Use of Upadacitinib to Treat a Severe Flare-Up of Rheumatoid Arthritis During Anti-PD-1 Immune Checkpoint Inhibitor Therapy for Stage IV Squamous Cell Carcinoma of the Lung
Abstract
1. Introduction
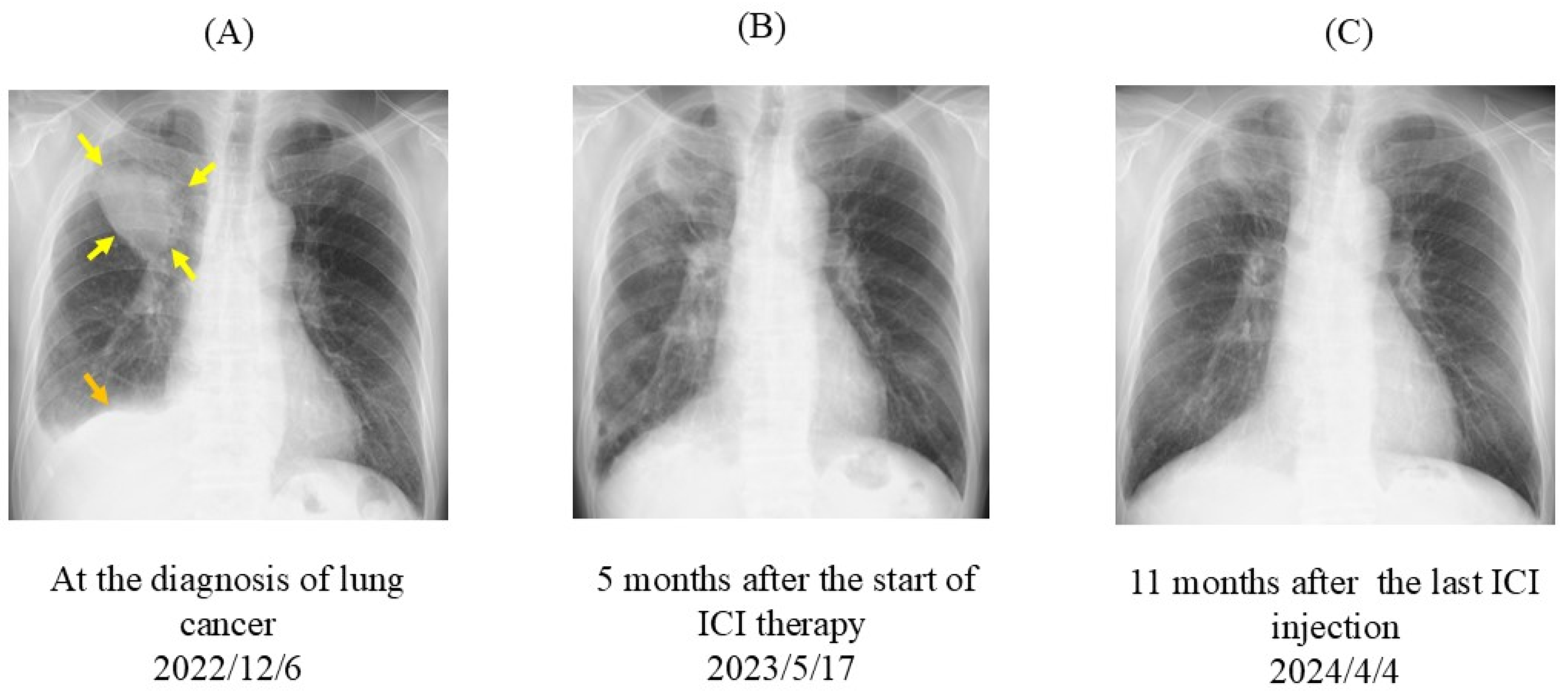
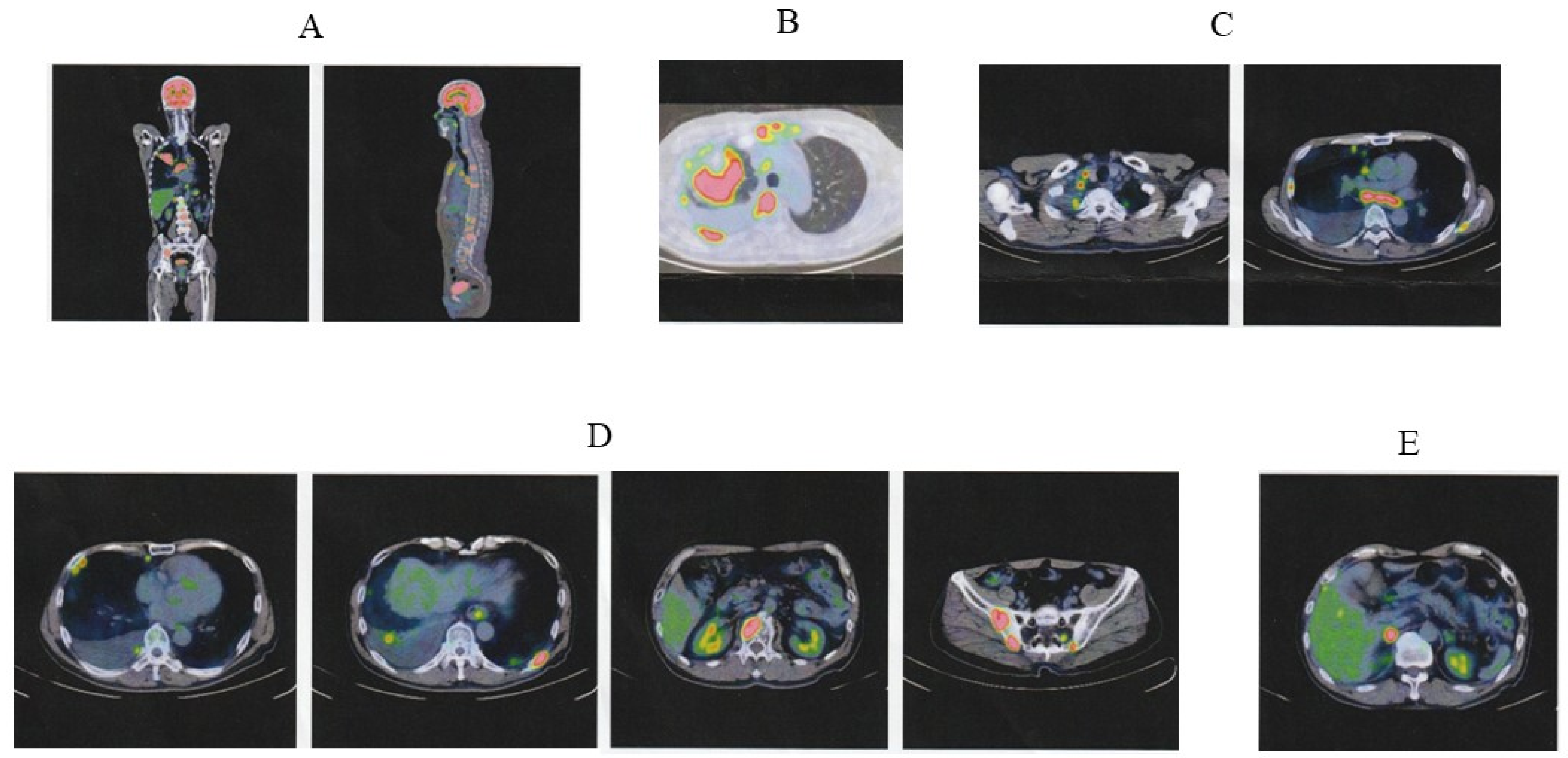
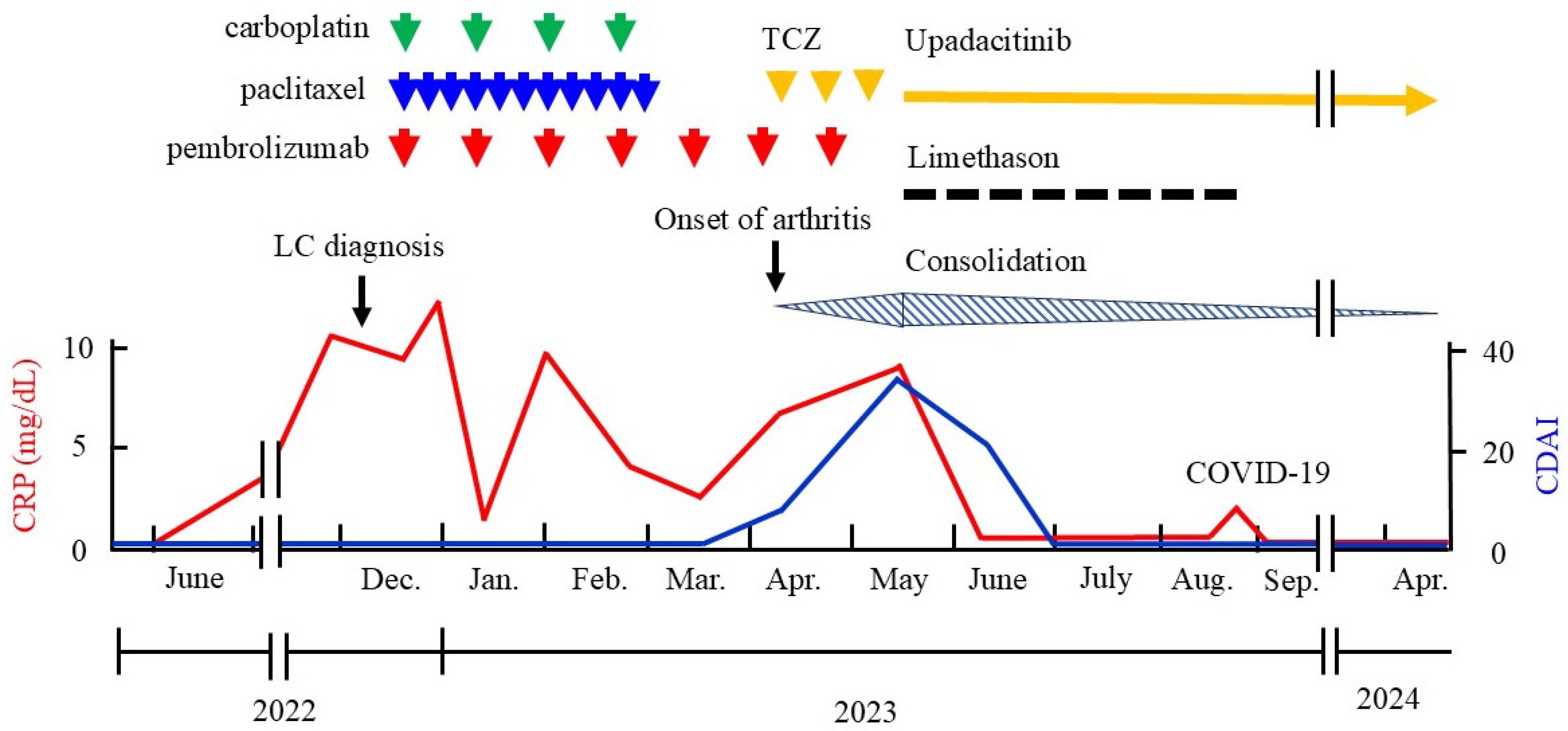
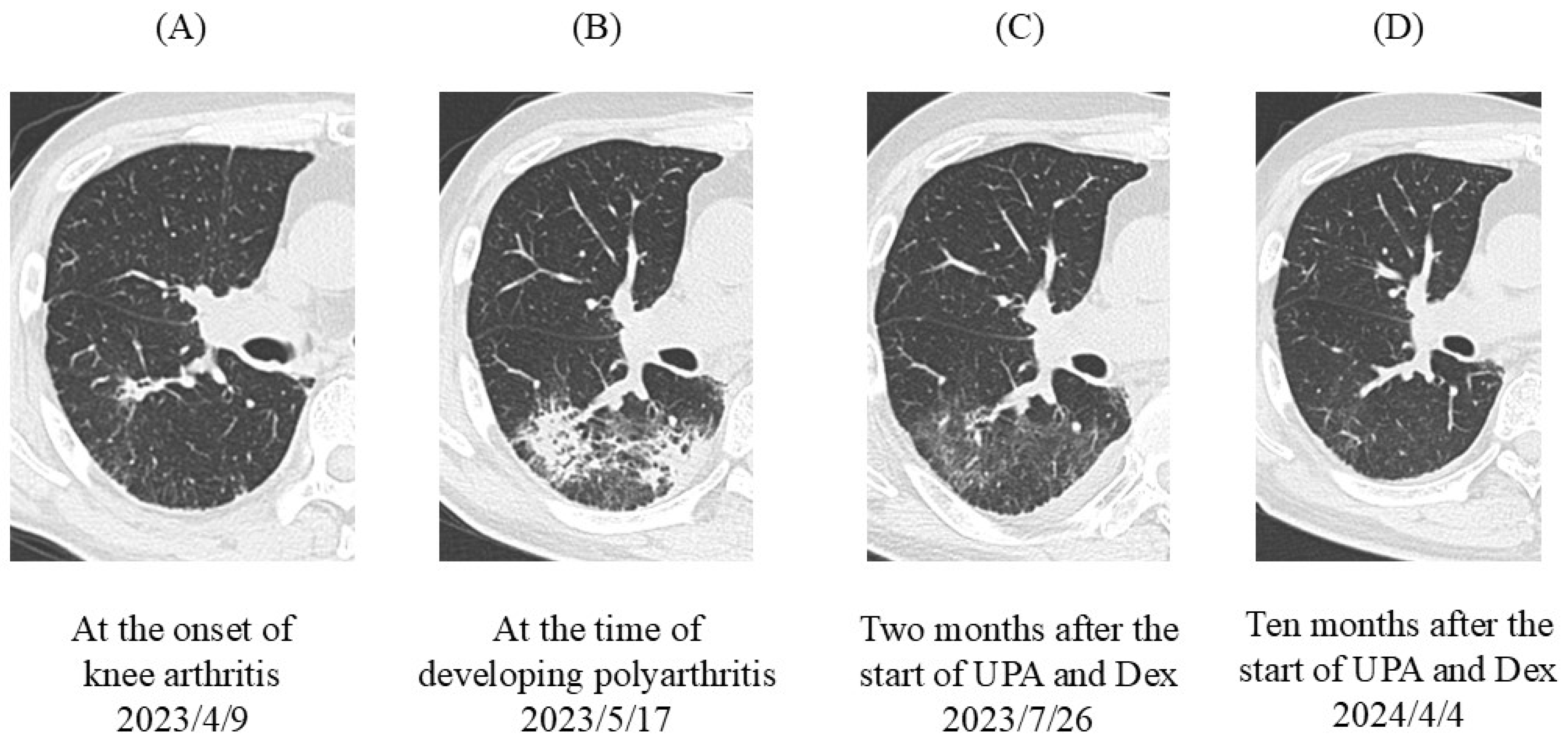
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewe, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 2023, 82, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Wilton, K.M.; Matteson, E.L. Malignancy incidence, management, and prevention in patients with rheumatoid arthritis. Rheumatol. Ther. 2017, 4, 333–347. [Google Scholar] [CrossRef] [PubMed]
- De Cock, D.; Hyrich, K. Malignancy and rheumatoid arthritis: Epidemiology, risk factors and management. Best Pract. Res. Clin. Rheumatol. 2018, 32, 869–886. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Li, C.I. Impact of rheumatoid arthritis and biologic and targeted synthetic disease modifying antirheumatic agents on cancer risk and recurrence. Curr. Opin. Rheumatol. 2021, 33, 292–299. [Google Scholar] [CrossRef]
- Bade, B.C.; Dela Cruz, C.S. Lung cancer 2020: Epidemiology, etiology, and prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef]
- Chatzidionysiou, K.; di Giuseppe, D.; Soderling, J.; Catrina, A.; Askling, J. Risk of lung cancer in rheumatoid arthritis and in relation to autoantibody positivity and smoking. RMD Open 2022, 8, e002465. [Google Scholar] [CrossRef]
- Choi, H.G.; Kang, H.S.; Lim, H.; Kim, J.H.; Kim, J.H.; Cho, S.J.; Nam, E.S.; Min, K.W.; Park, H.Y.; Kim, N.Y.; et al. Potential cancer risk in patients with rheumatoid arthritis: A longitudinal Korean population-based analysis. J. Pers. Med. 2022, 12, 965. [Google Scholar] [CrossRef]
- Wang, F.; Palmer, N.; Fox, K.; Liao, K.P.; Yu, K.H.; Kou, S.C. Large-scale real-world data analyses of cancer risks among patients with rheumatoid arthritis. Int. J. Cancer 2023, 153, 1139–1150. [Google Scholar] [CrossRef]
- Beydon, M.; Pinto, S.; De Rycke, Y.; Fautrel, B.; Mariette, X.; Seror, R.; Tubach, F. Risk of cancer for patients with rheumatoid arthritis versus general population: A national claims database cohort study. Lancet Reg. Health Eur. 2023, 35, 100768. [Google Scholar] [CrossRef]
- Cho, M.H.; Cho, J.H.; Eun, Y.; Han, K.; Jung, J.; Cho, I.Y.; Yoo, J.E.; Lee, H.; Kim, H.; Park, S.Y.; et al. Rheumatoid arthritis and risk of lung Cancer: A nationwide cohort study. J. Thorac. Oncol. 2024, 19, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.M.; Yang, Y.; Roul, P.; Sauer, B.C.; Cannon, G.W.; Kunkel, G.; Michaud, K.; Baker, J.F.; Mikuls, T.R.; England, B.R. A narrowing mortality gap: Temporal trends of cause-specific mortality in a national matched cohort study in US veteranswith rheumatoid arthritis. Arthritis Care Res. 2023, 75, 1648–1658. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Ueki, Y.; Hasegawa, M.; Nakamura, K.; Nakashima, K.; Hidaka, T.; Ishii, K.; Kobayashi, H.; Miyamura, T. Impact of combined pulmonary fibrosis and emphysema on lung cancer risk and mortality in rheumatoid arthritis: A multicenter retrospective cohort study. PLoS ONE 2024, 19, e0298573. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Hasegawa, M.; Sakai, F.; Nakashima, K.; Nakamura, K. Incidence of and predictive factors for lung cancer in patients with rheumatoid arthritis: A retrospective long-term follow-up study. Mod. Rheumatol. 2024, roae084. [Google Scholar] [CrossRef] [PubMed]
- Mamdani, H.; Matosevic, S.; Khalid, A.B.; Durm, G.; Jalal, S.I. Immunotherapy in lung cancer: Current landscape and future directions. Front. Immunol. 2022, 13, 823618. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chavez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suarez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers 2020, 6, 38. [Google Scholar] [CrossRef]
- Tison, A.; Garaud, S.; Chiche, L.; Cornec, D.; Kostine, M. Immune-checkpoint inhibitor use in patients with cancer and pre-existing autoimmune diseases. Nat. Rev. Rheumatol. 2022, 18, 641–656. [Google Scholar] [CrossRef]
- Onoi, K.; Chihara, Y.; Uchino, J.; Shimamoto, T.; Morimoto, Y.; Iwasaku, M.; Kaneko, Y.; Yamada, T.; Takayama, K. Immune checkpoint inhibitors for lung cancer treatment: A review. J. Clin. Med. 2020, 9, 1362. [Google Scholar] [CrossRef]
- Tang, S.; Qin, C.; Hu, H.; Liu, T.; He, Y.; Guo, H.; Yan, H.; Zhang, J.; Tang, S.; Zhou, H. Immune checkpoint inhibitors in non-small cell lung cancer: Progress, challenges, and prospects. Cells 2022, 11, 320. [Google Scholar] [CrossRef]
- Cai, Q.; Huo, G.W.; Zhu, F.Y.; Yue, P.; Yuan, D.Q.; Chen, P. Safety and efficacy of immune checkpoint inhibitors in advanced cancer patients with autoimmune disease: A meta-analysis. Hum. Vaccines Immunother. 2022, 18, 2145102. [Google Scholar] [CrossRef]
- Lopez-Olivo, M.A.; Kachira, J.J.; Abdel-Wahab, N.; Pundole, X.; Aldrich, J.D.; Carey, P.; Khan, M.; Geng, Y.; Pratt, G.; Suarez-Almazor, M.E. A systematic review and meta-analysis of observational studies and uncontrolled trials reporting on the use of checkpoint blockers in patients with cancer and pre-existing autoimmune disease. Eur. J. Cancer 2024, 207, 114148. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, N.; Shah, M.; Lopez-Olivo, M.A.; Suarez-Almazor, M.E. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: A systematic review. Ann. Intern. Med. 2018, 168, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Huang, H.; Xiao, S.; Fan, Y.; Deng, X.; Zhang, Z. Immune checkpoint inhibitors therapies in patients with cancer and preexisting autoimmune diseases: A meta-analysis of observational studies. Autoimmun. Rev. 2020, 19, 102687. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, S.; Ke, L.; Cui, H. Immune checkpoint inhibitors in cancer patients with rheumatologic preexisting autoimmune diseases: A systematic review and meta-analysis. BMC Cancer 2024, 24, 490. [Google Scholar] [CrossRef]
- Sparks, J.A. Pre-existing autoimmune diseases and immune checkpoint inhibitors for cancer treatment: Considerations about initiation, flares, immune-related adverse events, and cancer progression. Rheum. Dis. Clin. North Am. 2024, 50, 147–159. [Google Scholar] [CrossRef]
- Gadina, M.; Johnson, C.; Schwartz, D.; Bonelli, M.; Hasni, S.; Kanno, Y.; Changelian, P.; Laurence, A.; O’Shea, J.J. Translational and clinical advances in JAK-STAT biology: The present and future of jakinibs. J. Leukoc. Biol. 2018, 104, 499–514. [Google Scholar] [CrossRef]
- Bonelli, M.; Kerschbaumer, A.; Kastrati, K.; Ghoreschi, K.; Gadina, M.; Heinz, L.X.; Smolen, J.S.; Aletaha, D.; O’Shea, J.; Laurence, A. Selectivity, efficacy and safety of JAKinibs: New evidence for a still evolving story. Ann. Rheum. Dis. 2024, 83, 139–160. [Google Scholar] [CrossRef]
- Parker, B.S.; Rautela, J.; Hertzog, P.J. Antitumour actions of interferons: Implications for cancer therapy. Nat. Rev. Cancer 2016, 16, 131–144. [Google Scholar] [CrossRef]
- Ivashkiv, L.B. IFNgamma: Signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef]
- Mori, S.; Koga, Y.; Sugimoto, M. Organizing pneumonia in rheumatoid arthritis patients: A case-based review. Clin. Med. Insights Circ. Respir. Pulm. Med. 2015, 9, 69–80. [Google Scholar] [CrossRef]
- McCarter, K.R.; Arabelovic, S.; Wang, X.; Wolfgang, T.; Yoshida, K.; Qian, G.; Kowalski, E.N.; Vanni, K.M.M.; LeBoeuf, N.R.; Buchbinder, E.I.; et al. Immunomodulator use, risk factors and management of flares, and mortality for patients with pre-existing rheumatoid arthritis after immune checkpoint inhibitors for cancer. Semin. Arthritis Rheum. 2024, 64, 152335. [Google Scholar] [CrossRef] [PubMed]
- Efuni, E.; Cytryn, S.; Boland, P.; Niewold, T.B.; Pavlick, A.; Weber, J.; Sandigursky, S. Risk of toxicity after initiating immune checkpoint inhibitor treatment in patients with rheumatoid arthritis. J. Clin. Rheumatol. 2021, 27, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Dang, Q.M.; Watanabe, R.; Shiomi, M.; Fukumoto, K.; Nobashi, T.W.; Okano, T.; Yamada, S.; Hashimoto, M. Rheumatic immune-related adverse events due to immune checkpoint inhibitors: A 2023 update. Int. J. Mol. Sci. 2023, 24, 5643. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.C.; Choy, E.; Baraliakos, X.; Szekanecz, Z.; Xavier, R.M.; Isaacs, J.D.; Strengholt, S.; Parmentier, J.M.; Lippe, R.; Tanaka, Y. Differential properties of Janus kinase inhibitors in the treatment of immune-mediated inflammatory diseases. Rheumatology 2024, 63, 298–308. [Google Scholar] [CrossRef]
- Owen, K.L.; Brockwell, N.K.; Parker, B.S. JAK-STAT signaling: A double-edged sword of immune regulation and cancer progression. Cancers 2019, 11, 2002. [Google Scholar] [CrossRef]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017, 19, 1189–1201. [Google Scholar] [CrossRef]
- Minn, A.J.; Wherry, E.J. Combination cancer therapies with immune checkpoint blockade: Convergence on interferon signaling. Cell 2016, 165, 272–275. [Google Scholar] [CrossRef]
- Mimura, K.; Teh, J.L.; Okayama, H.; Shiraishi, K.; Kua, L.F.; Koh, V.; Smoot, D.T.; Ashktorab, H.; Oike, T.; Suzuki, Y.; et al. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci. 2018, 109, 43–53. [Google Scholar] [CrossRef]
- Memon, D.; Schoenfeld, A.J.; Ye, D.; Fromm, G.; Rizvi, H.; Zhang, X.; Keddar, M.R.; Mathew, D.; Yoo, K.J.; Qiu, J.; et al. Clinical and molecular features of acquired resistance to immunotherapy in non-small cell lung cancer. Cancer Cell 2024, 42, 209–224.e209. [Google Scholar] [CrossRef]
- Benci, J.L.; Xu, B.; Qiu, Y.; Wu, T.J.; Dada, H.; Twyman-Saint Victor, C.; Cucolo, L.; Lee, D.S.M.; Pauken, K.E.; Huang, A.C.; et al. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell 2016, 167, 1540–1554. [Google Scholar] [CrossRef]
- Zak, J.; Pratumchai, I.; Marro, B.S.; Marquardt, K.L.; Zavareh, R.B.; Lairson, L.L.; Oldstone, M.B.A.; Varner, J.A.; Hegerova, L.; Cao, Q.; et al. JAK inhibition enhances checkpoint blockade immunotherapy in patients with Hodgkin lymphoma. Science 2024, 384, eade8520. [Google Scholar] [CrossRef] [PubMed]
- Mathew, D.; Marmarelis, M.E.; Foley, C.; Bauml, J.M.; Ye, D.; Ghinnagow, R.; Ngiow, S.F.; Klapholz, M.; Jun, S.; Zhang, Z.; et al. Combined JAK inhibition and PD-1 immunotherapy for non-small cell lung cancer patients. Science 2024, 384, eadf1329. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Kumar, D.; Gupta, K.; Lofland, G.; Sharma, A.K.; Banka, D.S.; Hobbs, R.F.; Dannals, R.F.; Rowe, S.P.; Gabrielson, E.; et al. Gallium-68-labeled peptide PET quantifies tumor exposure of PD-L1 therapeutics. Clin. Cancer Res. 2023, 29, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Gupta, K.; Kumar, D.; Lofland, G.; Sharma, A.K.; Solnes, L.B.; Rowe, S.P.; Forde, P.M.; Pomper, M.G.; Gabrielson, E.W.; et al. Non-invasive PD-L1 quantification using [(18)F]DK222-PET imaging in cancer immunotherapy. J. Immunother. Cancer 2023, 11, e007535. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.; Floudas, A.; Murray, C.; Fabre, A.; Crown, J.; Fearon, U.; Veale, D. First use of tofacitinib to treat an immune checkpoint inhibitor-induced arthritis. BMJ Case Rep. 2021, 14, e238851. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, M.; Zou, Z.; Lin, J.; Zhang, N.; Zhao, L.; Zhou, J.; Zhou, H.; Zhou, X.; Jiao, X.; et al. Tofacitinib for the treatment of immune-related adverse events in cancer immunotherapy: A multi-center observational study. J. Transl. Med. 2024, 22, 803. [Google Scholar] [CrossRef]
- Curtis, J.R.; Yamaoka, K.; Chen, Y.H.; Bhatt, D.L.; Gunay, L.M.; Sugiyama, N.; Connell, C.A.; Wang, C.; Wu, J.; Menon, S.; et al. Malignancy risk with tofacitinib versus TNF inhibitors in rheumatoid arthritis: Results from the open-label, randomised controlled ORAL Surveillance trial. Ann. Rheum. Dis. 2023, 82, 331–343. [Google Scholar] [CrossRef]
- Harrington, R.; Harkins, P.; Conway, R. Janus kinase inhibitors in rheumatoid arthritis: An update on the efficacy and safety of tofacitinib, baricitinib and upadacitinib. J. Clin. Med. 2023, 12, 6690. [Google Scholar] [CrossRef]
- Mori, S.; Yoshitama, T.; Ueki, Y. Tofacitinib therapy for rheumatoid arthritis: A direct comparison study between biologic-naïve and experienced patients. Intern. Med. 2018, 57, 663–670. [Google Scholar] [CrossRef]
- Mori, S.; Urata, Y.; Yoshitama, T.; Ueki, Y. Tofacitinib versus tocilizumab in the treatment of biological-naive or previous biological-failure patients with methotrexate-refractory active rheumatoid arthritis. RMD Open 2021, 7, e001601. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mori, S.; Nakamura, K.; Shimamura, M.; Ohe, K. Use of Upadacitinib to Treat a Severe Flare-Up of Rheumatoid Arthritis During Anti-PD-1 Immune Checkpoint Inhibitor Therapy for Stage IV Squamous Cell Carcinoma of the Lung. J. Clin. Med. 2024, 13, 6257. https://doi.org/10.3390/jcm13206257
Mori S, Nakamura K, Shimamura M, Ohe K. Use of Upadacitinib to Treat a Severe Flare-Up of Rheumatoid Arthritis During Anti-PD-1 Immune Checkpoint Inhibitor Therapy for Stage IV Squamous Cell Carcinoma of the Lung. Journal of Clinical Medicine. 2024; 13(20):6257. https://doi.org/10.3390/jcm13206257
Chicago/Turabian StyleMori, Shunsuke, Kazuyoshi Nakamura, Minori Shimamura, and Kouhei Ohe. 2024. "Use of Upadacitinib to Treat a Severe Flare-Up of Rheumatoid Arthritis During Anti-PD-1 Immune Checkpoint Inhibitor Therapy for Stage IV Squamous Cell Carcinoma of the Lung" Journal of Clinical Medicine 13, no. 20: 6257. https://doi.org/10.3390/jcm13206257
APA StyleMori, S., Nakamura, K., Shimamura, M., & Ohe, K. (2024). Use of Upadacitinib to Treat a Severe Flare-Up of Rheumatoid Arthritis During Anti-PD-1 Immune Checkpoint Inhibitor Therapy for Stage IV Squamous Cell Carcinoma of the Lung. Journal of Clinical Medicine, 13(20), 6257. https://doi.org/10.3390/jcm13206257




