Predicting Survival Status in COVID-19 Patients: Machine Learning Models Development with Ventilator-Related and Biochemical Parameters from Early Stages: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Data Acquisition Procedures
2.3. Demographics, Biochemical Data, ABG Measurements, and Ventilator-Associated Parameters
2.4. Statistical Analysis
2.5. Survival Status Prediction and Feature Importance
3. Results
3.1. Demographics of Recruited Participants
3.2. Comparisons of Biochemical Measurements Between the Surviving and Non-Surviving Groups
3.3. Comparisons of ABG Measurements and Ventilator-Associated Parameters Between the Surviving and Non-Surviving Groups
3.4. Associations of Biochemical Data, ABG Measurements, and Ventilator-Associated Parameters with Survivability
3.5. Feature Importance Impacting Survival Status
3.6. Supplementary Analysis: Comparative Assessment of Biochemical Data, ABG Measurements, and Ventilator-Associated Parameters at Different Time Intervals Between the Surviving and Non-Surviving Groups
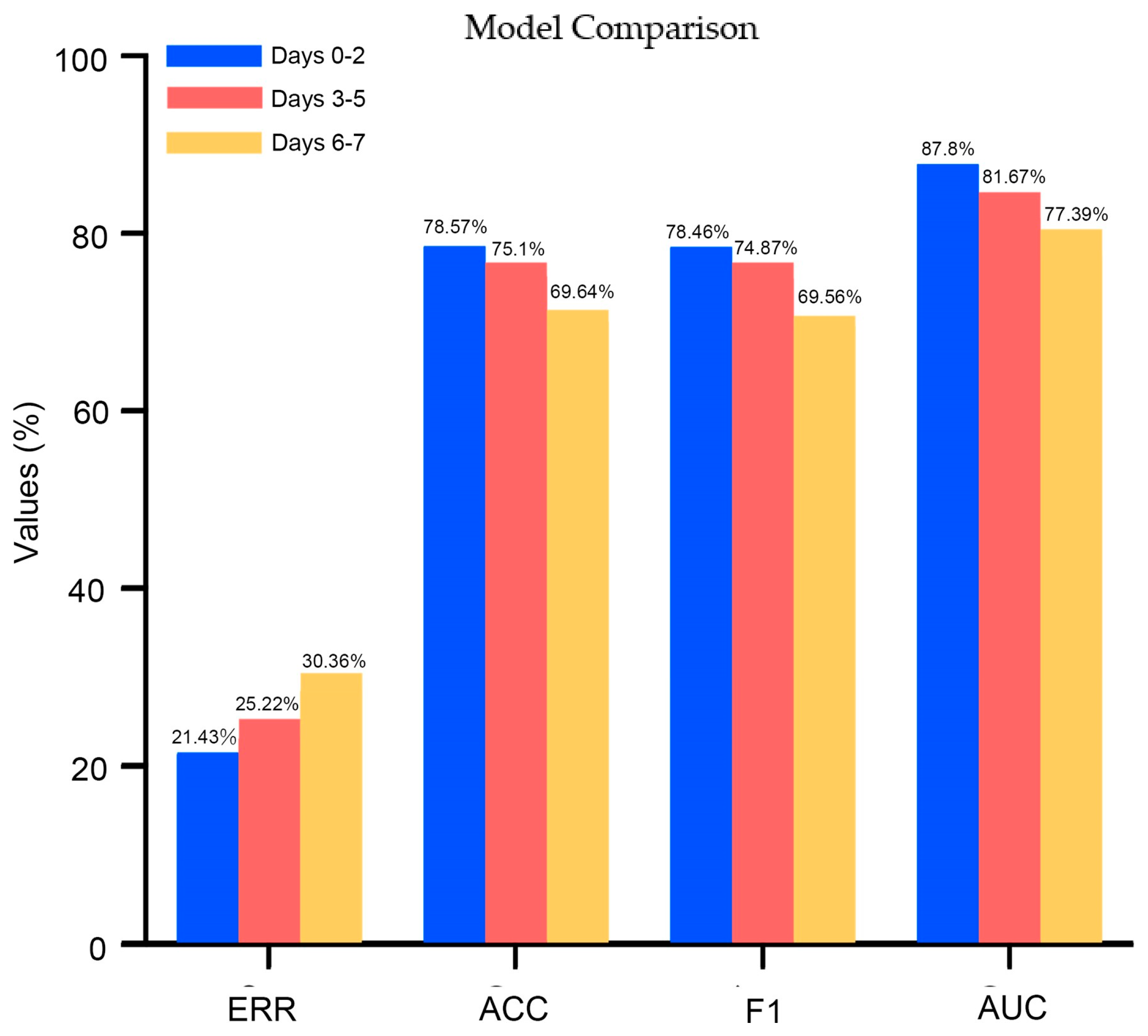
3.7. Supplementary Analysis: Feature Importance Impacting Survival Status Using Data at Different Time Intervals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Botta, M.; Tsonas, A.M.; Pillay, J.; Boers, L.S.; Algera, A.G.; Bos, L.D.; Dongelmans, D.A.; Hollmann, M.W.; Horn, J.; Vlaar, A.P. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): A national, multicentre, observational cohort study. Lancet Respir. Med. 2021, 9, 139–148. [Google Scholar] [CrossRef]
- Coronavirus Disease (COVID-19)—World Health Organization. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 14 July 2023).
- Docherty, A.B.; Mulholland, R.H.; Lone, N.I.; Cheyne, C.P.; De Angelis, D.; Diaz-Ordaz, K.; Donegan, C.; Drake, T.M.; Dunning, J.; Funk, S. Changes in in-hospital mortality in the first wave of COVID-19: A multicentre prospective observational cohort study using the WHO Clinical Characterisation Protocol UK. Lancet Respir. Med. 2021, 9, 773–785. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Johnson, D.W.; Wiggins, K.J.; Armstrong, K.A.; Campbell, S.B.; Isbel, N.M.; Hawley, C.M. Elevated white cell count at commencement of peritoneal dialysis predicts overall and cardiac mortality. Kidney Int. 2005, 67, 738–743. [Google Scholar] [CrossRef]
- Kaushal, K.; Kaur, H.; Sarma, P.; Bhattacharyya, A.; Sharma, D.J.; Prajapat, M.; Pathak, M.; Kothari, A.; Kumar, S.; Rana, S.; et al. Serum ferritin as a predictive biomarker in COVID-19. A systematic review, meta-analysis and meta-regression analysis. J. Crit. Care 2022, 67, 172–181. [Google Scholar] [CrossRef]
- Stringer, D.; Braude, P.; Myint, P.K.; Evans, L.; Collins, J.T.; Verduri, A.; Quinn, T.J.; Vilches-Moraga, A.; Stechman, M.J.; Pearce, L.; et al. The role of C-reactive protein as a prognostic marker in COVID-19. Int. J. Epidemiol. 2021, 50, 420–429. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Hung, C.-C.; Kuo, M.-C.; Tsai, J.-C.; Yeh, S.-M.; Hwang, S.-J.; Chiu, Y.-W.; Kuo, H.-T.; Chang, J.-M.; Chen, H.-C. Association of hsCRP, white blood cell count and ferritin with renal outcome in chronic kidney disease patients. PLoS ONE 2012, 7, e52775. [Google Scholar] [CrossRef]
- Tan, Y.; Zhou, J.; Zhou, Q.; Hu, L.; Long, Y. Role of eosinophils in the diagnosis and prognostic evaluation of COVID-19. J. Med. Virol. 2021, 93, 1105–1110. [Google Scholar] [CrossRef]
- Li, Q.; Ding, X.; Xia, G.; Chen, H.-G.; Chen, F.; Geng, Z.; Xu, L.; Lei, S.; Pan, A.; Wang, L.; et al. Eosinopenia and elevated C-reactive protein facilitate triage of COVID-19 patients in fever clinic: A retrospective case-control study. EClinicalMedicine 2020, 23, 100375. [Google Scholar] [CrossRef]
- Santotoribio, J.D.; Nunez-Jurado, D.; Lepe-Balsalobre, E. Evaluation of Routine Blood Tests for Diagnosis of Suspected Coronavirus Disease 2019. Clin. Lab 2020, 66, 1867–1875. [Google Scholar] [CrossRef]
- Yamada, T.; Wakabayashi, M.; Yamaji, T.; Chopra, N.; Mikami, T.; Miyashita, H.; Miyashita, S. Value of leukocytosis and elevated C-reactive protein in predicting severe coronavirus 2019 (COVID-19): A systematic review and meta-analysis. Clin. Chim. Acta 2020, 509, 235–243. [Google Scholar] [CrossRef]
- Kang, D.H.; Kim, G.H.J.; Park, S.-B.; Lee, S.-I.; Koh, J.S.; Brown, M.S.; Abtin, F.; McNitt-Gray, M.F.; Goldin, J.G.; Lee, J.S. Quantitative Computed Tomography Lung COVID Scores with Laboratory Markers: Utilization to Predict Rapid Progression and Monitor Longitudinal Changes in Patients with Coronavirus 2019 (COVID-19) Pneumonia. Biomedicines 2024, 12, 120. [Google Scholar] [CrossRef]
- Baratella, E.; Bussani, R.; Zanconati, F.; Marrocchio, C.; Fabiola, G.; Braga, L.; Maiocchi, S.; Berlot, G.; Volpe, M.C.; Moro, E. Radiological–pathological signatures of patients with COVID-19-related pneumomediastinum: Is there a role for the Sonic hedgehog and Wnt5a pathways? ERJ Open Res. 2021, 7, 00346-2021. [Google Scholar] [CrossRef]
- Kneyber, M.C.; Khemani, R.G.; Bhalla, A.; Blokpoel, R.G.; Cruces, P.; Dahmer, M.K.; Emeriaud, G.; Grunwell, J.; Ilia, S.; Katira, B.H. Understanding clinical and biological heterogeneity to advance precision medicine in paediatric acute respiratory distress syndrome. Lancet Respir. Med. 2023, 11, 197–212. [Google Scholar] [CrossRef]
- Charlson, M.; Pompei, P.; Ales, K.; MacKenzie, R. Charlson comorbidity index. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Gou, R.; Diao, Y.S.; Yin, Q.H.; Fan, W.X.; Liang, Y.P.; Chen, Y.; Wu, M.; Zang, L.; Li, L.; et al. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J. Zhejiang Univ. Sci. B 2014, 15, 58–66. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Su, C.-L.; Wang, Y.-H.; Wu, S.-M.; Liu, W.-T.; Hsu, W.-H.; Majumdar, A.; Stettler, M.; Chen, K.-Y.; Lee, Y.-T.; et al. Impact of lifetime air pollution exposure patterns on the risk of chronic disease. Environ. Res. 2023, 229, 115957. [Google Scholar] [CrossRef]
- Kong, Y.; Han, J.; Wu, X.; Zeng, H.; Liu, J.; Zhang, H. VEGF-D: A novel biomarker for detection of COVID-19 progression. Crit. Care 2020, 24, 373. [Google Scholar] [CrossRef]
- Goldstein, B.A.; Hubbard, A.E.; Cutler, A.; Barcellos, L.F. An application of Random Forests to a genome-wide association dataset: Methodological considerations & new findings. BMC Genet. 2010, 11, 49. [Google Scholar]
- Archer, K.J.; Kimes, R.V. Empirical characterization of random forest variable importance measures. Comput. Stat. Data Anal. 2008, 52, 2249–2260. [Google Scholar] [CrossRef]
- Elshazli, R.M.; Toraih, E.A.; Elgaml, A.; El-Mowafy, M.; El-Mesery, M.; Amin, M.N.; Hussein, M.H.; Killackey, M.T.; Fawzy, M.S.; Kandil, E. Diagnostic and prognostic value of hematological and immunological markers in COVID-19 infection: A meta-analysis of 6320 patients. PLoS ONE 2020, 15, e0238160. [Google Scholar] [CrossRef]
- Fujishima, S. Guideline-based management of acute respiratory failure and acute respiratory distress syndrome. J. Intensive Care 2023, 11, 10. [Google Scholar] [CrossRef]
- Gupta, B.; Jain, G.; Chandrakar, S.; Gupta, N.; Agarwal, A. Arterial blood gas as a predictor of mortality in COVID pneumonia patients initiated on noninvasive mechanical ventilation: A retrospective analysis. Indian J. Crit. Care Med. 2021, 25, 866. [Google Scholar]
- Sahetya, S.K.; Wu, T.D.; Morgan, B.; Herrera, P.; Roldan, R.; Paz, E.; Jaymez, A.A.; Chirinos, E.; Portugal, J.; Quispe, R.; et al. Mean airway pressure as a predictor of 90-day mortality in mechanically ventilated patients. Crit. Care Med. 2020, 48, 688. [Google Scholar] [CrossRef]
- Long, Y.; Su, L.; Zhang, Q.; Zhou, X.; Wang, H.; Cui, N.; Chai, W.; Wang, X.; Rui, X.; Liu, D. Elevated mean airway pressure and central venous pressure in the first day of mechanical ventilation indicated poor outcome. Crit. Care Med. 2017, 45, e485–e492. [Google Scholar] [CrossRef]
- Khan, W.; Safi, A.; Muneeb, M.; Mooghal, M.; Aftab, A.; Ahmed, J. Complications of invasive mechanical ventilation in critically Ill COVID-19 patients-A narrative review. Ann. Med. Surg. 2022, 80, 104201. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Biswas, M.; Rahaman, S.; Biswas, T.K.; Haque, Z.; Ibrahim, B. Association of sex, age, and comorbidities with mortality in COVID-19 patients: A systematic review and meta-analysis. Intervirology 2021, 64, 36–47. [Google Scholar] [CrossRef]
- Aleem, A.; Ab, A.S.; Slenker, A.K. Emerging Variants of SARS-CoV-2 and Novel Therapeutics against Coronavirus (COVID-19). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Manchanda, V.; Mitra, S.; Rafique, I.; Sharma, A.; Dhakad, M.S.; Saxena, S.; Kapoor, S.; Kumar, S. Is Omicron really mild?–Comparative analysis of comorbidities and disease outcomes associated with SARS-CoV-2 Omicron (B. 1.1. 529) and Delta (B. 1.617. 2) variants. Indian J. Med. Microbiol. 2023, 45, 100391. [Google Scholar] [CrossRef]

| Categorical Variables | Survival Group (n = 29) | Non-Survival Group (n = 27) | p |
|---|---|---|---|
| Age (year) a | 69.17 ± 10.6 | 73.59 ± 7.82 | 0.08 |
| Sex (male/female) b | 22/7 | 19/12 | 0.23 |
| Body mass index (kg/m2) c | 25.43 ± 4.5 | 27.58 ± 8.48 | 0.47 |
| GCS (score) c | 12.17 ± 4.28 | 12.15 ± 4.61 | 0.99 |
| CCI (score) c | 0.52 ± 0.63 | 0.81 ± 0.88 | 0.23 |
| Hospital day (n) c | 18.31 ± 15.36 | 19.85 ± 13.57 | 0.33 |
| Comorbidities (n, %) b | |||
| Cardiovascular diseases | 8 (27.59%) | 4 (14.81%) | 0.24 |
| Hypertension | 13 (44.83%) | 18 (66.67%) | 0.11 |
| Chronic heart failure | 0 (0.0%) | 1 (3.7%) | 0.97 |
| COPD | 1 (3.45%) | 1 (3.7%) | 0.51 |
| Asthma | 0 (0.0%) | 2 (7.41%) | 0.44 |
| Diabetes mellitus | 9 (31.03%) | 10 (37.04%) | 0.64 |
| Acute kidney injury | 8 (27.59%) | 12 (44.44%) | 0.19 |
| Dementia | 1 (3.45%) | 2 (7.41%) | 0.95 |
| Tobacco usage (n, %) b | 0.17 | ||
| Current smoker | 1 (3.45%) | 5 (18.52%) | |
| Non-smoker | 28 (96.55%) | 22 (81.48%) |
| Categorical Variables | Survival Group (n = 29) | Non-Survival Group (n = 27) | p |
|---|---|---|---|
| CRP (mg/L) | |||
| Mean of Days 0–2 | 12.62 ± 8.15 | 7.51 ± 6.34 | <0.05 |
| Mean of Days 3–5 | 6.06 ± 6.3 | 4.51 ± 6.23 | 0.29 |
| Mean of Days 6–7 | 3.27 ± 4.78 | 4.91 ± 6.23 | 0.53 |
| Ferritin (ng/mL) | |||
| Mean of Days 0–2 | 1370.4 ± 864.34 | 3701.1 ± 12137.88 | 0.39 |
| Mean of Days 3–5 | 962.19 ± 582.5 | 2420.93 ± 7551.5 | 0.58 |
| Mean of Days 6–7 | 784.98 ± 438.32 | 1254.21 ± 2167.77 | 0.34 |
| WBC (103/μL) | |||
| Mean of Days 0–2 | 10.24 ± 5.47 | 11.39 ± 4.9 | 0.34 |
| Mean of Days 3–5 | 10.23 ± 5.15 | 13.16 ± 5.15 | <0.05 |
| Mean of Days 6–7 | 10.55 ± 4.52 | 13.63 ± 6.31 | <0.05 |
| Categorical Variables | Survival Group (n = 29) | Non-Survival Group (n = 27) | p |
|---|---|---|---|
| pH | |||
| Mean of Days 0–2 | 7.37 ± 0.07 | 7.32 ± 0.07 | <0.05 |
| Mean of Days 3–5 | 7.44 ± 0.13 | 7.41 ± 0.09 | 0.17 |
| Mean of Days 6–7 | 7.43 ± 0.22 | 7.45 ± 0.22 | 0.88 |
| PaO2 (mmHg) | |||
| Mean of Days 0–2 | 152.24 ± 66.6 | 132.51 ± 39.59 | 0.29 |
| Mean of Days 3–5 | 130.32 ± 55.56 | 109.95 ± 37.91 | 0.31 |
| Mean of Days 6–7 | 126.11 ± 54.51 | 105.18 ± 42.39 | 0.12 |
| PaCO2 (mmHg) | |||
| Mean of Days 0–2 | 41.2 ± 9.0 | 45.25 ± 9.16 | 0.06 |
| Mean of Days 3–5 | 42.12 ± 8.43 | 45.41 ± 10.24 | 0.13 |
| Mean of Days 6–7 | 43.15 ± 7.44 | 47.12 ± 9.49 | 0.09 |
| HCO3− (mEq/L) | |||
| Mean of Days 0–2 | 23.01 ± 3.77 | 22.53 ± 3.49 | 0.62 |
| Mean of Days 3–5 | 25.77 ± 4.42 | 25.18 ± 3.94 | 0.59 |
| Mean of Days 6–7 | 27.0 ± 6.79 | 27.04 ± 6.37 | 0.74 |
| P/F ratio | |||
| Mean of Days 0–2 | 245.09 ± 117.36 | 201.98 ± 79.74 | 0.21 |
| Mean of Days 3–5 | 284.5 ± 165.67 | 213.48 ± 95.2 | 0.31 |
| Mean of Days 6–7 | 298.97 ± 158.06 | 190.82 ± 93.9 | <0.05 |
| Mean airway pressure (mmHg) | |||
| Mean of Days 0–2 | 16.65 ± 3.82 | 17.96 ± 3.11 | 0.25 |
| Mean of Days 3–5 | 16.71 ± 4.45 | 17.81 ± 4.39 | 0.42 |
| Mean of Days 6–7 | 16.08 ± 4.01 | 18.14 ± 4.31 | 0.07 |
| Categorical Variables | Crude OR (95% CI) a | Adjusted OR (95% CI) b |
|---|---|---|
| CRP (mg/L) | ||
| Mean of Days 0–2 | 1.1 (1.02 to 1.21) * | 1.11 (1.01 to 1.22) * |
| Mean of Days 3–5 | 1.04 (0.95 to 1.14) | 1.09 (0.98 to 1.22) |
| Mean of Days 6–7 | 0.94 (0.85 to 1.05) | 0.96 (0.86 to 1.08) |
| Ferritin (ng/mL) | ||
| Mean of Days 0–2 | 0.57 (0.09 to 3.67) | 0.53 (0.06 to 4.83) |
| Mean of Days 3–5 | 0.56 (0.09 to 3.65) | 0.49 (0.04 to 6.25) |
| Mean of Days 6–7 | 0.68 (0.32 to 1.43) | 0.71 (0.33 to 1.51) |
| WBC (103/μL) | ||
| Mean of Days 0–2 | 0.96 (0.86 to 1.06) | 0.97 (0.86 to 1.08) |
| Mean of Days 3–5 | 0.89 (0.8 to 1.0) | 0.89 (0.79 to 1.01) |
| Mean of Days 6–7 | 0.89 (0.8 to 1.0) | 0.88 (0.78 to 0.99) |
| Categorical Variables | Crude OR (95% CI) a | Adjusted OR (95% CI) b |
|---|---|---|
| PH | ||
| Mean of Days 0–2 | 2.08 (1.11 to 3.89) * | 2.22 (1.08 to 4.59) * |
| Mean of Days 3–5 | 1.32 (0.76 to 2.28) | 1.11 (0.58 to 2.11) |
| Mean of Days 6–7 | 0.96 (0.57 to 1.62) | 1.14 (0.62 to 2.11) |
| PaO2 (mmHg) | ||
| Mean of Days 0–2 | 1.48 (0.82 to 2.7) | 1.8 (0.91 to 3.55) |
| Mean of Days 3–5 | 1.56 (0.89 to 2.76) | 2.32 (1.11 to 4.86) * |
| Mean of Days 6–7 | 1.61 (0.88 to 2.93) | 2.11 (1.06 to 4.21) * |
| PaCO2 (mmHg) | ||
| Mean of Days 0–2 | 0.95 (0.9 to 1.01) | 0.91 (0.84 to 0.99) * |
| Mean of Days 3–5 | 0.96 (0.91 to 1.02) | 0.95 (0.89 to 1.02) |
| Mean of Days 6–7 | 0.94 (0.88 to 1.01) | 0.92 (0.85 to 0.99) * |
| HCO3− (mEq/L) | ||
| Mean of Days 0–2 | 1.04 (0.9 to 1.2) | 0.98 (0.82 to 1.16) |
| Mean of Days 3–5 | 1.04 (0.91 to 1.18) | 0.99 (0.85 to 1.15) |
| Mean of Days 6–7 | 1.01 (0.92 to 1.08) | 0.98 (0.89 to 1.07) |
| P/F ratio | ||
| Mean of Days 0–2 | 1.59 (0.88 to 2.88) | 2.24 (1.02 to 4.93) * |
| Mean of Days 3–5 | 1.79 (0.95 to 3.36) | 2.91 (1.23 to 6.89) * |
| Mean of Days 6–7 | 2.84 (1.29 to 6.23) * | 3.84 (1.54 to 9.53) * |
| Mean airway pressure (mmHg) | ||
| Mean of Days 0–2 | 0.68 (0.41 to 1.17) | 0.49 (0.24 to 0.99) * |
| Mean of Days 3–5 | 0.78 (0.46 to 1.32) | 0.71 (0.36 to 1.33) |
| Mean of Days 6–7 | 0.61 (0.35 to 1.05) | 0.53 (0.26 to 1.07) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, S.-H.; Tsai, C.-Y.; Hsu, W.-H.; Chung, C.-L.; Li, H.-Y.; Chen, Z.; Chien, R.; Cheng, W.-H. Predicting Survival Status in COVID-19 Patients: Machine Learning Models Development with Ventilator-Related and Biochemical Parameters from Early Stages: A Pilot Study. J. Clin. Med. 2024, 13, 6190. https://doi.org/10.3390/jcm13206190
Chou S-H, Tsai C-Y, Hsu W-H, Chung C-L, Li H-Y, Chen Z, Chien R, Cheng W-H. Predicting Survival Status in COVID-19 Patients: Machine Learning Models Development with Ventilator-Related and Biochemical Parameters from Early Stages: A Pilot Study. Journal of Clinical Medicine. 2024; 13(20):6190. https://doi.org/10.3390/jcm13206190
Chicago/Turabian StyleChou, Shin-Ho, Cheng-Yu Tsai, Wen-Hua Hsu, Chi-Li Chung, Hsin-Yu Li, Zhihe Chen, Rachel Chien, and Wun-Hao Cheng. 2024. "Predicting Survival Status in COVID-19 Patients: Machine Learning Models Development with Ventilator-Related and Biochemical Parameters from Early Stages: A Pilot Study" Journal of Clinical Medicine 13, no. 20: 6190. https://doi.org/10.3390/jcm13206190
APA StyleChou, S.-H., Tsai, C.-Y., Hsu, W.-H., Chung, C.-L., Li, H.-Y., Chen, Z., Chien, R., & Cheng, W.-H. (2024). Predicting Survival Status in COVID-19 Patients: Machine Learning Models Development with Ventilator-Related and Biochemical Parameters from Early Stages: A Pilot Study. Journal of Clinical Medicine, 13(20), 6190. https://doi.org/10.3390/jcm13206190






