonists (GLP-1RAs) on gastric emptying using ultrasound. After an eight-hour fast, 70% of participants on semaglutide showed solid gastric contents in the supine position, compared to 10% of controls (risk ratio [RR] = 3.50, 95% CI 1.26–9.65, p = 0.02). In the lateral position, these numbers were 90% for semaglutide users vs. 20% for controls (RR 7.36, 95% CI 1.13- 47.7, p = 0.005). Participants subsequently had a gastric ultrasound study performed two hours after clear liquid intake. No lateral position differences were noted, but in the supine position, 90% of controls had an empty stomach versus 30% of the semaglutide group (p = 0.02). The semaglutide group’s gastric solids had a layered/yogurt-like consistency on gastric ultrasound imaging. These findings suggest GLP-1RAs may slow gastric emptying, potentially increasing aspiration risk during anesthesia despite adequate fasting. | Not mentioned |
| Sen et al. JAMA Surgery. 2024 [35] | Propspective cros-sectional study | 124 patients (median age 56 yo [IQR, 46–65 yo], 60% female), of which 50% (62 patients) were taking once-weekly GLP-1 RA and the remainder 50% were non-users. GLP-1 RA group: median age 59 yo [IQR, 48–65 yo], 60% female; Control group: median age 53 yo [IQR, 43–64 yo], 61% female | Median BMI 33.9 [IQR, 30.7–39.2] | Diabetes, GERD, home opioid use, pain, ASA physical status classification. Exclusion criteria included patients with altered gastric anatomy (i.e., prior gastric surgery), pregnancy, recent trauma (<1 month), or inability to lie in the right lateral decubitus position for gastric ultrasound. | Semaglutide, dulaglutide, tirzepatide | Not mentioned | Standard fasting guidelines | Of 62 patient taking GLP-1 RAs, all but 7 patients took their GLP-1 RA in the last 7 days. The remainder of the patients had withheld their GLP-1 RA for 8–15 days. | Not mentioned | Retained gastric content was higher in the GLP-1 RA compared to the non-user group (56% versus 19%) evaluated by preprocedural gastric ultrasound. There was no significant association between the duration of withholding GLP-1 RA and prevalence of retained gastric contents. | Not mentioned |
| Anazco et al. Clinical Gastroenterology and Hepatology. 2023 [37] | Retrospective, multiple hospital cohort study | 2968 unique patients taking GLP-1 RA prescribed prior to endoscopies totaling 4134 endoscopic procedures (average age and sex distribution not mentioned for cohort) | Average BMI of patients not mentioned | Included all patients who had been prescribed any injectable GLP-1 RA and had a prior upper endoscopy after initiation of GLP-1 RA. History of patients who aspirated included ASA III, diabetes, and/or GERD. Excluded patients who had aspirations mentioned in clinical notes prior to the first upper endoscopy event. | Not specifically mentioned. The patients initially identified as having GLP-1 RA prescriptions (irrespective of endoscopy) for the following medications: lixisenatide, tirzepatide, exenatide, liraglutide, dulaglutide, semaglutide (dosing, frequency, duration). | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not rigorously assessed except in the context of 2 definite pulmonary aspiration events from 4134 endoscopic procedures reviewed after GLP-1 RA prescription using a previously validated automatic search algorithm of the electronic medical record system. One of the two patients who had an aspiration event had reported retained food content in stomach and duodenum during upper endoscopy performed under MAC. The other patient who had an aspiration rate was noted to have ectasia with bleeding and underwent argon plasma coagulation (APC). | Using a previously validated automatic search algorithm of the electronic medical record system, the authors identified 2 definite episodes of pulmonary aspirations from 4134 endoscopic procedures. The aspiration rate was 4.8 cases per 10,000 endoscopies in comparison to a prior retrospective study from the institution which revealed an aspiration rate of 4.6 cases per 10,000 endoscopies previously using the same automatic search algorithm. They defined an aspiration event as presence of bilious material or particulate matter in the airway during visualization, and/or radiographic evidence of post-procedural pulmonary infiltrates. The 2 aspiration events occurred in one patient who was a 47 year old female, ASA III, with normal weight, on dulaglutide (3.0 mg weekly, started 30 months ago) for diabetes (HbA1c 6.7) that had an upper endoscopy for abdominal pain and diarrhea to rule out celiac disease, was found to have retained food contents in stomach and duodenum during upper endoscopy, had massive vomiting upon scope removal with direct visualization of aspirated contents in the airways and chest imaging compatible with aspiration, and required intubation and ICU admission for 3 days, with vasopressors, and was discharged from the hospital after 5 days with oxygen; the other patient was a 72 year old female, ASA III, with obesity (class II) and GERD, taking semaglutide (0.5 mg weekly, started 3 months ago) for obesity and no symptoms on initiation of GLP-1 RA, had presented for upper endoscopy for iron deficiency anemia and possible GAVE, found to have diffuse gastric antral vascular actasia and bleeding requiring APC, did not have visualization of retained food contents in stomach and duodenum, had persistent hypoxemia after the procedure with imaging compatible with aspiration, not requiring ICU admission, and was discharged home after 8 days of hospitalization. |
| Bi et al. Digestive Diseases and Sciences. 2021 [38] | Retrospective study | 2150 patients (mean age 55 yo ± 16; 67% female) | BMI not noted | Medications known to impair gastric antral motility and/or cause delayed gastric emptying (GE) including GLP-1 agonists, opioids, antiacids (i.e., histamine receptor antagonists and PPIs), cardiovascular medications (i.e., beta and calcium channel blockers), and others. Patients on GLP-1 agonists represented a small fractions of patients, with exact number not reported. Of note, patients with diabetes were excluded from GLP-1 RA and retained gastric food analysis Excluded patients who had neither structural foregut abnormalities nor medical comorbidities associated with retained gastric food in the multivariate analysis of prevalence (i.e., diabetes, amyloidosis, or a diagnosis of gastroparesis) | Not mentioned | Not specifically mentioned. Of note, patients with diabetes were excluded from GLP-1 RA and retained gastric food analysis. | Not mentioned; assumed adherence to standard pre-procedural fasting (6–8 h) | Not mentioned | Not mentioned. Study included patients presenting to endoscopy unit for any indication. | GLP-1 RAs were not found to have a statistically significant association with retained gastric food (RGF) during EGD on multivariate analysis, although they showed significance on univariate analysis. However, due to the small number of patients taking GLP-1 agonists in the study, the odds ratio did not reach statistical significance, suggesting a potential association that warrants further investigation. Opioids, antacids, and cardiovascular medications were associated with RGF on univariate and multivariate analysis. | Not evaluated |
| Kobori et al. 2023. Journal of Diabetes Investigation [39] | Matched pair case-control study | 1128 individuals with diabetes, had EGD performed, and taking GLP-1 RA matched against control group not taking GLP-1 RA over a 2-year period (median age 70 [IQR 62–76] for GLP RA group, 72 yo yo [IQR 63–77] for non-GLP-1 RA group, approximately 80% male in both groups) | Not mentioned | Diabetes (median HbA1c was 7.3) Excluded patients with history of esophageal or gastric operation, unable to fast and no A1c within 3 months prior to EGD Patients unable to fast were also excluded. | Dulaglutide 0.75 mg, liraglutide <0.3 to 1.8 mg, semaglutide 0.25–1.0 mg, oral semaglutide 3–7 mg, lixisenatide <10–20 ug, exenatide 20 ug (frequency and duration note noted) | Not specifically mentioned | >=12 h | Not specifically mentioned | Not mentioned; indications for EGD procedures not mentioned | The occurrence of gastric residue was significantly higher in those receiving GLP-1 RA treatment (5.4% vs. 0.49%, p = 0.004). The specific GLP-1 RAs used among the 11 patients with gastric residue included liraglutide (1.8 mg daily, 2 of 19 patients or 10.5%), dulaglutide (0.75 mg weekly, 5 of 90 patients or 5.6%), and semaglutide (0.5 mg weekly, 2 of 17 patients or 11.8%; 1.0 mg weekly, 2 of 9 patients or 22.2%). Patients with gastric residue were notably younger than those without. The distribution of GLP-1 RA treatments among these patients indicated varying percentages of gastric residue occurrence, with semaglutide 1.0 mg weekly showing the highest rate (22.2%) among the specific doses and types of GLP-1 RAs mentioned. No gastric residue was reported in patients treated with lower doses of liraglutide (≤1.5 mg), lower doses of semaglutide (0.25 mg), any dose of oral semaglutide, lixisenatide, or exenatide. | Not mentioned |
| Silveira et al. Journal of Clinical Anesthesia. 2023 [40] | Retrospective observational study | 404 patients (33 in semglutide group, 371 in non-semaglutide group) (median age 50.8 (percentile 25–75% 39–64) and 48.5% female) | Obesity (BMI > 30) observed in 19.9% patients, median (percentile 25–75%) BMI was 26.2 (22.98–28.73) | Patients with medical issues included diabetes, PPI use, prior abdominal surgeries, treated hypothyroidism, psychiatric illness, and ASA 1–3. Included patients presenting for colonscopy in addition to EGD, as well as just EGD. Exclusion criteria included conditions such as gastric outlet obstruction, gastrointestinal mechanical obstruction, active gastrointestinal bleeding, recent abdominal surgery within 2 months, emergency procedures, ASA-PS >= IV, ICU admission, as well as other medical conditions (i.e., chronic renal and liver disease, pregnancy, chronic opioid use, etc) and the use of medications (i.e., tricyclic antidepressants, opioids, prokinetics, histamine H2-receptor antagonists) known to affect gastric emptying, except for semaglutide. Patients using GLP-1 agonists other than semaglutide were also excluded. | Semaglutide (dose, frequency, and duration not reported) | Primary indication for weight loss (87.8%), followed by diabetes (12.2%) | Median fasting (percentile 25–75%) for clear fluids 9.3 (5.0–12.8) hours and 14.5 (12.2–28.7) hours for solids. | Patients instructed to stop 10–14 days prior to elective procedure but some unable to follow instructions for a variety of reasons (i.e., last minute notice to fill case cancellations); mean interruption time approximately 10 days | Semalutide group: 27.3% with digestive symptoms; non-semaglutide group: 4.5% with digestive symptoms | The retrospective study of patients undergoing upper endoscopies found that semaglutide use perioperatively significantly increased residual gastric content (RGC) (defined as any amount of solid content, or >0.8 mL/Kg measured from aspiration/suction canister) compared to non-users (24.2% vs. 5.1%, p < 0.001), but not the subjective amount of RGC found on EGD by visual estimation (small, medium, large). Propensity weighted analysis revealed increased risk for RGC with factors such as the use of semaglutides (Prevalence Ratio (PR) = 5.15, 95% CI (1.92–12.92), p < 0.001), preoperative digestive symptoms (including nausea/vomiting, dyspepsia, abdominal distension) (PR = 3.56. 95% CI 2.25–5.78, p < 0.001), semaglutide use and digestive symptoms (PR = 16.5, 95% CI 9.08–34.91, p < 0.001), semaglutide use and no digestive symptoms (PR = 9.68, 95% CI 5.6–17.66, p < 0.001), and no semaglutide use and digestive symptoms (PR = 4.94, 95% CI 1.32–15.77, p = 0.0098); while patients undergoing both EGD and colonoscopy showed a protective effect against RGC (PR = 0.25, 95% CI 0.16–0.39, p < 0.001). Pre-endoscopy digestive symptoms were associated with increased RGC, suggesting their relevance in identifying higher risk patients, especially those using semaglutide perioperatively. The duration of preoperative semaglutide cessation did not impact the presence or absence of RGC (10.5 ± 5.5 and 10.2 ± 5.6 days, p = 0.54). | There was one reported case (0.24%) of pulmonary aspiration under deep sedation for upper endoscopy in a 63-year-old man with obesity (BMI 37.7), prior history of gastric bypass, and semaglutide use (last taken 11 days prior) who had adequately fasted (12.4 h for solids and clear fluids) and reported no digestive symptoms pre-procedure. No negative sequelae were specifically reported for this aspiration event. Of note, their institutional practice is to stop the procedure or intubate to prevent aspiration if excessive gastric residue is found. |
| Stark et al. Annals of Pharmacotherapy. 2021 [41] | Retrospective cohort study with matched controls | 59 patients prescribed GLP-1 RAs over 5 year period, January 2015, matched 1:2 against 118 control group for diagnosis of diabetes and/or cirrhosis given their effects on delayed gastric emptying (mean age 64 yo ± 10 and 83% males for GLP-1 RAs, 66 yo ± 10.2 and 94% males for matched controls) | Mean BMI of 33 for both groups | Diabetes, cirrhosis, concomitant medications that slow GI transit, concomitant prokinetic medications, GERD, Barrett’s esophagus, dysphagia, and gastric polyps. Excluded patients with bowel obstruction; prior diagnosis of gastroparesis; history of esophageal, gastric, or thoracic surgery; or if the indication for EGD was for food impaction, foreign body, or active GI bleed. | Duglatide, liraglutide, exenatide, semaglutide (dose, frequency, and duration of use not specified) | Does not mention specifically | Not mentioned | Not mentioned | Not specifically mentioned. Indications for EGD procedures included anemia, GERD, Barrett’s esophagus, dysphagia, abdominal pain, history of gastric polyps, surgical screening, and other. | The primary endpoint, the odds of observing retained food content during EGD, showed a higher, yet not statistically significant, incidence in the treatment group compared to the control (6.8% vs. 1.7%; Odds Ratio [OR] 4.22, 95% Confidence Interval [CI] 0.87–20.34). Specifically, cases of retained food involved patients on either dulaglutide or liraglutide therapy (two instances noted for each medication). Secondary endpoints evaluated the necessity for gastric lavage or a repeat EGD due to inadequate visualization. Both groups had a single instance of gastric lavage (1.7% vs.0.8%, p = 0.62), which removed retained food to achieve satisfactory visibility. No instances necessitating a repeat EGD were recorded. | Not mentioned |
| Wu et al. CJA. 2024 [42] | Retrospective cohort study | GLP-1 RA group: 64 patients; Control (patients started on GLP-1 RA within 1000 days after EGD): 69 patients GLP-1 RA group: median age 64.1 yo [IQR, 56.6–68.9], 62% female; Control group: median age 58.5 yo [IQR, 45.7–67.6 yo], 53% female | GLP-1 RA group: median BMI 34.0 [IQR, 30.0–38.9]; Control group: median BMI 34.0 [IQR, 30.0–37.6] | Incidence of diabetes (96% GLP-1 RA vs. 25% control), insulin use (31% GLP-1 RA vs. 2% control), oral diabetes medications (41% GLP-1 RA vs. 22% control), nausea on presentation (12% GLP-1 RA vs. 2% control), ASA status III or IV (81% GLP-1 RA vs. 57% control) | In GLP-1 RA group: 70 procedures performed in patients taking semaglutide, 11 liraglutide, 6 dulaglutide, 1 tirzepatide, and 2 combination of two different drugs | Not mentioned | GLP-1 RA group: median 16 h [IQR, 14–19]; Control group: median 16 h [IQR, 14–19 h] | Not mentioned; of note, no instructions to withhold GLP-1 Ras prior to the procedure | Nausea on presentation in 12% GLP-1 RA vs. 2% control group patients | Visible gastric content on EGD was reported in 17 cases (19%) in GLP-1 RA group and 5 cases (5%) in control group (p = 0.004). In GLP RA group, 4 of 71 procedures that started off as MAC, residual gastric contents resulted in emergent endotracheal intubation, and in one case a pulmonary aspiration event which resulted in transfer to ICU; extubated 4 h later and discharged the next day. In the non-user group, there were no such reported events. | Pulmonary aspiration reported in 1 case in GLP-1 RA group (total of 90 procedures from 64 patients) and none in control group (total of 102 procedures from 69 patients). In the one case of a pulmonary aspiration event previously reported in Klein et al., the patient was emergently intubated, had bronchoscopy performed which revealed food remains in the trachea and bronchi, was transferred to the ICU, extubated 4 h later, and discharged the next day. |
| Avraham et al. Anaesthesia Rports. 2024 [43] | Case Series | 2 (Patient 1: 70 yo M) Patient 2: 25 yo F) | Patient 1: BMI 35 Patient 2: BMI 32 | Patient 1: Type 2 diabetes (HbA1c 7%) Patient 2: none reported | Patient 1: semaglutide 1 mg weekly subcutaneous injections Patient 2: semaglutide 1 mg weekly subcutaneous injections | Patient 1: Not mentioned Patient 2: weight loss | Patient 1: 12 h Patient 2: >8 h | Patient 1: 6 days prior to procedure Patient 2: 4 days prior to procedure | Patient 1: No nausea and vomiting Patient 2: Not mentioned | Patient 1: No mention of presence or absence of residual gastric contents on EGD for ERCP following regurgitant episode during laryngoscopy. Nasogastric suctioning performed following regurgitatn epsidoe with no gastric residual volume reported Patient 2: Nasogastric suctioning performed following regurgitant episode following LMA removal following completion of surgery with no gastric residual volume reported. | Patient 1: During laryngosocopy following RSI for ERCP, large volume of regurgitant contents with particulate food noted. CXR with bilateral infiltrates. Patient transferred to ICU following procedure and extubated the following day, and was discharged from hospital after a week. Patient 2: Regurgitation of solid and liquids following LMA removal after completion of surgery for I&D of breast abscess. Patient head tilted and sucitoned, rapid sequence intubation and then extubated awake. Transferred to PACU, with reportedly normal chest xray and observation that was normal. |
| Kalas et al. J Investig Med High Impact Case Rep. 2021 [44] | Case Series | 2 patients (Patient 1: 52 yo F; Patient 2: 57 yo F) | Not mentioned | Patient 1: Type 2 diabetes (A1c 5.7), medications including PPIs and antispasmodics (anticholinergics) (not clear from report if taking at the time) Patient 2: Type 2 diabetes (A1c 8.2) | Kalas et al.: Patient 1: semaglutide subcutaneous weekly (dose not mentioned), unclear duration relative to intial endoscopy procedure; Patient 2: dulaglutide subcutaneous weekly (dose not mentioned), unclear duration relative to initial endoscopy procedure | Not mentioned for both cases | Not mentioned for both cases | Not mentioned for both cases | Patient 1: postprandial epigastric pain, fullness, bloating, and nausea Patient 2: abdominal bloating, nausea, and vomiting | Patient 1: Prior upper and lower endoscopies negative for obstruction. CT abdomen and HIDA scan and abdominal doppler blood flow study unremarkable. Initial scintigraphic GES revealed delayed gastric emptying with 24% isotope retention at 4 h (normal residual <10%). Semaglutide was held for 6 weeks, leading to symptom resolution and a repeat GES showing normalization of gastric emptying. Patient 2: Prior upper and lower endoscopies no obstruction or other abnormalities. A 4 h GES showed 35% isotope retention at 4 h, indicating delayed gastric emptying. Dulaglutide was stopped for 4 weeks, resulting in gradual symptom resolution. A subsequent GES confirmed normal gastric emptying. | Not mentioned for both patients |
| Kittner et al. The Journal of Bone and Joint Surgery. 2023. [45] | Case Series | 3 patients (Patient 1: 75 yo M; Patient 2: 72 yo F; Patient 3: 61 yo M) | All 3 patients obese, BMI not noted | All patients had Type 2 diabetes (A1c not noted) | Semaglutide (dose, frequency, and duration not mentioned) | Not mentioned. Of note, all patients were obese and had diabetes. | Patient 1: 11 h Patient 2: 10 h Patient 3: 14 h | Case 1: last taken day before surgery Case 2: last taken 6 days prior to surgery Case 3: last taken day before surgery | All patients noted new GI symptoms since starting GLP-1 RAs | Gastric ultrasound revealed retained solids for all three patients preoperatively who were presenting for orthopedic procedures, resulting in case postponement. Of note, authors note that this article does not include data on non-orthopedic patients who underwent gastric ultrasound during the same timeframe at their institution and revealed 7 out of 8 patients (87.5%) on GLP-1 RAs with adequate fasting periods who had retained solid food. | No aspiration events; cases postponed after gastric ultrasound revealed retained solids. |
| Raven et al. Am J Med. 2023 [46] | Clinical communication to the editor on case series | 2 patients (Patient 1: 62 yo M; Patient 2: 61 yo M) | Both patients with BMI 30 | Patient 1: history of esophagitis on PPI and hypothyroidism on thyroxine at stable doses Patient 2: history of GERD, HIV on HAART | Patient 1: semaglutide 1 mg (frequency unknown), duration unclear but mentioned it was started week prior to procedure Patient 2: liraglutide daily subcutaneous (dose and duration unknown) | Not mentioned | Patient 1: 13 h for first EGD, 12 h for repeat EGD Patient 2: fasted 10 h | Patient 1: no withholding for 1st EGD (had started week prior to procedure), withheld for 3 weeks prior to 2nd EGD Patient 2: not mentioned | Patient 1: None specifically mentioned. Indication for EGD for 1st patient was routine follow-up for esophagitis. Patient 2: indication for EGD was investigation of reflux | Patient 1: Initial EGD revealed stomach full of solid full and procedure aborted. Repeat EGD when semaglutide held for 3 weeks revealed empty stomach after 12 h of fasting. Patient 2: EGD found stomach full of solid food, and procedure aborted. | Not specifically mentioned. EGD procedures aborted when EGD found stomach full of solid food for both patients. |
| Wilson et al. A&A Practice. 2023 [47] | Case series | 2 (Patien1: 55 yo F; Patient 2: 34 yo F) | Patient 1: BMI 48.84 Patient 2: BMI 50.1 | Patient 1: Type 2 diabetes (no A1c reported), ketamine used for sedation (associated with increased secretions) Patient 2: Type 2 diabetes (A1c 5.9), asymptomatic GERD, thyroid surgery (given post-op regurgitation) | Patient 1: dulaglutide 1.5 mg subcutaneously weekly (duration not specified) Patient 2: semaglutide 7 mg oral daily (duration not specified) | Both patients: diabetes | Patient 1: solids for 10 solids, clear liquids for 4 h Patient 2: solids for 16 h, clear liquids for 5 h | Patient 1: Taken her dulaglutide injection in the past week Patient 2: Held oral semaglutide on morning of surgery | Both patients: no symptoms of gastroparesis, no abdominal surgeries, or abnormalities | None | Patient 1: Developed oropharyngeal secretions and labored respirations after regional anesthesia and initiating monitored anesthesia care with fentanyl, midazolam, propofol, and ketamine for foot arthrodesis, prompting conversion to general anesthesia. Upon intubation, bilious particulate matter was suctioned from the oropharynx, and gastric contents were evacuated. No respiratory symptoms post-procedure were reported, and patient was discharged after 2 h in recovery. Patient 2: Not specifically mentioned. After thyroidectomy under general anesthesia, the patient after extubation experienced multiple episodes of projectile vomiting with bile-tinted particulate matter. Lung auscultation was unremarkable, and the patient received supportive care with suctioning and oxygen therapy, and was discharged the following day per protocol for thyroidectomy procedures. |
| Almustanyir et al. Cureus. 2020 [48] | Case report | 1 (18 yo F) | BMI 31.9 | Type 2 diabetes (A1c 8.4) | Liraglutide (dose, route, frequency, and duration of use not specified) | Diabetes and obesity | Not mentioned | Not mentioned | Gastroparesis symptoms after initial dose of liraglutide | EGD with no proof of an obstructing lesion X-ray with non-obstructive gas pattern with intestinal dilation. CT abdomen with isolated moderate-grade gastric distention, with smooth, thin wall and no evidence of distal gastric, pyloric, or proximal duodenal obstructing masses. Gastric suctioning through NGT and discontinuation of liraglutide and short regimen of antiemetics resulted in symptom resolution. | Not mentioned |
| Espinoza et al. J ECT. 2024 [49] | Case report | 1 (64 yo F) | Class 3 Obesity (BMI >40) | Hypothyroidism | Semaglutide subcutaneously weekly (dosing and duration of use not mentioned) | Obesity | Adequate | Not held, last taken days prior | Not mentioned | Not assessed in patient presenting for ECT | ECT performed after risk/benefit discussion with patient. Uneventful ECT treatment performed with monitored anesthesia care with mask ventilation, without any significant anesthesia modifications. She has subsequently had 9 uneventful ECT treatments with semaglutide held 1 week prior to treatments and resumed after treatments and has had continued weight loss. |
| Fujino et al. Cureus. 2023. [50] | Case report | 1 (31 yo F) | BMI 45.6 | Type 2 diabetes (A1 5.9) | Semaglutide 0.25 mg weekly, duration approximately 1 month | Obesity | Solids for 10 h for first endoscope; for second endoscopy, liquid fast for 36 h | Did not hold, taken as prescribed for first endoscopy; for second endoscopy, last taken 7 days prior to procedure (only held on day of procedure) | No symptoms; of note, EGD performed in preparation for bariatric surgery | EGD initially performed in preparation for bariatric surgery revealed food residue in the stomach, leading to procedure being aborted to minimize risk for aspiration. A month later, repeat EGD performed after 36 h liquid diet fasting period and semaglutide last taken 7 days prior revealed no food residue in stomach. | No aspiration event during EGD when food residue in stomach noted and procedure aborted |
| Giron-Arango et al. A A Pract. 2024 [51] | Case report | 1 (74 yo M) | BMI 36 | Type 2 diabetes, noted to not have history of GERD and gastroparesis | Semaglutide 1 mg subcutaneously weekly | Diabetes | 14 h solids, 5 h fluid | Not held, last dose day prior to surgery | Not mentioned | Increased gastric volume assessed by gastric ultrasound consistent with full stomach, given metoclopramide, performed urological procedure under spinal without sedation after risk/benefit discussion with patient | None |
| Ishihara et al. Curues. 2022 [53] | Case report | 1 (74 yo F) | Not mentioned | Type 2 diabetes (A1c 8.2); of note, also taking sitagliptin (DPP-4 inhibitor) | Liraglutide 0.6 mg (frequency not noted), started taking 4 days prior to symptoms onset | Not specifically noted, likely diabetes | Not mentioned | Not mentioned | Nausea, decreased appetite, abdominal distention, sore throat | NGT placed with 600 cc output, A gastroscopy was performed to investigate the cause of nausea, which showed a patent pylorus along with reflux esophagitis. An NGT was placed with 600 mL of output. EGD to workup nausea was remarkable for reflux esophagitis and no obstruction. CT abdomen revealed fluid accumulation from the stomach to the duodenum without bowel dilation or obstruction. Nausea resolved by third day after NGT placed and suctioned, metoclopramide treatment, and halting diabetic medications and oral nutrition. | Not mentioned |
| Gulak et al. Can J Anesth. 2023 [52] | Case report | 1 patient (48 yo F) | BMI 28 | Hypothyroidism, on levothyroxine. Noted to not be diabetic | Semaglutide 0.5 mg subcutaneous weekly, prescribed 5 months ago | Weight loss | 20 h for solids, 8 h for clears | No withholding, last taken 2 days prior to surgery | No gastrointestinal side effects | OGT placed after regurgitation of excess of 200 mL of clear fluid following induction drained minimal gastric content. | The patient regurgitated a large volume, greater than 200 mL of clear fluid, which occurred after less than 30 s of gentle mask ventilation following standard sequence induction for a scheduled breast procedure. The patient was turned to the lateral decubitus position, suctioned, and then intubated. A flexible fiberoptic bronchoscopy revealed no evidence of aspiration. The surgery proceeded without complications, and the patient’s oxygenation and ventilation remained stable throughout. The patient was administered, metoclopramide and an OGT was placed before extubation, draining minimal gastric content. The patient was successfully extubated, transferred to postanesthesia care unit (PACU), and was discharged home the next day in stable condition. |
| Klein et al. Can J Anesth. 2023 [54] | Case report | 1 (42 yo M) | BMI 37 | Barrett’s esophagus, on PPI and histamine receptor antagonist; OSA, on CPAP; mixed anxiety and depressive disorder, on medications with anticholinergic effects (paroxetine); prior history of lung abscess likely secondary to aspiration in setting of heavy alcohol use, now sober for 4 years. Of note, patient did not have diabetes | Semaglutide 1.7 mg subcutaneous weekly, started taking two months ago with dosing progressively increased to current dosing regimen | Weight loss | >18 h | Not mentioned. | None mentioned | The patient underwent a repeat EGD for treatment of dysplastic mucosa under deep sedation where he was found to have copious quantities of liquid and solid in the stomach and experienced an aspiration event noted in the next column, which was different compared to his prior EGDs which were uncomplicated and his stomach was empty. | Following large quantities of liquid and solid in the stomach on EGD, the stomach was suctioned and the patient was intubated using a rapid sequence induction with succinylcholine. Bronchoscopy was performed, and food remains were removed from the trachea and bronchi. The patient was transferred to the ICU intubated and sedated, and subsequently extubated four hours later with no sequelae, remained asymptotic and discharged the next day. |
| Rai et al. Cureus. 2018 [55] | Case report | 1 (52 yo M) | Not mentioned | Type 2 diabetes (A1c 7.0) Noted to have not been taking narcotics prior to and during hospitalization | Liraglutide 1.2 mg subcutaneously daily, duration not specified other than recently started | Diabetes | Not mentioned | Not mentioned | Nausea, abdominal distention, abdominal pain | NGT placed with 1 L fluid suctioned. EGD unremarkable except for mild irritation in the gastric body likely related to trauma from nasogastric tube. Resolution of symptoms with conservative management including discontinuation of liraglutide, gastric suctioning, anti-emetic and prokinetic therapy | Not mentioned |
| Weber et al. British Journal of Anaesthesia. 2023 [56] | Correspondence with editor—case report | 1 (59 yo F) | Obesity and BMI not noted | No diabetes | Tirzepatide (dose and frequency not mentioned), no details on duration other than the drug was recently started | Weight loss | Noted to be appropriate | Not mentioned | Not mentioned | OGT placed after gastric aspirate in oropharynx triggered conversion from general anesthesia with an LMA to endotracheal tube (details in next column); patient had a large volume emesis that resulted in a total of 750 mL of undigested food and gastric contents suggesting delayed gastric emptying. | The patient underwent a hysteroscopy with polyp resection and experienced significant respiratory and gastrointestinal complications. Initially, a supraglottic airway was placed, but gastric aspirate was observed in the oropharynx shortly thereafter, necessitating tracheal intubation. Following intubation, the patient experienced a large volume emesis containing undigested food. An OGT was then inserted, removing an additional 500 mL of thick gastric aspirate. At the procedure’s conclusion, approximately 750 mL of undigested food and gastric contents were collected, indicating a significant aspiration event. Despite these complications, a subsequent chest radiograph revealed no signs of pneumonia or pneumonitis. The patient was admitted for overnight monitoring and was able to be weaned to room air quickly. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, M.G.; Ripoll, J.G.; Lopez, E.; Krishnan, K.; Bittner, E.A. A Scoping Review of GLP-1 Receptor Agonists: Are They Associated with Increased Gastric Contents, Regurgitation, and Aspiration Events? J. Clin. Med. 2024, 13, 6336. https://doi.org/10.3390/jcm13216336
Chang MG, Ripoll JG, Lopez E, Krishnan K, Bittner EA. A Scoping Review of GLP-1 Receptor Agonists: Are They Associated with Increased Gastric Contents, Regurgitation, and Aspiration Events? Journal of Clinical Medicine. 2024; 13(21):6336. https://doi.org/10.3390/jcm13216336
Chicago/Turabian StyleChang, Marvin G., Juan G. Ripoll, Ernesto Lopez, Kumar Krishnan, and Edward A. Bittner. 2024. "A Scoping Review of GLP-1 Receptor Agonists: Are They Associated with Increased Gastric Contents, Regurgitation, and Aspiration Events?" Journal of Clinical Medicine 13, no. 21: 6336. https://doi.org/10.3390/jcm13216336
APA StyleChang, M. G., Ripoll, J. G., Lopez, E., Krishnan, K., & Bittner, E. A. (2024). A Scoping Review of GLP-1 Receptor Agonists: Are They Associated with Increased Gastric Contents, Regurgitation, and Aspiration Events? Journal of Clinical Medicine, 13(21), 6336. https://doi.org/10.3390/jcm13216336







