The Performance of Continuous Glucose Monitoring During the Intraoperative Period: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
2.3. Source of Evidence and Search Strategy
2.4. Data Extraction
2.5. Data Synthesis
3. Results
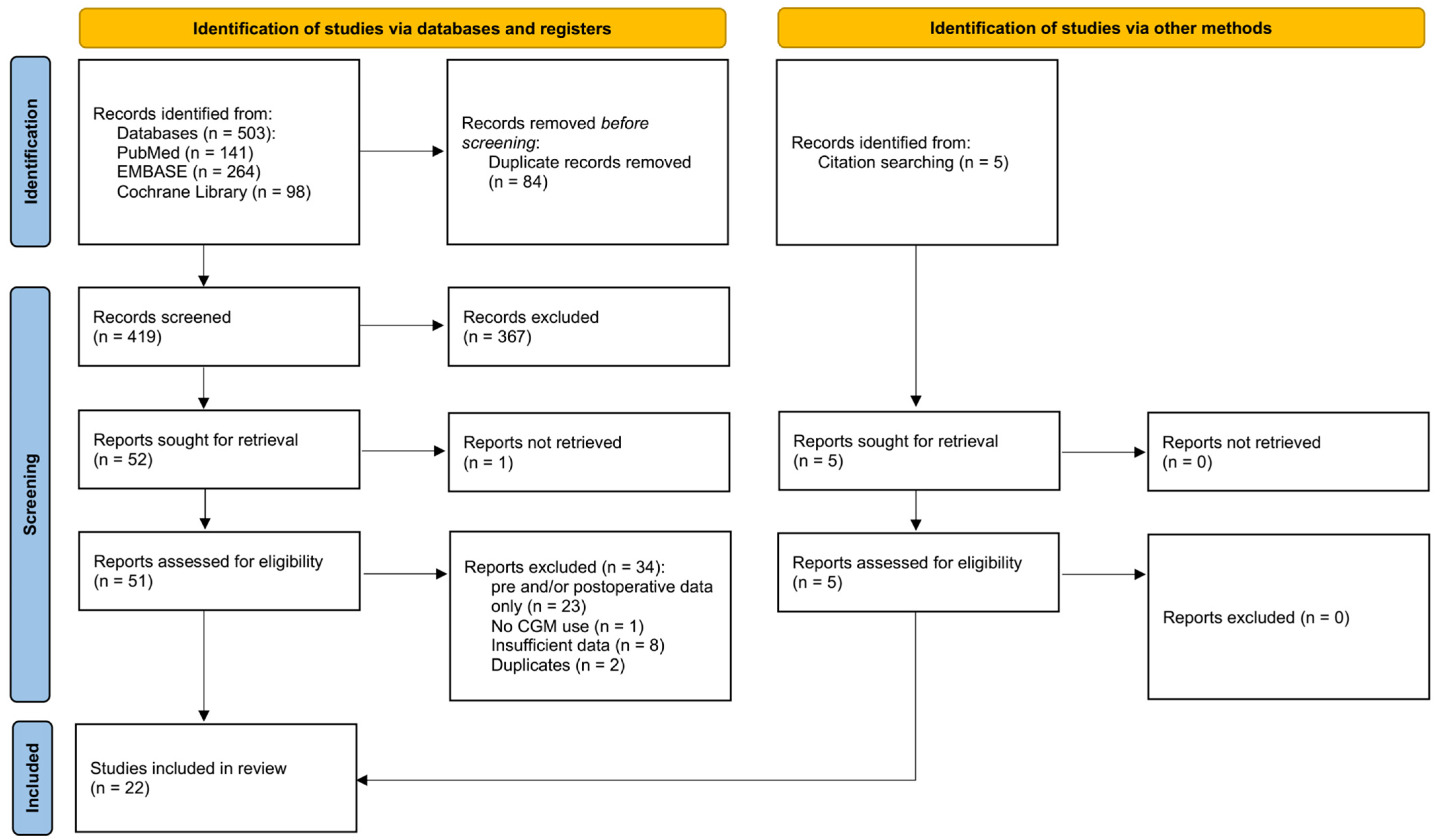
3.1. Selection of Sources of Evidence
3.2. Study Characteristics
3.3. CGM Characteristics
3.4. Technical Reliability
3.5. Accuracy
3.6. Adverse Device Effects
3.7. Efficacy
4. Discussion
4.1. Summary of Main Findings
4.2. Comparison with Existing Literature
4.3. Clinical Implications
4.4. Technical Limitations of CGM in the Intraoperative Period
4.5. Strengths and Limitations of the Review
4.6. Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sreedharan, R.; Khanna, S.; Shaw, A. Perioperative glycemic management in adults presenting for elective cardiac and non-cardiac surgery. Perioper. Med. 2023, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Thongsuk, Y.; Hwang, N.C. Perioperative Glycemic Management in Cardiac Surgery: A Narrative Review. J. Cardiothorac. Vasc. Anesth. 2024, 38, 248–267. [Google Scholar] [CrossRef] [PubMed]
- Galindo, R.J.; Fayfman, M.; Umpierrez, G.E. Perioperative Management of Hyperglycemia and Diabetes in Cardiac Surgery Patients. Endocrinol. Metab. Clin. North Am. 2018, 47, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Bock, M.; Johansson, T.; Fritsch, G.; Flamm, M.; Hansbauer, B.; Mann, E.; Sönnichsen, A. The impact of preoperative testing for blood glucose concentration and haemoglobin A1c on mortality, changes in management and complications in noncardiac elective surgery: A systematic review. Eur. J. Anaesthesiol. 2015, 32, 152–159. [Google Scholar] [CrossRef]
- Jones, C.E.; Graham, L.A.; Morris, M.S.; Richman, J.S.; Hollis, R.H.; Wahl, T.S.; Copeland, L.A.; Burns, E.A.; Itani, K.M.F.; Hawn, M.T. Association Between Preoperative Hemoglobin A1c Levels, Postoperative Hyperglycemia, and Readmissions Following Gastrointestinal Surgery. JAMA Surg. 2017, 152, 1031–1038. [Google Scholar] [CrossRef]
- Ivascu, R.; Torsin, L.I.; Hostiuc, L.; Nitipir, C.; Corneci, D.; Dutu, M. The Surgical Stress Response and Anesthesia: A Narrative Review. J. Clin. Med. 2024, 13, 3017. [Google Scholar] [CrossRef]
- Toner, A.J.; Ganeshanathan, V.; Chan, M.T.; Ho, K.M.; Corcoran, T.B. Safety of Perioperative Glucocorticoids in Elective Noncardiac Surgery: A Systematic Review and Meta-analysis. Anesthesiology 2017, 126, 234–248. [Google Scholar] [CrossRef]
- Suzuki, K.; Sato, Y.; Kai, S.; Nishi, K.; Adachi, T.; Matsuo, Y.; Hirota, K. Volatile anesthetics suppress glucose-stimulated insulin secretion in MIN6 cells by inhibiting glucose-induced activation of hypoxia-inducible factor 1. PeerJ 2015, 3, e1498. [Google Scholar] [CrossRef]
- Vogt, A.P.; Bally, L. Perioperative glucose management: Current status and future directions. Best Pract. Res. Clin. Anaesthesiol. 2020, 34, 213–224. [Google Scholar] [CrossRef]
- Crowley, K.; Scanaill, P.; Hermanides, J.; Buggy, D.J. Current practice in the perioperative management of patients with diabetes mellitus: A narrative review. Br. J. Anaesth. 2023, 131, 242–252. [Google Scholar] [CrossRef]
- Chang, S.; Xu, M.; Wang, Y.; Zhang, Y. Acute Glycemic Variability and Early Outcomes After Cardiac Surgery: A Meta-Analysis. Horm. Metab. Res. 2023, 55, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Van Den Boom, W.; Schroeder, R.A.; Manning, M.W.; Setji, T.L.; Fiestan, G.-O.; Dunson, D.B. Effect of A1C and Glucose on Postoperative Mortality in Noncardiac and Cardiac Surgeries. Diabetes Care 2018, 41, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Ascione, R.; Rogers, C.A.; Rajakaruna, C.; Angelini, G.D. Inadequate Blood Glucose Control Is Associated With In-Hospital Mortality and Morbidity in Diabetic and Nondiabetic Patients Undergoing Cardiac Surgery. Circulation 2008, 118, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Nair, B.G.; Neradilek, M.B.; Newman, S.F.; Horibe, M. Association between acute phase perioperative glucose parameters and postoperative outcomes in diabetic and non-diabetic patients undergoing non-cardiac surgery. Am. J. Surg. 2019, 218, 302–310. [Google Scholar] [CrossRef]
- Lee, H.; Kim, M.-J.; Lee, I.-K.; Hong, C.-W.; Jeon, J.-H. Impact of hyperglycemia on immune cell function: A comprehensive review. Diabetol. Int. 2024, 1–16. [Google Scholar] [CrossRef]
- Duggan, E.W.; Carlson, K.; Umpierrez, G.E. Perioperative Hyperglycemia Management: An Update. Anesthesiology 2017, 126, 547–560. [Google Scholar] [CrossRef]
- Jackson, D.A.; Michael, T.; Vieira de Abreu, A.; Agrawal, R.; Bortolato, M.; Fisher, S.J. Prevention of Severe Hypoglycemia-Induced Brain Damage and Cognitive Impairment With Verapamil. Diabetes 2018, 67, 2107–2112. [Google Scholar] [CrossRef]
- Finfer, S.; Chittock, D.R.; Su, S.Y.; Blair, D.; Foster, D.; Dhingra, V.; Bellomo, R.; Cook, D.; Dodek, P.; Henderson, W.R.; et al. Intensive versus conventional glucose control in critically ill patients. N. Engl. J. Med. 2009, 360, 1283–1297. [Google Scholar] [CrossRef]
- Miles, M.E.; Rice, M.J. Recent advances in perioperative glucose monitoring. Curr. Opin. Anaesthesiol. 2017, 30, 718–722. [Google Scholar] [CrossRef]
- Longo, R.R.; Joshi, R. The Devil Is in the Details: Use, Limitations, and Implementation of Continuous Glucose Monitoring in the Inpatient Setting. Diabetes Spectr. 2022, 35, 405–419. [Google Scholar] [CrossRef]
- Zelada, H.; Perez-Guzman, M.C.; Chernavvsky, D.R.; Galindo, R.J. Continuous glucose monitoring for inpatient diabetes management: An update on current evidence and practice. Endocr. Connect. 2023, 12, e230180. [Google Scholar] [CrossRef] [PubMed]
- Mihai, D.A.; Stefan, D.S.; Stegaru, D.; Bernea, G.E.; Vacaroiu, I.A.; Papacocea, T.; Lupușoru, M.O.D.; Nica, A.E.; Stiru, O.; Dragos, D.; et al. Continuous glucose monitoring devices: A brief presentation (Review). Exp. Ther. Med. 2022, 23, 174. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.M. Using Continuous Glucose Monitoring in Clinical Practice. Clin. Diabetes 2020, 38, 429–438. [Google Scholar] [CrossRef]
- van Steen, S.C.; Rijkenberg, S.; Limpens, J.; van der Voort, P.H.; Hermanides, J.; DeVries, J.H. The Clinical Benefits and Accuracy of Continuous Glucose Monitoring Systems in Critically Ill Patients-A Systematic Scoping Review. Sensors 2017, 17, 146. [Google Scholar] [CrossRef]
- Clubbs Coldron, B.; Coates, V.; Khamis, A.; Macrury, S. Use of Continuous Glucose Monitoring in Non-ICU Hospital Settings for People With Diabetes: A Scoping Review of Emerging Benefits and Issues. J. Diabetes Sci. Technol. 2023, 17, 467–473. [Google Scholar] [CrossRef]
- Shehav-Zaltzman, G.; Segal, G.; Konvalina, N.; Tirosh, A. Remote Glucose Monitoring of Hospitalized, Quarantined Patients With Diabetes and COVID-19. Diabetes Care 2020, 43, e75–e76. [Google Scholar] [CrossRef]
- Singh, L.G.; Satyarengga, M.; Marcano, I.; Scott, W.H.; Pinault, L.F.; Feng, Z.; Sorkin, J.D.; Umpierrez, G.E.; Spanakis, E.K. Reducing Inpatient Hypoglycemia in the General Wards Using Real-time Continuous Glucose Monitoring: The Glucose Telemetry System, a Randomized Clinical Trial. Diabetes Care 2020, 43, 2736–2743. [Google Scholar] [CrossRef]
- Fortmann, A.L.; Spierling Bagsic, S.R.; Talavera, L.; Garcia, I.M.; Sandoval, H.; Hottinger, A.; Philis-Tsimikas, A. Glucose as the Fifth Vital Sign: A Randomized Controlled Trial of Continuous Glucose Monitoring in a Non-ICU Hospital Setting. Diabetes Care 2020, 43, 2873–2877. [Google Scholar] [CrossRef]
- Agarwal, S.; Mathew, J.; Davis, G.M.; Shephardson, A.; Levine, A.; Louard, R.; Urrutia, A.; Perez-Guzman, C.; Umpierrez, G.E.; Peng, L.; et al. Continuous Glucose Monitoring in the Intensive Care Unit During the COVID-19 Pandemic. Diabetes Care 2021, 44, 847–849. [Google Scholar] [CrossRef]
- Voglová Hagerf, B.; Protus, M.; Nemetova, L.; Mraz, M.; Kieslichova, E.; Uchytilova, E.; Indrova, V.; Lelito, J.; Girman, P.; Haluzík, M.; et al. Accuracy and Feasibility of Real-time Continuous Glucose Monitoring in Critically Ill Patients After Abdominal Surgery and Solid Organ Transplantation. Diabetes Care 2024, 47, 956–963. [Google Scholar] [CrossRef]
- Krinsley, J.S.; Chase, J.G.; Gunst, J.; Martensson, J.; Schultz, M.J.; Taccone, F.S.; Wernerman, J.; Bohe, J.; De Block, C.; Desaive, T.; et al. Continuous glucose monitoring in the ICU: Clinical considerations and consensus. Critical Care 2017, 21, 197. [Google Scholar] [CrossRef] [PubMed]
- De Backer, D.; Hajjar, L.; Monnet, X. Vasoconstriction in septic shock. Intensive Care Med. 2024, 50, 459–462. [Google Scholar] [CrossRef]
- Galindo, R.J.; Umpierrez, G.E.; Rushakoff, R.J.; Basu, A.; Lohnes, S.; Nichols, J.H.; Spanakis, E.K.; Espinoza, J.; Palermo, N.E.; Awadjie, D.G.; et al. Continuous Glucose Monitors and Automated Insulin Dosing Systems in the Hospital Consensus Guideline. J. Diabetes Sci. Technol. 2020, 14, 1035–1064. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Kawahito, S.; Mita, N.; Soga, T.; Yagi, S.; Kakuta, N.; Satomi, S.; Kinoshita, H.; Takaishi, K.; Kitagawa, T.; Kitahata, H. Accuracy and reliability of continuous blood glucose monitoring during pediatric cardiopulmonary bypass. J. Artif. Organs 2019, 22, 353–356. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Metrics for Continuous Interstitial Glucose Monitoring (CLSI Guideline POCT05), 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Aust, H.; Dinges, G.; Nardi-Hiebl, S.; Koch, T.; Lattermann, R.; Schricker, T.; Eberhart, L.H.J. Feasibility and precision of subcutaneous continuous glucose monitoring in patients undergoing CABG surgery. J. Cardiothorac. Vasc. Anesth. 2014, 28, 1264–1272. [Google Scholar] [CrossRef]
- Blixt, C.; Rooyackers, O.; Isaksson, B.; Wernerman, J. Continuous on-line glucose measurement by microdialysis in a central vein. A pilot study. Crit. Care 2013, 17, R87. [Google Scholar] [CrossRef]
- Carlsson, C.J.; Nørgaard, K.; Oxbøll, A.B.; Søgaard, M.I.V.; Achiam, M.P.; Jørgensen, L.N.; Eiberg, J.P.; Palm, H.; Sørensen, H.B.D.; Meyhof, C.S.; et al. Continuous Glucose Monitoring Reveals Perioperative Hypoglycemia in Most Patients With Diabetes Undergoing Major Surgery: A Prospective Cohort Study. Ann. Surg. 2023, 277, 603–611. [Google Scholar] [CrossRef]
- DiGiusto, M.; Wolf, R.M.; Arcara, K.M.; Vanderhoek, S.M. Use of Continuous Glucose Monitoring to Facilitate Perioperative Glycemic Management: A Case Report. A&A Pract. 2021, 15, e01438. [Google Scholar] [CrossRef]
- Guensch, D.P.; Tripyla, A.; Fischer, K.; Vogt, A.P.; Bally, L. First insights into the performance of the Dexcom G6 continuous glucose monitoring system during cardiac surgery using hypothermic extracorporal circulation. Diabetes Obes. Metab. 2021, 23, 294–295. [Google Scholar] [CrossRef] [PubMed]
- Herzig, D.; Vettoretti, M.; Guensch, D.P.; Melmer, A.; Schürch, D.; Roos, J.; Goerg, A.M.C.; Krutkyte, G.; Cecchini, L.; Facchinetti, A.; et al. Performance of the Dexcom G6 Continuous Glucose Monitoring System During Cardiac Surgery Using Hypothermic Extracorporeal Circulation. Diabetes Care 2023, 46, 864–867. [Google Scholar] [CrossRef] [PubMed]
- Kalmovich, B.; Bar-Dayan, Y.; Boaz, M.; Wainstein, J. Continuous glucose monitoring in patients undergoing cardiac surgery. Diabetes Technol. Ther. 2012, 14, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Perez-Guzman, M.C.; Duggan, E.; Gibanica, S.; Cardona, S.; Corujo-Rodriguez, A.; Faloye, A.; Halkos, M.; Umpierrez, G.E.; Peng, L.; Davis, G.M.; et al. Continuous Glucose Monitoring in the Operating Room and Cardiac Intensive Care Unit. Diabetes Care 2021, 44, e50–e52. [Google Scholar] [CrossRef]
- Piper, H.G.; Alexander, J.L.; Shukla, A.; Pigula, F.; Costello, J.M.; Laussen, P.C.; Jaksic, T.; Agus, M.S. Real-time continuous glucose monitoring in pediatric patients during and after cardiac surgery. Pediatrics 2006, 118, 1176–1184. [Google Scholar] [CrossRef]
- Polderman, J.A.W.; Ma, X.L.; Eshuis, W.J.; Hollmann, M.W.; Devries, J.H.; Preckel, B.; Hermanides, J. Efficacy of continuous intravenous glucose monitoring in perioperative glycaemic control: A randomized controlled study. Br. J. Anaesth. 2017, 118, 264–266. [Google Scholar] [CrossRef]
- Poljakova, I.; Elsikova, E.; Chlup, R.; Kalabus, S.; Hasala, P.; Zapletalova, J. Glucose sensing module—Is it time to integrate it into real-time perioperative monitoring? An observational pilot study with subcutaneous sensors. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2013, 157, 346–357. [Google Scholar] [CrossRef]
- Price, C.E.; Fanelli, J.E.; Aloi, J.A.; Anzola, S.C.; Vishneski, S.R.; Saha, A.K.; Woody, C.C.; Segal, S. Feasibility of intraoperative continuous glucose monitoring: An observational study in general surgery patients. J. Clin. Anesth. 2023, 87, 111090. [Google Scholar] [CrossRef]
- Saha, P.; Beardsall, K. Perioperative continuous glucose monitoring in a preterm infant. BMJ Case Rep. 2018, 2018, bcr-2018. [Google Scholar] [CrossRef]
- Schierenbeck, F.; Franco-Cereceda, A.; Liska, J. Accuracy of 2 Different Continuous Glucose Monitoring Systems in Patients Undergoing Cardiac Surgery. J. Diabetes Sci. Technol. 2017, 11, 108–116. [Google Scholar] [CrossRef]
- Schierenbeck, F.; Öwall, A.; Franco-Cereceda, A.; Liska, J. Evaluation of a continuous blood glucose monitoring system using a central venous catheter with an integrated microdialysis function. Diabetes Technol. Ther. 2013, 15, 26–31. [Google Scholar] [CrossRef]
- Sindhvananda, W.; Poopuangpairoj, W.; Jaiprasat, T.; Ongcharit, P. Comparison of glucose control by added liraglutide to only insulin infusion in diabetic patient undergoing cardiac surgery: A preliminary randomized-controlled trial. Ann. Card. Anaesth. 2023, 26, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Song, I.K.; Lee, J.H.; Kang, J.E.; Park, Y.H.; Kim, H.S.; Kim, J.T. Continuous glucose monitoring system in the operating room and intensive care unit: Any difference according to measurement sites? J. Clin. Monit. Comput. 2017, 31, 187–194. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Wakabayashi, R.; Urasawa, M.; Maruyama, Y.; Shimizu, S.; Kawamata, M. Perioperative Characteristics of the Accuracy of Subcutaneous Continuous Glucose Monitoring: Pilot Study in Neurosurgery and Cardiac Surgery. Diabetes Technol. Ther. 2018, 20, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, Y.; Kiuchi, C.; Suzuki, M.; Maruyama, Y.; Wakabayashi, R.; Ohno, Y.; Takahata, S.; Shibazaki, T.; Kawamata, M. Glucose Management during Insulinoma Resection Using Real-Time Subcutaneous Continuous Glucose Monitoring. Case Rep. Anesthesiol. 2018, 2018, 6248467. [Google Scholar] [CrossRef] [PubMed]
- Tripyla, A.; Herzig, D.; Joachim, D.; Nakas, C.T.; Amiet, F.; Andreou, A.; Gloor, B.; Vogt, A.; Bally, L. Performance of a factory-calibrated, real-time continuous glucose monitoring system during elective abdominal surgery. Diabetes Obes. Metab. 2020, 22, 1678–1682. [Google Scholar] [CrossRef]
- Vriesendorp, T.M.; DeVries, J.H.; Holleman, F.; Dzoljic, M.; Hoekstra, J.B. The use of two continuous glucose sensors during and after surgery. Diabetes Technol. Ther. 2005, 7, 315–322. [Google Scholar] [CrossRef]
- Wasiq, M.A.; Behura, S.S.; Sahoo, S.; Panda, S.K. Comparison of Continuous Real Time Blood Glucose Measurement With Venous Laboratory Blood Glucose Level in Neonates During Perioperative Period. Indian. Pediatr. 2022, 59, 620–622. [Google Scholar] [CrossRef]
- ISO 15197:2003; In Vitro Diagnostic Test Systems—Requirements for Blood-Glucose Monitoring Systems for Self-Testing in Managing Diabetes Mellitus. Available online: https://www.iso.org/standard/26309.html (accessed on 3 May 2024).
- ISO 15197:2013; In Vitro Diagnostic Test Systems—Requirements for Blood-Glucose Monitoring Systems for Self-Testing in Managing Diabetes Mellitus. Available online: https://www.iso.org/standard/54976.html (accessed on 3 May 2024).
- Clarke, W.L.; Cox, D.; Gonder-Frederick, L.A.; Carter, W.; Pohl, S.L. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care 1987, 10, 622–628. [Google Scholar] [CrossRef]
- Martens, T.; Beck, R.W.; Bailey, R.; Ruedy, K.J.; Calhoun, P.; Peters, A.L.; Pop-Busui, R.; Philis-Tsimikas, A.; Bao, S.; Umpierrez, G.; et al. Effect of Continuous Glucose Monitoring on Glycemic Control in Patients With Type 2 Diabetes Treated With Basal Insulin: A Randomized Clinical Trial. Jama 2021, 325, 2262–2272. [Google Scholar] [CrossRef]
- Deshmukh, H.; Wilmot, E.G.; Gregory, R.; Barnes, D.; Narendran, P.; Saunders, S.; Furlong, N.; Kamaruddin, S.; Banatwalla, R.; Herring, R.; et al. Effect of Flash Glucose Monitoring on Glycemic Control, Hypoglycemia, Diabetes-Related Distress, and Resource Utilization in the Association of British Clinical Diabetologists (ABCD) Nationwide Audit. Diabetes Care 2020, 43, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Polonsky, W.H.; Hessler, D.; Ruedy, K.J.; Beck, R.W. The Impact of Continuous Glucose Monitoring on Markers of Quality of Life in Adults With Type 1 Diabetes: Further Findings From the DIAMOND Randomized Clinical Trial. Diabetes Care 2017, 40, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Lind, M.; Polonsky, W.; Hirsch, I.B.; Heise, T.; Bolinder, J.; Dahlqvist, S.; Schwarz, E.; Ólafsdóttir, A.F.; Frid, A.; Wedel, H.; et al. Continuous Glucose Monitoring vs Conventional Therapy for Glycemic Control in Adults With Type 1 Diabetes Treated With Multiple Daily Insulin Injections: The GOLD Randomized Clinical Trial. JAMA 2017, 317, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.J.; Coursin, D.B. Continuous measurement of glucose: Facts and challenges. Anesthesiology 2012, 116, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Arroyo, L.; Faulds, E.; Perez-Guzman, M.C.; Davis, G.M.; Dungan, K.; Pasquel, F.J. Continuous Glucose Monitoring in the Intensive Care Unit. J. Diabetes Sci. Technol. 2023, 17, 667–678. [Google Scholar] [CrossRef]
- MacDonald, D.B.; Mackin, M.J. Intraoperative glucose management: When to monitor and who to treat? Can. J. Anaesth. 2023, 70, 177–182. [Google Scholar] [CrossRef]
- Hermanns, N.; Heinemann, L.; Freckmann, G.; Waldenmaier, D.; Ehrmann, D. Impact of CGM on the Management of Hypoglycemia Problems: Overview and Secondary Analysis of the HypoDE Study. J. Diabetes Sci. Technol. 2019, 13, 636–644. [Google Scholar] [CrossRef]
- Maiorino, M.I.; Signoriello, S.; Maio, A.; Chiodini, P.; Bellastella, G.; Scappaticcio, L.; Longo, M.; Giugliano, D.; Esposito, K. Effects of Continuous Glucose Monitoring on Metrics of Glycemic Control in Diabetes: A Systematic Review With Meta-analysis of Randomized Controlled Trials. Diabetes Care 2020, 43, 1146–1156. [Google Scholar] [CrossRef]
- Choi, H.; Park, C.S.; Huh, J.; Koo, J.; Jeon, J.; Kim, E.; Jung, S.; Kim, H.W.; Lim, J.Y.; Hwang, W. Intraoperative Glycemic Variability and Mean Glucose are Predictors for Postoperative Delirium After Cardiac Surgery: A Retrospective Cohort Study. Clin. Interv. Aging 2022, 17, 79–95. [Google Scholar] [CrossRef]
- Nam, K.; Jeon, Y.; Kim, W.H.; Jung, D.E.; Kwon, S.M.; Kang, P.; Cho, Y.J.; Kim, T.K. Intraoperative glucose variability, but not average glucose concentration, may be a risk factor for acute kidney injury after cardiac surgery: A retrospective study. Can. J. Anaesth. 2019, 66, 921–933. [Google Scholar] [CrossRef]
- Clement, K.C.; Suarez-Pierre, A.; Sebestyen, K.; Alejo, D.; DiNatale, J.; Whitman, G.J.R.; Matthew, T.L.; Lawton, J.S. Increased Glucose Variability Is Associated With Major Adverse Events After Coronary Artery Bypass. Ann. Thorac. Surg. 2019, 108, 1307–1313. [Google Scholar] [CrossRef]
- Kalra, S.; Bajwa, S.J.; Baruah, M.; Sehgal, V. Hypoglycaemia in anesthesiology practice: Diagnostic, preventive, and management strategies. Saudi J. Anaesth. 2013, 7, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Danne, T.; Nimri, R.; Battelino, T.; Bergenstal, R.M.; Close, K.L.; DeVries, J.H.; Garg, S.; Heinemann, L.; Hirsch, I.; Amiel, S.A.; et al. International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care 2017, 40, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Welsh, J.B.; Lu, S.; Gray, J.M. Safety and Functional Integrity of Continuous Glucose Monitoring Components After Simulated Radiologic Procedures. J. Diabetes Sci. Technol. 2021, 15, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Migdal, A.L.; Spanakis, E.K.; Galindo, R.J.; Davis, G.; Singh, L.G.; Satyarengga, M.; Scott, W.H.; Fayfman, M.; Pasquel, F.J.; Albury, B.; et al. Accuracy and Precision of Continuous Glucose Monitoring in Hospitalized Patients Undergoing Radiology Procedures. J. Diabetes Sci. Technol. 2020, 14, 1135–1136. [Google Scholar] [CrossRef]
- Heinemann, L. Interferences With CGM Systems: Practical Relevance? J. Diabetes Sci. Technol. 2022, 16, 271–274. [Google Scholar] [CrossRef]
- Battelino, T.; Alexander, C.M.; Amiel, S.A.; Arreaza-Rubin, G.; Beck, R.W.; Bergenstal, R.M.; Buckingham, B.A.; Carroll, J.; Ceriello, A.; Chow, E.; et al. Continuous glucose monitoring and metrics for clinical trials: An international consensus statement. Lancet Diabetes Endocrinol. 2023, 11, 42–57. [Google Scholar] [CrossRef]
- Matzka, M.; Ørtenblad, N.; Lenk, M.; Sperlich, B. Accuracy of a continuous glucose monitoring system applied before, during, and after an intense leg-squat session with low- and high-carbohydrate availability in young adults without diabetes. Eur. J. Appl. Physiol. 2024, 1–13. [Google Scholar] [CrossRef]
- Layne, J.E.; Jepson, L.H.; Carite, A.M.; Parkin, C.G.; Bergenstal, R.M. Long-Term Improvements in Glycemic Control with Dexcom CGM Use in Adults with Noninsulin-Treated Type 2 Diabetes. Diabetes Technol. Ther. 2024. [Google Scholar] [CrossRef]
- Cordero, T.L.; Dai, Z.; Arrieta, A.; Niu, F.; Vella, M.; Shin, J.; Rhinehart, A.S.; McVean, J.; Lee, S.W.; Slover, R.H.; et al. Glycemic Outcomes During Early Use of the MiniMed™ 780G Advanced Hybrid Closed-Loop System with Guardian™ 4 Sensor. Diabetes Technol. Ther. 2023, 25, 652–658. [Google Scholar] [CrossRef]
- CFR—Code of Federal Regulation Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=862.1355 (accessed on 3 May 2024).
| Category | Search Terms |
|---|---|
| Population | |
| 1 | “Surgical Procedures, Operative”[Mesh] |
| 2 | “Surgical Procedures, Operative”[TW] OR “Surgical Procedures”[TW] OR “Procedures, Surgical”[TW] OR “Procedure, Surgical”[TW] OR “Surgical Procedure”[TW] OR “Operative Procedures”[TW] OR “Operative Procedure”[TW] OR “Procedure, Operative”[TW] OR “Procedures, Operative”[TW] OR “Operative Surgical Procedure”[TW] OR “Operative Surgical Procedures”[TW] OR “Procedure, Operative Surgical”[TW] OR “Procedures, Operative Surgical”[TW] OR “Surgical Procedure, Operative”[TW] |
| 3 | “General Surgery”[Mesh] |
| 4 | “General Surgery”[TW] OR “Surgery, General”[TW] OR “Surgery”[TW] OR “general surgery patient”[TW] OR “general surgery patients”[TW] |
| 5 | “surgery” [Subheading] |
| 6 | “operations”[TW] OR “invasive procedures”[TW] OR “operative therapy”[TW] OR “preoperative procedures”[TW] OR “intraoperative procedures”[TW] OR “peroperative procedures”[TW] OR “perioperative procedures”[TW] |
| 7 Combine | 1 OR 2 OR 3 OR 4 OR 5 OR 6 |
| Concept | |
| 8 | “Continuous Glucose Monitoring”[Mesh] |
| 9 | “Continuous Glucose Monitoring”[TW] OR “Glucose Monitoring, Continuous”[TW] OR “Monitoring, Continuous Glucose”[TW] OR “Monitorings, Continuous Glucose”[TW] OR “Continuous Glucose Monitoring Device”[TW] OR “CGM Device”[TW] OR “CGM Devices”[TW] OR “Device, CGM”[TW] OR “Devices, CGM”[TW] OR “CGM”[TW] |
| 10 | (“Blood Glucose”[Mesh] OR “Blood Glucose”[TW]) AND “Continuous”[TW] |
| 11 | “Continuous Blood Glucose” |
| 12 Combine | 8 OR 9 OR 10 OR 11 |
| Context | |
| 13 | “Perioperative Period”[Mesh] |
| 14 | “Perioperative Period”[TW] OR “Period, Perioperative”[TW] OR “Periods, Perioperative”[TW] OR “Perioperative Periods”[TW] |
| 15 | “Preoperative Period”[Mesh] OR “Preoperative Period”[TW] OR “Period, Preoperative”[TW] |
| 16 | “Intraoperative Period”[Mesh] |
| 17 | “Intraoperative Period”[TW] OR “Intraoperative Periods”[TW] OR “Period, Intraoperative”[TW] OR “Periods, Intraoperative”[TW] |
| 18 | “Postoperative Period”[Mesh] |
| 19 | “Postoperative Period”[TW] OR “Period, Postoperative”[TW] OR “Periods, Postoperative”[TW] OR “Postoperative Periods”[TW] |
| 20 Combine | 13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19 |
| 21 Combine | 7 AND 12 AND 20 |
| Category | Parameter | Description |
|---|---|---|
| Technical reliability | Sensor survival | The ability of CGM to correctly function until the end of its intended use. |
| Data availability | The ability of CGM to provide the expected number of glucose measurements without interruptions. | |
| Accuracy | Mean and/or median bias/absolute difference | Average difference between CGM value and comparator value. |
| Mean and/or median absolute relative difference (MARD) | Average difference between CGM value and comparator value divided by the comparator value. | |
| Error grid analysis (Clarke, consensus, surveillance, and continuous glucose) | Values in Zone A and B considered clinically acceptable. | |
| Agreement rate | Proportion of CGM values within certain limits (e.g., ±15 mg/dL or ±15%) of comparator values. | |
| Limits of agreement | Indicate how closely CGM values and comparator values agree using the Bland–Altman method (mean ± 1.96 standard deviation). | |
| Association | Using correlation and/or regression. | |
| Adverse device effects | Adverse device effects | Occurrence of adverse events related to or caused by CGM. |
| Efficacy | Average glucose level | Assessed in comparison with a comparator by at least one metric of the average glucose level. |
| Time in range | Assessed in comparison with a comparator by time spent in different glucose ranges. |
| First Author Year | Origin | Design | Population | Surgery Type | Aims |
|---|---|---|---|---|---|
| Aust 2014 [38] | Germany | Prospective cohort | Adults (n = 10) | Cardiac (n = 10) | To evaluate if subcutaneous CGM is feasible in cardiac surgery and if reliable glucose values are reported under hypothermic extracorporeal circulation. |
| Blixt 2013 [39] | Sweden | Prospective cohort | Adults (n = 10) | Abdominal (n = 10) | To test a central venous catheter with a microdialysis membrane in combination with an online analyzer and monitor as a principle for CGM. |
| Carlsson 2023 [40] | Denmark | Prospective cohort | Adults (n = 70) | Abdominal (n = 45), orthopedic (n = 11), vascular (n = 14) | To investigate the frequency and duration of hypo- and hyperglycemia, assessed by CGM during and after major surgery. |
| DiGiusto 2021 [41] | USA | Case report | Children (n = 2) | Abdominal (n = 2) | To assess the accuracy of CGM compared to capillary POC and arterial blood analysis in two cases where CGM was utilized as an adjunct method of perioperative glucose monitoring. |
| Guensch 2021 [42] | Switzerland | Case report | Adults (n = 2) | Cardiac (n = 2) | To present the first insights into the performance of the Dexcom G6 sensor during cardiac surgery with mild and deep hypothermia. |
| Herzig 2023 [43] | Switzerland | Prospective cohort | Adults (n = 16) | Cardiac (n = 16) | To test the accuracy of the Dexcom G6 CGM sensor in patients undergoing cardiac surgery using hypothermic extracorporeal circulation. |
| Kalmovich 2012 [44] | Israel | Prospective cohort | Adults (n = 32) | Cardiac (n = 32) | To examine and monitor the glycemic response in patients undergoing cardiac surgery during the perioperative period, using 24 h monitoring with a CGM and evaluating its accuracy and reliability. |
| Perez-Guzman 2021 [45] | USA | Prospective cohort | Adults (n = 15) | Cardiac (n = 15) | To evaluate the performance of CGM in adults without DM undergoing scheduled or urgent coronary artery bypass graft surgery. |
| Piper 2006 [46] | USA | Prospective cohort | Children (n = 20) | Cardiac (n = 20) | To validate a subcutaneous sensor for real-time CGM in pediatric patients during and after cardiac surgery. |
| Polderman 2017 [47] | The Netherlands | RCT | Adults (n = 36) | Major abdominal or cardiothoracic | To investigate the efficacy of perioperative CGM via peripheral intravenous sampling in patients with DM type 2 compared with standard care. |
| Poljakova 2013 [48] | Czech Republic | Prospective cohort | Adults (n = 17) | Orthopedics (n = 13), vascular (n = 4) | To explore the feasibility of subcutaneous CGM in perioperative settings. |
| Price 2023 [49] | USA | Prospective cohort | Adults (n = 76) | Abdominal (n = 13), cardiac (n = 5), otolaryngologic (n = 6), gynecological (n = 5), neuro (n = 14), ophthalmologic (n = 1), orthopedic (n = 6), plastic (n = 5), thoracic (n = 3), urologic (n = 8), vascular (n = 10) | To compare the performance of two CGM devices to contemporaneous capillary blood glucose sampling in patients with DM undergoing major non-cardiac surgery. |
| Saha 2018 [50] | United Kingdom | Case report | Neonate (n = 1) | Abdominal (n = 1) | To report on a preterm infant who uniquely underwent surgery while wearing a CGM, blinded to the clinical team. |
| Schierenbeck 2017 [51] | Sweden | Prospective cohort | Adults (n = 24) | Cardiac (n = 24) | To compare two different CGM systems: the FreeStyle Libre subcutaneous CGM and the Eirus intravascular microdialysis CGM in patients undergoing cardiac surgery. |
| Schierenbeck 2013 [52] | Sweden | Prospective cohort | Adults (n = 30) | Cardiac (n =30) | To evaluate the accuracy of intravascular microdialysis CGM in patients undergoing cardiac surgery. |
| Sindhvananda 2023 [53] | Thailand | RCT | Adults (n = 64) | Cardiac (n = 64) | To compare perioperative blood glucose and glycemic variability between added liraglutide and only-insulin infusion in DM patients undergoing cardiac surgery. |
| Song 2017 [54] | South Korea | Prospective cohort | Adults (n = 22) | Cardiac (n = 22) | To evaluate the accuracy and performance of the CGM system depending on different measurement sites in the OR and in the ICU. |
| Sugiyama 2018 [55] | Japan | Prospective cohort | Adults (n = 30) | Cardiac (n = 15), neuro (n = 15) | To evaluate the accuracy of a subcutaneous CGM system during different types of surgeries. |
| Sugiyama 2018 [56] | Japan | Case report | Child (n = 1) | Abdominal (n = 1) | To present a case in which real-time subcutaneous CGM, in combination with intermittent blood glucose measurement, was used for glycemic control during surgery for insulinoma resection. |
| Tripyla 2020 [57] | Switzerland | Prospective cohort | Adults (n = 20) | Abdominal (n = 20) | To assess the performance of the CGM system Dexcom G6 during elective abdominal surgery. |
| Vriesendorp 2005 [58] | The Netherlands | Prospective cohort | Adults (n = 8) | Abdominal (n = 8) | To examine whether CGM is feasible and reliable during and after major surgical procedures using two commercially available sensors. |
| Wasiq 2022 [59] | India | Prospective cohort | Neonates (n = 10) | Abdominal (n = 6), cardiac (n = 1), neuro (n = 2), urologic (n = 1) | To compare the blood glucose level by CGM with laboratory blood glucose testing in neonates during the perioperative period. |
| First Author Year | Manufacturer | Sensor Model | Location | Period CGM Values Collected |
|---|---|---|---|---|
| Aust 2014 [38] | Medtronic (Minneapolis, MN, USA) | CGMS system Gold | Subcutaneous | From 1 day before surgery to 72 h after surgery. |
| Blixt 2013 [39] | Eirus (Solna, Sweden) | Microdialysis system | Intravascular | OR to ward for a total of 20 h. |
| Carlsson 2023 [40] | Dexcom (San Diego, CA, USA) | G6 | Subcutaneous | From 1 day before surgery to POD 8 or hospital discharge. |
| DiGiusto 2021 [41] | Dexcom (San Diego, CA, USA) | G6 | Subcutaneous | OR to immediate postoperative. |
| Guensch 2021 [42] | Dexcom (San Diego, CA, USA) | G6 | Subcutaneous | Intraoperative only. |
| Herzig 2023 [43] | Dexcom (San Diego, CA, USA) | G6 | Subcutaneous | Intraoperative only. |
| Kalmovich 2012 [44] | Medtronic (Minneapolis, MN, USA) | CGMS system Gold | Subcutaneous | Intraoperative only. |
| Perez-Guzman 2021 [45] | Dexcom (San Diego, CA, USA) | G6 | Subcutaneous | Perioperative. |
| Piper 2006 [46] | Medtronic (Minneapolis, MN, USA) | Guardian REAL-Time | Subcutaneous | From OR to a maximum of 72 h or until ICU discharge. |
| Polderman 2017 [47] | Edwards Lifescience (Irvine, CA, USA) | GlucoClear | Intravascular | From OR to PACU discharge. |
| Poljakova 2013 [48] | Medtronic (Minneapolis, MN, USA) | Guardian REAL-Time | Subcutaneous | From OR to 30 min after surgery. |
| Price 2023 [49] | Dexcom (San Diego, CA, USA) | G6 | Subcutaneous | From OR to PACU discharge. |
| Abbott (Abbott Park, IL, USA) | Freestyle Libre 2.0 | |||
| Saha 2018 [50] | Medtronic (Minneapolis, MN, USA) | Enlite | Subcutaneous | On the day of surgery. |
| Schierenbeck 2017 [51] | Eirus (Solna, Sweden) | Microdialysis system | Intravascular | From OR to POD 1. |
| Abbott (Abbott Park, IL, USA) | Freestyle libre | Subcutaneous | From 1 day before surgery to POD 1. | |
| Schierenbeck 2013 [52] | Eirus (Solna, Sweden) | Microdialysis system | Intravascular | From OR to 48 h after surgery or until catheter removal. |
| Sindhvananda 2023 [53] | Medtronic (Minneapolis, MN, USA) | Enlite | Subcutaneous | From 1 day before surgery to POD 3. |
| Song 2017 [54] | Medtronic (Minneapolis, MN, USA) | Guardian REAL-Time | Subcutaneous | From OR to 72 h after surgery or until ICU discharge. |
| Sugiyama 2018 [55] | Medtronic (Minneapolis, MN, USA) | Enlite | Subcutaneous | On the day of surgery. |
| Sugiyama 2018 [56] | Medtronic (Minneapolis, MN, USA) | Enlite | Subcutaneous | Intraoperative only. |
| Tripyla 2020 [57] | Dexcom (San Diego, CA, USA) | G6 | Subcutaneous | From OR to 2 h after surgery. |
| Vriesendorp 2005 [58] | Medtronic (Minneapolis, MN, USA) | CGMS system Gold | Subcutaneous | Intraoperative only. |
| A. Menarini Diagnostics (Florence, Italy) | GlucoDay | Subcutaneous | ||
| Wasiq 2022 [59] | Abbott (Abbott Park, IL, USA) | Freestyle Libre | Subcutaneous | From 2 h before surgery to 72 h after surgery. |
| First Author Year | Sensor Model | Sensor Survival | Data Availability | Study Specific |
|---|---|---|---|---|
| Aust 2014 [38] | CGMS system Gold | 10/10 (100%) | 98.5% | NR |
| Carlsson 2023 [40] | G6 | NR | 96% (92, 98) | NR |
| DiGiusto 2021 [41] | G6 | 1/2 (50%) | NR | Failure to transmit data for a 30 min period shortly after induction in one patient. |
| Guensch 2021 [42] | G6 | 2/2 (100%) | NR | NR |
| Herzig 2023 [43] | G6 | 16/16 (100%) | 90.1% | NR |
| Kalmovich 2012 [44] | CGMS system Gold | NR | NR | “Split curve” phenomenon: 10/32 (31%; defined as hypoglycemic values reported by CGM, but much higher values in actual blood glucose). |
| Piper 2006 [46] | Guardian REAL-Time | NR | NR | Device alarm: 10/20 (50%; due to use of electrocautery). |
| Polderman 2017 [47] | GlucoClear | NR | NR | Sensor failure: 9/37 (24.3%; defined as missing sensor data for > 50% of the intraoperative or postoperative period or when the difference from POC measurements on two consecutive time points was > 45 mg/dL) |
| Poljakova 2013 [48] | Guardian REAL-Time | 17/17 (100%) | NR | NR |
| Price 2023 [49] | G6 | 64/76 (84.2%) | NR | NR |
| Freestyle Libre 2.0 | ||||
| Saha 2018 [50] | Enlite | 1/1 (100%) | NR | NR |
| Schierenbeck 2017 [51] | Microdialysis system | NR | NR | Interruption: 22/24 (91.7%), data gap duration: 13 ± 19 min. |
| Freestyle libre | NR | NR | Interruption: 1/24 (4.2%; due to excessive sweating causing sensor detachment). | |
| Schierenbeck 2013 [52] | Microdialysis system | 29/30 (96.7%) | NR | NR |
| Sindhvananda 2023 [53] | Enlite | 60/64 (93.8%) | NR | NR |
| Song 2017 [54] | Guardian REAL-Time | Abdomen: 19/22 (86.4%) | Abdomen: 58.7% | NR |
| Thigh: 22/22 (100%) | Thigh: 72.9% | |||
| Sugiyama 2018 [55] | Enlite | 1/1 (100%) | NR | NR |
| Tripyla 2020 [57] | G6 | 19/20 (95%) | 98.6% (95.9, 100) | NR |
| Vriesendorp 2005 [58] | CGMS system Gold | 7/8 (87.5%) | NR | Technical failure: 66% (defined as missing data). |
| GlucoDay | 8/16 (50%) | Technical failure: shoulder 10%, upper leg 63% (defined as missing data or broken fiber). |
| First Author Year | Sensor Model | Comparator Method | Matched Measurements | MARD (%) | Agreement (%) | Mean Bias (mg/dL) | Limits of Agreement (mg/dL) | Error Grid Analysis (%) | Correlation or Regression |
|---|---|---|---|---|---|---|---|---|---|
| Aust 2014 [38] | CGMS system Gold | Arterial BGA Capillary POC | Overall: 342 | NR | NR | NR | NR | Clarke: 99.1 | Pearson’s: 0.87 (95% CI: 0.844, 0.895) |
| CPB: 59 | Clarke: 100 | Pearson’s: 0.76 (95% CI: 0.624, 0.851) | |||||||
| Blixt 1 2013 [39] | Microdialysis system | Arterial CL | 195 | (1) 8.8 ± 8.4 | NR | NR | (1) ± 42.1 | (3) Clarke: 100 | Pearson’s: 0.89 (p < 0.001) |
| (2) 6.8 ± 9.3 | (2) ± 34.9 | (4) Clarke: 100 | Pearson’s: 0.92 (p < 0.001) | ||||||
| DiGiusto 2 2021 [41] | G6 | Arterial BGA | NR | NR | NR | (3) 33.22 (4) 17.78 | (3) 19.65 to 46.79 (4) 2.47 to 38.02 | NR | R2: 0.9365 (p < 0.01) R2: 0.6057 (p < 0.01) |
| Capillary POC | (3) 20.11 (4) 23.38 | (3) 13.45 to 53.67 (4) 12.24 to 34.51 | R2: 0.4752 (p = 0.0239) R2: 0.9095 (p < 0.01) | ||||||
| Guensch 2 2021 [42] | G6 | Venous BGA | 16 | (3) 4.3 ± 3.8 (4) 8.1 ± 5.6 | NR | NR | NR | NR | NR |
| Herzig 2023 [43] | G6 | Arterial BGA | Overall: 256 | 23.8 | NR | NR | NR | Clarke: 86.3 | NR |
| ECC: 154 | 29.1 | ||||||||
| DHCA: 10 | 41.6 | ||||||||
| Kalmovich 2012 [44] | CGMS system Gold | Venous BGA | NR | 19.2 | NR | NR | NR | NR | NR |
| Perez-Guzman 2021 [45] | G6 | Arterial BGA, capillary POC | 149 | 12.9 | 15/15: 69 20/20: 82 30/30: 94 | NR | NR | Clarke: 98.6 | NR |
| Piper 2006 [46] | Guardian REAL-Time | Arterial BGA | 246 | 17.6 | NR | NR | NR | Clarke: 98.8 Consensus: 99.6 | Pearson’s: 0.787 (p < 0.001) |
| Polderman 2017 [47] | GlucoClear | Capillary POC | NR | 7.8 [5.5, 10.4] | NR | −13.9 | −64.3 to 36.6 | NR | NR |
| Poljakova 2013 [48] | Guardian REAL-Time | Capillary POC | 51 | NR | NR | NR | NR | NR | Pearson’s: 0.866 |
| Price 2023 [49] | G6 Freestyle Libre 2.0 | Capillary POC | 323 | NR | NR | −18.27 | −82.47 to 45.93 | NR | Pearson’s: 0.731 (95% CI: 0.675, 0.778) |
| Schierenbeck 2017 [51] | Microdialysis system | Arterial BGA | 514 | 6.5 ± 8.2 | 15/15: 90 | 0.9 ± 15.1 | −27 to 29 | Clarke: 100 | NR |
| Freestyle Libre | 578 | 30.5 ± 12.4 | 15/15: 7 | −43.4 ± 20 | −82 to −4.5 | Clarke: 99.1 | |||
| Schierenbeck 2013 [52] | Microdialysis system | Arterial BGA | 607 | 5.6 | 20/15: 97.2 | 2.2 | −10.4 to 14.8 | Clarke: 100 | NR |
| Song 2017 [54] | Guardian REAL-Time | Arterial BGA | Abdomen: 270 | 27.4 ± 20.1 | NR | 20.6 | −143.8 to 185.0 | Clarke: 89.0 | Pearson’s: 0.45 (p < 0.001) |
| Thigh: 331 | 29.7 ± 51.3 | −7.8 | −148.0 to 132.4 | Clarke: 89.3 | Pearson’s: 0.33 (p = 0.004) | ||||
| Sugiyama 2018 [55] | Enlite | Arterial POC Capillary POC | Neuro: 144 | NR | NR | −8.3 | −37.1 to 20.6 | Clarke: 100 | NR |
| Cardiac: 147 | −23.5 | −77.3 to 3.03 | Clarke: 99.3 | ||||||
| Tripyla 2020 [57] | G6 | Capillary POC | 523 | 12.7 ± 5.4 | 15/15: 67.4 ± 24.5 | 9.0 | −9.0 to 48.6 | Clarke: 99.2 ± 2.6 | NR |
| Vriesendorp 3 2005 [58] | CGMS system Gold | Arterial BGA | NR | 13 | NR | NR | NR | Clarke: 100 | NR |
| GlucoDay | (5) 10 (6) 15 | (5) Clarke: 99.1 (6) Clarke: 87.0 | |||||||
| Wasiq 2022 [59] | Freestyle Libre | Venous CL | 40 | NR | NR | 23.8 | −5.3 to 52.9 | NR | Interclass: 0.953 (p < 0.001) |
| Capillary POC | 8.4 | 25 to 37.8 | Interclass: 0.956 (p < 0.001) |
| First Author Year | Sensor Model | Predefined Definition | Incidence |
|---|---|---|---|
| Aust 2014 [38] | CGMS System Gold | No | 0/10 (0%) |
| Blixt 2013 [39] | Microdialysis system | No | 0/10 (0%) |
| Carlsson 2023 [40] | G6 | No | 0/70 (0%) |
| Herzig 2023 [43] | G6 | No | 0/0 (0%) |
| Piper 2006 [46] | Guardian REAL-Time | Adverse skin reaction, infection, or sensor dislodgment. | 0/20 (0%) |
| Poljakova 2013 [48] | Guardian REAL-Time | No | 0/17 (0%) |
| Schierenbeck 2013 [52] | Microdialysis system | No | 0/30 (0%) |
| Song 2017 [54] | Guardian REAL-Time | Adverse skin reaction, infection, or bleeding. | 0/44 (0%) |
| Wasiq 2022 [59] | Freestyle Libre | Local infection or thrombophlebitis. | 0/10 (0%) |
| Tripyla 2020 [57] | G6 | No | 2/20 (10%) Due to self-limited bleeding after sensor insertion, mild pruritus, and skin irritation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.A.; Kim, M.; Kim, N.J.; Huh, J.; Jeong, J.-O.; Hwang, W.; Choi, H. The Performance of Continuous Glucose Monitoring During the Intraoperative Period: A Scoping Review. J. Clin. Med. 2024, 13, 6169. https://doi.org/10.3390/jcm13206169
Lim HA, Kim M, Kim NJ, Huh J, Jeong J-O, Hwang W, Choi H. The Performance of Continuous Glucose Monitoring During the Intraoperative Period: A Scoping Review. Journal of Clinical Medicine. 2024; 13(20):6169. https://doi.org/10.3390/jcm13206169
Chicago/Turabian StyleLim, Hyun Ah, Minjoo Kim, Na Jin Kim, Jaewon Huh, Jin-Oh Jeong, Wonjung Hwang, and Hoon Choi. 2024. "The Performance of Continuous Glucose Monitoring During the Intraoperative Period: A Scoping Review" Journal of Clinical Medicine 13, no. 20: 6169. https://doi.org/10.3390/jcm13206169
APA StyleLim, H. A., Kim, M., Kim, N. J., Huh, J., Jeong, J.-O., Hwang, W., & Choi, H. (2024). The Performance of Continuous Glucose Monitoring During the Intraoperative Period: A Scoping Review. Journal of Clinical Medicine, 13(20), 6169. https://doi.org/10.3390/jcm13206169







