Clinical Meaningfulness of an Algorithm-Based Service for Analyzing Treatment Response in Patients with Metastatic Cancer Using FDG PET/CT
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data
2.2. Standard Radiology Report Analysis
2.3. Algorithm-Based Service Analysis
3. Results
3.1. Patient Information
3.2. Standard Radiology Report Analysis
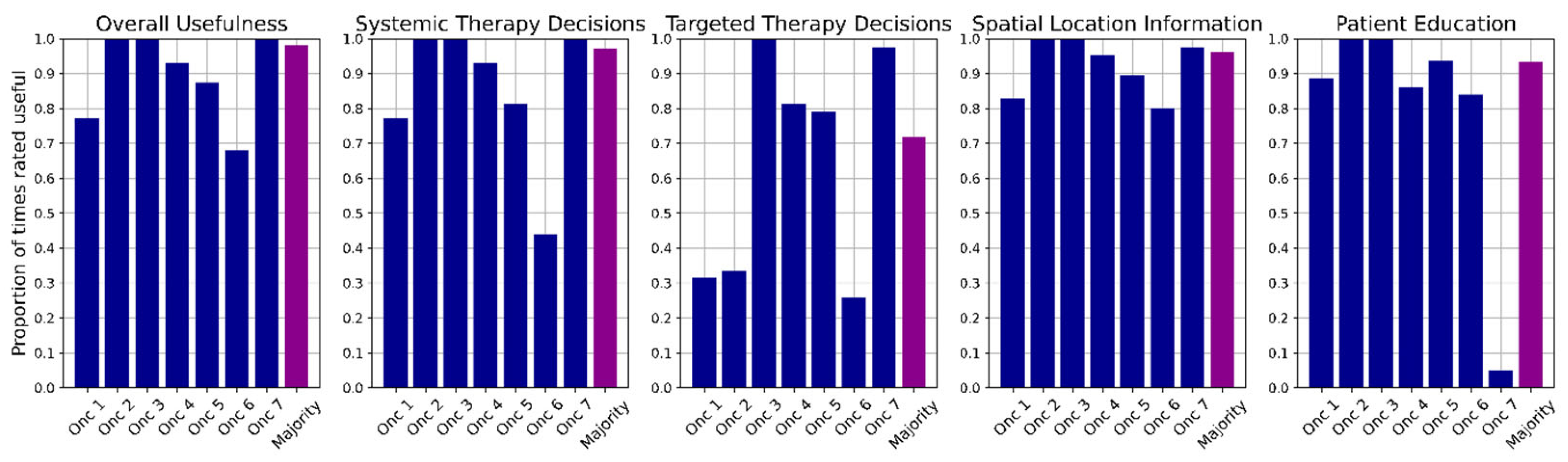
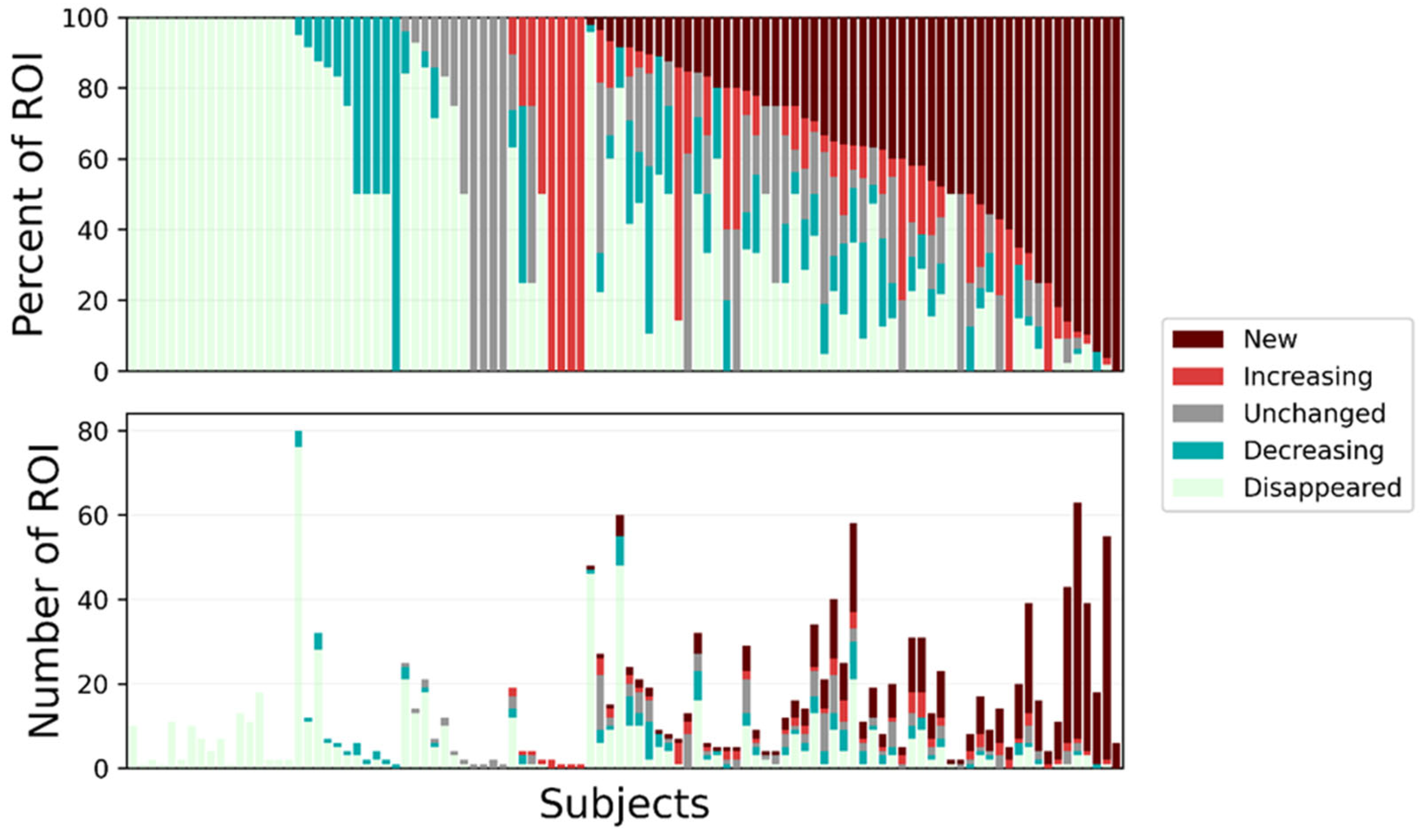
3.3. Algorithm-Based Service Analysis
4. Discussion
4.1. Clinical Usefulness of Algorithm-Based Analysis
4.2. Radiology Report Analysis
4.3. Clinical Implementation and Future Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grzywa, T.M.; Paskal, W.; Włodarski, P.K. Intratumor and Intertumor Heterogeneity in Melanoma. Transl. Oncol. 2017, 10, 956–975. [Google Scholar] [CrossRef]
- Saito, Y.; Horiuchi, S.; Morooka, H.; Ibi, T.; Takahashi, N.; Ikeya, T.; Shimizu, Y.; Hoshi, E. Inter-Tumor Heterogeneity of PD-L1 Expression in Non-Small Cell Lung Cancer. J. Thorac. Dis. 2019, 11, 4982–4991. [Google Scholar] [CrossRef] [PubMed]
- Hendlisz, A.; Deleporte, A.; Delaunoit, T.; Maréchal, R.; Peeters, M.; Holbrechts, S.; Van den Eynde, M.; Houbiers, G.; Filleul, B.; Van Laethem, J.-L.; et al. The Prognostic Significance of Metabolic Response Heterogeneity in Metastatic Colorectal Cancer. PLoS ONE 2015, 10, e0138341. [Google Scholar] [CrossRef] [PubMed]
- Roider, T.; Seufert, J.; Uvarovskii, A.; Frauhammer, F.; Bordas, M.; Abedpour, N.; Stolarczyk, M.; Mallm, J.-P.; Herbst, S.A.; Bruch, P.-M.; et al. Dissecting Intratumour Heterogeneity of Nodal B-Cell Lymphomas at the Transcriptional, Genetic and Drug-Response Levels. Nat. Cell Biol. 2020, 22, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulos, C.E.; Heath, E.I.; Ferrari, A.; Sperger, J.M.; Singh, A.; Perlman, S.B.; Roth, A.R.; Perk, T.G.; Modelska, K.; Porcari, A.; et al. Exploring Spatial-Temporal Changes in 18F-Sodium Fluoride PET/CT and Circulating Tumor Cells in Metastatic Castration-Resistant Prostate Cancer Treated with Enzalutamide. J. Clin. Oncol. 2020, 38, 3662–3671. [Google Scholar] [CrossRef]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET Response Criteria in Solid Tumors. J. Nucl. Med. 2009, 50 (Suppl. S1), 122s–150s. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Glabbeke, M.V.; van Oosterom, T.; Christian, M.C.; et al. New Guidelines to Evaluate the Response to Treatment in Solid Tumors. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef]
- Dell’Oro, M.; Huff, D.T.; Lokre, O.; Kendrick, J.; Govindan, R.; Ong, J.S.L.; Ebert, M.A.; Perk, T.G.; Francis, R.J. Assessing the Heterogeneity of Response of [68Ga]Ga-PSMA-11 PET/CT Lesions in Patients with Biochemical Recurrence of Prostate Cancer. Clin. Genitourin. Cancer 2024, 22, 102155. [Google Scholar] [CrossRef]
- Lokre, O.; Perk, T.G.; Weisman, A.J.; Govindan, R.M.; Chen, S.; Chen, M.; Eickhoff, J.; Liu, G.; Jeraj, R. Quantitative Evaluation of Lesion Response Heterogeneity for Superior Prognostication of Clinical Outcome. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 3505–3517. [Google Scholar] [CrossRef]
- Santoro-Fernandes, V.; Schott, B.; Deatsch, A.; Keigley, Q.; Francken, T.; Iyer, R.; Fountzilas, C.; Perlman, S.; Jeraj, R. Models Using Comprehensive, Lesion-Level, Longitudinal [68Ga]Ga-DOTA-TATE PET-Derived Features Lead to Superior Outcome Prediction in Neuroendocrine Tumor Patients Treated with [177Lu]Lu-DOTA-TATE. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 3428–3439. [Google Scholar] [CrossRef] [PubMed]
- Huff, D.T.; Santoro-Fernandes, V.; Chen, S.; Chen, M.; Kashuk, C.; Weisman, A.J.; Jeraj, R.; Perk, T.G. Performance of an Automated Registration-Based Method for Longitudinal Lesion Matching and Comparison to Inter-Reader Variability. Phys. Med. Biol. 2023, 68, 175031. [Google Scholar] [CrossRef] [PubMed]
- Sundar, L.K.S.; Yu, J.; Muzik, O.; Kulterer, O.C.; Fueger, B.; Kifjak, D.; Nakuz, T.; Shin, H.M.; Sima, A.K.; Kitzmantl, D.; et al. Fully Automated, Semantic Segmentation of Whole-Body 18 F-FDG PET/CT Images Based on Data-Centric Artificial Intelligence. J. Nucl. Med. 2022, 63, 1941–1948. [Google Scholar] [CrossRef]
- Blanc-Durand, P.; Jégou, S.; Kanoun, S.; Berriolo-Riedinger, A.; Bodet-Milin, C.; Kraeber-Bodéré, F.; Carlier, T.; Le Gouill, S.; Casasnovas, R.O.; Meignan, M.; et al. Fully Automatic Segmentation of Diffuse Large B Cell Lymphoma Lesions on 3D FDG-PET/CT for Total Metabolic Tumour Volume Prediction Using a Convolutional Neural Network. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1362–1370. [Google Scholar] [CrossRef]
- Flaus, A.; Habouzit, V.; De Leiris, N.; Vuillez, J.; Leccia, M.; Perrot, J.; Prevot, N.; Cachin, F. FDG PET Biomarkers for Prediction of Survival in Metastatic Melanoma Prior to Anti-PD1 Immunotherapy. Sci. Rep. 2021, 11, 18795. [Google Scholar] [CrossRef]
- Park, J.; Kang, S.K.; Hwang, D.; Choi, H.; Ha, S.; Seo, J.M.; Eo, J.S.; Lee, J.S. Automatic Lung Cancer Segmentation in [18F]FDG PET/CT Using a Two-Stage Deep Learning Approach. Nucl. Med. Mol. Imaging 2023, 57, 86–93. [Google Scholar] [CrossRef]
- Andrearczyk, V.; Oreiller, V.; Boughdad, S.; Le Rest, C.C.; Tankyevych, O.; Elhalawani, H.; Jreige, M.; Prior, J.O.; Vallières, M.; Visvikis, D.; et al. Automatic Head and Neck Tumor Segmentation and Outcome Prediction Relying on FDG-PET/CT Images: Findings from the Second Edition of the HECKTOR Challenge. Med. Image Anal. 2023, 90, 102972. [Google Scholar] [CrossRef]
- Sibille, L.; Seifert, R.; Avramovic, N.; Vehren, T.; Spottiswoode, B.; Zuehlsdorff, S.; Schäfers, M. 18F-FDG PET/CT Uptake Classification in Lymphoma and Lung Cancer by Using Deep Convolutional Neural Networks. Radiology 2019, 294, 445–452. [Google Scholar] [CrossRef]
- Isensee, F.; Jaeger, P.F.; Kohl, S.A.A.; Petersen, J.; Maier-Hein, K.H. nnU-Net: A Self-Configuring Method for Deep Learning-Based Biomedical Image Segmentation. Nat. Methods 2021, 18, 203–211. [Google Scholar] [CrossRef]
- Patel, Z.; Schroeder, J.A.; Bunch, P.M.; Evans, J.K.; Steber, C.R.; Johnson, A.G.; Farris, J.C.; Hughes, R.T. Discordance Between Oncology Clinician–Perceived and Radiologist-Intended Meaning of the Postradiotherapy Positron Emission Tomography/Computed Tomography Freeform Report for Head and Neck Cancer. JAMA Otolaryngol. Head Neck Surg. 2022, 148, 927. [Google Scholar] [CrossRef]
- Patel, B.N.; Lopez, J.M.; Jiang, B.G.; Roth, C.J.; Nelson, R.C. Image-Rich Radiology Reports: A Value-Based Model to Improve Clinical Workflow. J. Am. Coll. Radiol. 2017, 14, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Tie, X.; Shin, M.; Pirasteh, A.; Ibrahim, N.; Huemann, Z.; Castellino, S.M.; Kelly, K.M.; Garrett, J.; Hu, J.; Cho, S.Y.; et al. Personalized Impression Generation for PET Reports Using Large Language Models. J. Digit. Imaging Inform. Med. 2024, 37, 471–488. [Google Scholar] [CrossRef]
- Gunn, A.J.; Sahani, D.V.; Bennett, S.E.; Choy, G. Recent Measures to Improve Radiology Reporting: Perspectives From Primary Care Physicians. J. Am. Coll. Radiol. 2013, 10, 122–127. [Google Scholar] [CrossRef]
- Freudenberg, L.S.; Hicks, R.J.; Beyer, T. An International Survey on Clinical Reporting of PET/CT Examinations: A Starting Point for Cross-Specialty Engagement. J. Nucl. Med. 2019, 60, 480–485. [Google Scholar] [CrossRef]
- Weisman, A.J.; Huff, D.T.; Govindan, R.M.; Chen, S.; Perk, T.G. Multi-Organ Segmentation of CT via Convolutional Neural Network: Impact of Training Setting and Scanner Manufacturer. Biomed. Phys. Eng. Express 2023, 9, 065021. [Google Scholar] [CrossRef]
- Perk, T.; Chen, S.; Harmon, S.; Lin, C.; Bradshaw, T.; Perlman, S.; Liu, G.; Jeraj, R. A Statistically Optimized Regional Thresholding Method (SORT) for Bone Lesion Detection in 18 F-NaF PET/CT Imaging. Phys. Med. Biol. 2018, 63, 225018. [Google Scholar] [CrossRef]
- Santoro-Fernandes, V.; Huff, D.T.; Scarpelli, M.L.; Perk, T.G.; Albertini, M.R.; Perlman, S.; Yip, S.S.F.; Jeraj, R. Development and Validation of a Longitudinal Soft-Tissue Metastatic Lesion Matching Algorithm. Phys. Med. Biol. 2021, 66, 155017. [Google Scholar] [CrossRef]
- Guida, M.; Bartolomeo, N.; De Risi, I.; Fucci, L.; Armenio, A.; Filannino, R.; Ruggieri, E.; Macina, F.; Traversa, M.; Nardone, A.; et al. The Management of Oligoprogression in the Landscape of New Therapies for Metastatic Melanoma. Cancers 2019, 11, 1559. [Google Scholar] [CrossRef]
- Gunderman, R.B. The True Purpose of a Radiology Report. J. Am. Coll. Radiol. 2018, 15, 1450. [Google Scholar] [CrossRef]
- Callen, A.L.; Dupont, S.M.; Price, A.; Laguna, B.; McCoy, D.; Do, B.; Talbott, J.; Kohli, M.; Narvid, J. Between Always and Never: Evaluating Uncertainty in Radiology Reports Using Natural Language Processing. J. Digit. Imaging 2020, 33, 1194–1201. [Google Scholar] [CrossRef]
- Berlin, L. Pitfalls of the Vague Radiology Report. Am. J. Roentgenol. 2000, 174, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Karantanis, D.; Kalkanis, D.; Czernin, J.; Herrmann, K.; Pomykala, K.L.; Bogsrud, T.V.; Subramaniam, R.M.; Lowe, V.J.; Allen-Auerbach, M.S. Perceived Misinterpretation Rates in Oncologic 18 F-FDG PET/CT Studies: A Survey of Referring Physicians. J. Nucl. Med. 2014, 55, 1925–1929. [Google Scholar] [CrossRef]
- Gandy, N.; Arshad, M.A.; Wallitt, K.L.; Dubash, S.; Khan, S.; Barwick, T.D. Immunotherapy-Related Adverse Effects on 18F-FDG PET/CT Imaging. Br. J. Radiol. 2020, 93, 20190832. [Google Scholar] [CrossRef]
- Balasubramanian Harisankar, C.; Preethi, R.; John, J. Metabolic Flare Phenomenon on 18 Fluoride-Fluorodeoxy Glucose Positron Emission Tomography-Computed Tomography Scans in a Patient with Bilateral Breast Cancer Treated with Second-Line Chemotherapy and Bevacizumab. Indian J. Nucl. Med. 2015, 30, 145. [Google Scholar] [CrossRef]
- Okuyama, C.; Higashi, T.; Ishizu, K.; Saga, T. FDG–PET Findings Associated with Various Medical Procedures and Treatments. Jpn. J. Radiol. 2023, 41, 459–476. [Google Scholar] [CrossRef]


| Category | Information | Description and Clinical Relevance |
|---|---|---|
| Nuclear medicine basics | Fasting glucose levels (mg/dL) | Ensures adequate uptake of radiotracer. |
| Activity of injected radiotracer (mCi) | Ensures an appropriate dose was given for adequate biodistribution. | |
| Quantification of reference regions (liver or blood pool) | Establishes a background value for lesion quantification. | |
| Location of injection site, specifically left/right arm | Aids in determining whether abnormal uptake is related to radiotracer injection or extravasation. | |
| Organization | Separation of reported information by anatomical parts | No specific requirements of how many anatomic sections were included. Important for readability of reports. |
| Reason for exam (cancer type, restaging/recurrence) | Important to ensure correct interpretation of exam. | |
| Patient treatment (e.g., chemotherapy, immunotherapy) | Important to ensure treatment-related effects are considered when reading the scan (e.g., immunotherapy-related adverse events). | |
| Quantification | Numerical value for the number of lesions on the scans | Important to determine disease progression and for patient education. Note: scans marked as having “no lesions” were counted as containing this information. |
| Quantification of lesion size at single time point | Numerical value required for at least one lesion, no restrictions on units (e.g., cm vs mm) required. Important to understand clinical relevance of lesion. | |
| Quantification of lesion size across the two time points | Two numerical values required (one for each scan) for at least one lesion unless lesion was described as new or disappeared. Important to understand change in response to therapy and clinical relevance. | |
| Quantification of lesion SUV at a single time point | Numerical SUV value required for at least one lesion, no restriction on type of measurement (e.g., maximum or mean). Important to understand clinical relevance of lesion. | |
| Quantification of lesion SUV across the two time points | Two numerical values required (one for each scan) for at least one lesion unless lesion was described as new or disappeared. Important to understand change in response to therapy and clinical relevance. | |
| Impression | Recommended follow-up | Not important for every scan, but useful for equivocal findings. |
| Clear statement on overall patient response (e.g., complete response, partial response/improvement, stable, progression, new disease) | Important to ensure scan is interpreted correctly without differences in perceived messaging. |
| Cancer type, n | Breast cancer, n = 57 Lung cancer, n = 41 Head & neck cancer, n = 27 Prostate cancer, n = 26 Melanoma, n = 24 | Colorectal cancer, n = 17 Other, n = 16 Lymphoma, n = 13 Gynecological cancer, n = 7 |
| Patient sex, n Female/Male | 113/115 | |
| Patient age, years Median (range) | 67 (25–88) | |
| Patient weight, kg Median (range) | 76.9 (44.0–132.9) | |
| Patient race, n | Unreported, n = 185 White, n = 39 Hispanic, n = 2 Black, n = 1 Asian, n = 1 | |
| Scanner model, n | Siemens Healthineers Biograph 20, n = 193 Siemens Healthineers Biograph 40, n = 80 Canon Medical Systems Celesteion, n = 69 Siemens Healthineers TruePoint (1093), n = 35 Canon Medical Systems Cartesion Prime, n = 19 GE HealthCare Discovery ST, n = 20 Siemens Healthineers Biograph 6, n = 8 Siemens Healthineers Biograph Horizon, n = 9 Siemens Healthineers Biograph HiRes (1080), n = 2 Unreported, n = 21 | |
| Category | Information | Number (Out of 228) | Percentage of Reports (%) | 95% Confidence Intervals (%) |
|---|---|---|---|---|
| Nuclear medicine basics | Patient glucose information (mg/dL) | 226 | 99 | (96.5, 99.8) |
| Injected dose (mCi) | 228 | 100 | (97.9, 100.0) | |
| Quantification of reference regions | 97 | 43 | (36.1, 49.3) | |
| Organization | Location of injection site | 25 | 11 | (7.4, 15.9) |
| Separated by anatomy | 180 | 79 | (73.0, 83.9) | |
| Quantification | Reason for exam | 228 | 100 | (97.9, 100.0) |
| Patient treatment | 81 | 36 | (29.4, 42.2) | |
| Number of lesions | 15 | 7 | (3.9, 10.8) | |
| Lesion size at single time point | 178 | 78 | (72.0, 83.1) | |
| Lesion size across two time points | 119 | 52 | (45.5, 58.8) | |
| Lesion SUV at a single time point | 217 | 95 | (91.3, 97.4) | |
| Lesion SUV across two time points | 151 | 66 | (59.6, 72.3) | |
| Impression | Recommended follow-up | 46 | 20 | (15.3, 26.1) |
| Overall patient response | 105 | 46 | (39.5, 52.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bupathi, M.; Garmezy, B.; Lattanzi, M.; Kieler, M.; Ibrahim, N.; Perk, T.G.; Weisman, A.J.; Perlman, S.B. Clinical Meaningfulness of an Algorithm-Based Service for Analyzing Treatment Response in Patients with Metastatic Cancer Using FDG PET/CT. J. Clin. Med. 2024, 13, 6168. https://doi.org/10.3390/jcm13206168
Bupathi M, Garmezy B, Lattanzi M, Kieler M, Ibrahim N, Perk TG, Weisman AJ, Perlman SB. Clinical Meaningfulness of an Algorithm-Based Service for Analyzing Treatment Response in Patients with Metastatic Cancer Using FDG PET/CT. Journal of Clinical Medicine. 2024; 13(20):6168. https://doi.org/10.3390/jcm13206168
Chicago/Turabian StyleBupathi, Manojkumar, Benjamin Garmezy, Michael Lattanzi, Minnie Kieler, Nevein Ibrahim, Timothy G. Perk, Amy J. Weisman, and Scott B. Perlman. 2024. "Clinical Meaningfulness of an Algorithm-Based Service for Analyzing Treatment Response in Patients with Metastatic Cancer Using FDG PET/CT" Journal of Clinical Medicine 13, no. 20: 6168. https://doi.org/10.3390/jcm13206168
APA StyleBupathi, M., Garmezy, B., Lattanzi, M., Kieler, M., Ibrahim, N., Perk, T. G., Weisman, A. J., & Perlman, S. B. (2024). Clinical Meaningfulness of an Algorithm-Based Service for Analyzing Treatment Response in Patients with Metastatic Cancer Using FDG PET/CT. Journal of Clinical Medicine, 13(20), 6168. https://doi.org/10.3390/jcm13206168






