Efficacy of Thiocolchicoside for Musculoskeletal Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
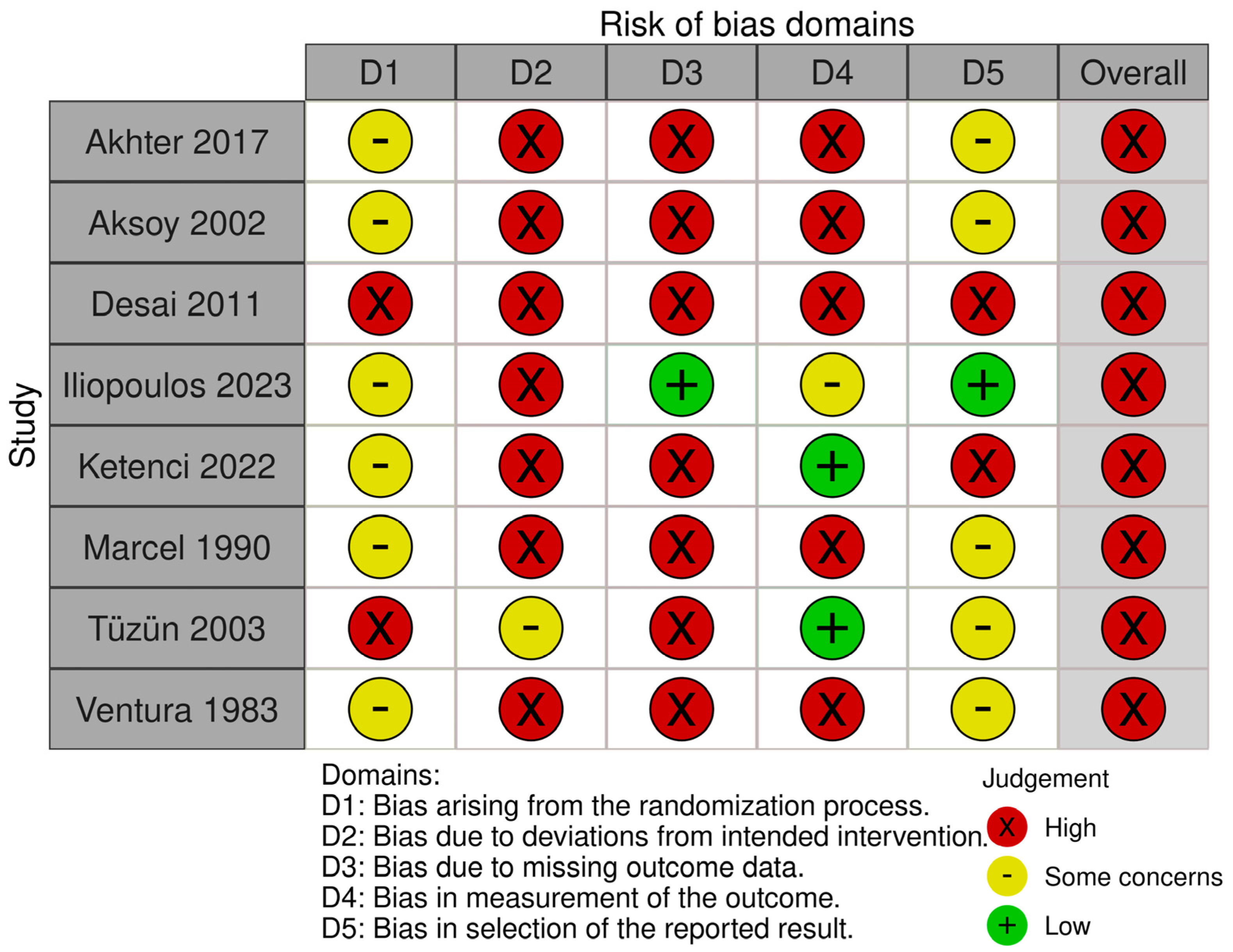
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- PubChem. National Institutes of Health. Thiocolchicoside. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Thiocolchicoside (accessed on 19 July 2024).
- European Medicines Agency. Thiocolchicoside-containing medicines—Referral. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/thiocolchicoside-containing-medicines#key-facts (accessed on 19 July 2024).
- Thiocolchicoside: Review of adverse effects. Prescrire Int. 2016, 25, 41–43.
- Agenzia Italiana del Farmaco. Nota Informativa Importante su Medicinali Contenenti Tiocolchicoside. Available online: https://www.aifa.gov.it/-/nota-informativa-importante-su-medicinali-contenenti-tiocolchicoside (accessed on 19 July 2024).
- Druet-Cabanac, A.; Sophie, J.L.; Afshari, R.; Sahnoun, R.; Kouao-Kanga, G.; Toussi, M.; Granados, D. A drug utilization study of thiocolchicoside-containing medicinal products for systemic use in France and Italy: A cross-sectional electronic medical records database study. Pharmacoepidemiol. Drug Saf. 2023, 32, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Akhter, N.; Zahid Siddiq, M. Comparative efficacy of diclofenac sodium alone and in combination with thiocolchicoside in patients with low back pain. Med. Forum Mon. 2017, 28, 93–96. [Google Scholar]
- Iliopoulos, K.; Koufaki, P.; Tsilikas, S.; Avramidis, K.; Tsagkalis, A.; Mavragani, C.; Zintzaras, E. A randomized controlled trial evaluating the short-term efficacy of a single-administration intramuscular injection with the fixed combination of thiocolchicoside-diclofenac versus diclofenac monotherapy in patients with acute moderate-to-severe low back pain. BMC Musculoskelet. Disord. 2023, 24, 476. [Google Scholar] [CrossRef]
- Marcel, C.; Rezvani, Y.; Revel, M. Evaluation of thiocolchicoside as monotherapy in low back pain. Results of a randomized study versus placebo. Presse Med. 1990, 19, 1133–1136. [Google Scholar] [PubMed]
- Tuzun, F.; Unalan, H.; Oner, N.; Ozguzel, H.; Kirazli, Y.; Icagasioglu, A.; Kuran, B.; Tuzun, S.; Basar, G. Multicenter, randomized, double-blinded, placebo-controlled trial of thiocolchicoside in acute low back pain. Jt. Bone Spine 2003, 70, 356–361. [Google Scholar] [CrossRef]
- Zeng, L.; Brignardello-Petersen, R.; Hultcrantz, M.; Mustafa, R.A.; Murad, M.H.; Iorio, A.; Traversy, G.; Akl, E.A.; Mayer, M.; Schunemann, H.J.; et al. GRADE Guidance 34: Update on rating imprecision using a minimally contextualized approach. J. Clin. Epidemiol. 2022, 150, 216–224. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schunemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Bozada, T., Jr.; Borden, J.; Workman, J.; Del Cid, M.; Malinowski, J.; Luechtefeld, T. Sysrev: A FAIR Platform for Data Curation and Systematic Evidence Review. Front. Artif. Intell. 2021, 4, 685298. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Farrah, K.; Young, K.; Tunis, M.C.; Zhao, L. Risk of bias tools in systematic reviews of health interventions: An analysis of PROSPERO-registered protocols. Syst. Rev. 2019, 8, 280. [Google Scholar] [CrossRef]
- Wang, Y.; Ghadimi, M.; Wang, Q.; Hou, L.; Zeraatkar, D.; Iqbal, A.; Ho, C.; Yao, L.; Hu, M.; Ye, Z.; et al. Instruments assessing risk of bias of randomized trials frequently included items that are not addressing risk of bias issues. J. Clin. Epidemiol. 2022, 152, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Hershkovich, O.; Grevitt, M.P.; Lotan, R. Schober Test and Its Modifications Revisited-What Are We Actually Measuring? Computerized Tomography-Based Analysis. J. Clin. Med. 2022, 11, 6895. [Google Scholar] [CrossRef]
- Aksoy, C.; Karan, A.; Diraçoǧlu, D. Low back pain: Results of an open clinical trial comparing the standard treatment alone to the combination of standard treatment and thiocolchicoside. J. Orthop. Traumatol. 2002, 3, 103–108. [Google Scholar] [CrossRef]
- Desai, A.A.; Sachdeva, P.D.; Arora, B. A comparative study of combined use of aceclofenac along with thiocolchicoside and aceclofenac alone in patients diagnosed of low back pain. Int. J. Pharm. Sci. 2011, 2, 141–150. [Google Scholar]
- Furukawa, T.A.; Barbui, C.; Cipriani, A.; Brambilla, P.; Watanabe, N. Imputing missing standard deviations in meta-analyses can provide accurate results. J. Clin. Epidemiol. 2006, 59, 7–10. [Google Scholar] [CrossRef]
- The Cochrane Collaboration. Review Manager (RevMan), Version 5.4; The Cochrane Collaboration: London, UK, 2020. [Google Scholar]
- Guyatt, G.; Zhao, Y.; Mayer, M.; Briel, M.; Mustafa, R.; Izcovich, A.; Hultcrantz, M.; Iorio, A.; Alba, A.C.; Foroutan, F.; et al. GRADE guidance 36: Updates to GRADE’s approach to addressing inconsistency. J. Clin. Epidemiol. 2023, 158, 70–83. [Google Scholar] [CrossRef]
- Olsen, M.F.; Bjerre, E.; Hansen, M.D.; Hilden, J.; Landler, N.E.; Tendal, B.; Hrobjartsson, A. Pain relief that matters to patients: Systematic review of empirical studies assessing the minimum clinically important difference in acute pain. BMC Med. 2017, 15, 35. [Google Scholar] [CrossRef]
- Ketenci, A.; Sindel, D.; Tulay Koca, T.; Yavuz Karahan, A.; Erdal, A.; Aydin, E.; Sarifakioglu, B.; Ustaomer, K.; Talay Calis, H.; Sarikaya, S.; et al. A multi-center, double-blind, randomized parallel-group Phase IV study comparing the efficacy and safety of thiocolchicoside ointment versus placebo in patients with chronic mechanical low back pain and an acute muscle spasm. Turk. J. Phys. Med. Rehabil. 2022, 68, 456–463. [Google Scholar] [CrossRef]
- Ventura, R.; Leonardi, M.; Mastropaolo, C. Controlled clinical trial of thiocolchicoside in orthopedics. Ortop. E Traumatol. Oggi 1983, 3, 64–72. [Google Scholar]
- Thiocolchicoside Injection and Capsule in Treatment of Acute Low Back Pain. Available online: https://clinicaltrials.gov/study/NCT00917436?intr=NCT00917436&rank=1 (accessed on 30 July 2024).
- An Investigator Initiated Study Comparing Individual Use of Etorocoxib (to Reduce Pain and Swelling) and Thiocolchicoside (a Muscle Relaxant) against Combined Use of Etoroxocib and Thiocolchicoside in Patients with Pain in Different Regions of the Spine Along with Muscle Rigidity and Spams. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2023/08/056416 (accessed on 30 July 2024).
- A Randomised, Two Arm, Openlabel, Active Controlled, Multicentric Clinical Study to Evaluate the Efficacy and Safety of a Topical Fixed Dose Combination of a Muscle Relaxant Thiocolchicoside and a Non Steroidal Anti-Inflammatory Drug, Diclofenac in Comparison with Diclofenac Topical Gel (1.16% w/w) in Acute Non-Specific Low Back Pain. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2011/11/002116 (accessed on 30 July 2024).
- Efe, C.; Purnak, T.; Ozaslan, E.; Milanlioglu, A. Thiocolchicoside-induced liver injury. Clinics 2011, 66, 521–522. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giavina-Bianchi, P.; Giavina-Bianchi, M.; Tanno, L.K.; Ensina, L.F.; Motta, A.A.; Kalil, J. Epileptic seizure after treatment with thiocolchicoside. Ther. Clin. Risk Manag. 2009, 5, 635–637. [Google Scholar] [CrossRef] [PubMed][Green Version]
- The Medicines Utilisation Monitoring Centre. National Report on Medicines Use in Italy. Year 2022. Available online: https://www.aifa.gov.it/documents/20142/1967301/Rapporto-OsMed-2022.pdf (accessed on 30 July 2024).
- Cashin, A.G.; Wand, B.M.; O’Connell, N.E.; Lee, H.; Rizzo, R.R.N.; Bagg, M.K.; O’Hagan, E.; Maher, C.G.; Furlan, A.D.; van Tulder, M.W.; et al. Pharmacological treatments for low back pain in adults: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2023, 4, CD013815. [Google Scholar] [CrossRef]
- Ijzelenberg, W.; Oosterhuis, T.; Hayden, J.A.; Koes, B.W.; van Tulder, M.W.; Rubinstein, S.M.; de Zoete, A. Exercise therapy for treatment of acute non-specific low back pain. Cochrane Database Syst. Rev. 2023, 8, CD009365. [Google Scholar] [CrossRef]
- Derry, S.; Moore, R.A.; Gaskell, H.; McIntyre, M.; Wiffen, P.J. Topical NSAIDs for acute musculoskeletal pain in adults. Cochrane Database Syst. Rev. 2015, 6, CD007402. [Google Scholar] [CrossRef]



| First Author | Year | Country | Funding | Study Population | Sample | Mean Age | % Female Participants | Intervention | Comparison | Outcomes and Effect Sizes |
|---|---|---|---|---|---|---|---|---|---|---|
| Akhter [6] | 2017 | Pakistan | Not reported | Adults (>18 y) with acute LBP with muscle spasms | 288 ITT | Not reported | 53 | Thiocolchicoside (4 mg) + Diclofenac (75 mg) IM injection, twice daily | Diclofenac (75 mg) IM injection | Day 3 VAS: MD = −0.43 [−0.87, 0.01] |
| Day 7 VAS: MD = −0.41 [−0.68, −0.14] | ||||||||||
| Day 3 HTF distance: MD = −1.69 [−2.25, −1.13] | ||||||||||
| Day 7 HTF distance: MD = −1.58 [−1.95, −1.21] | ||||||||||
| Aksoy [19] | 2002 | Turkey | Not reported | Adults (18–65 y) with acute or sub-acute LBP | 372 ITT, 329 PP | 40 ± 11 y | 64 | Thiocolchicoside capsules (8 mg), twice daily + NSAID | Standard treatment (NSAID or BDZ or corticosteroid) | Day 7 VAS: MD = −0.70 [−1.51, 0.11] * |
| Day 31 VAS: MD = −0.50 [−1.28, 0.28] * | ||||||||||
| Day 7 RMDQ scores: MD = −4.50 A * | ||||||||||
| Day 31 RMDQ scores: MD = −5.00 A * | ||||||||||
| Desai [20] | 2011 | India | Not reported | Adults (18–55 y) with acute LBP | 40 ITT, 40 PP | M: 39 y; F: 38 y | 63 | Thiocolchicoside (4 mg) + Aceclofenac (100 mg) IM injection, twice daily | Aceclofenac IM injection | Day 7 mean VAS: MD = −0.13 [−1.71, 1.46] ** |
| Day 7 mean pain during movement score: MD = −0.1 B | ||||||||||
| Day 7 mean movement restriction score: MD = −0.35 B | ||||||||||
| Iliopoulos [7] | 2023 | Greece | Win Medica S.A. | Adults (>18 y) with acute LBP | 134 ITT, 123 PP | 52 ± 11 y | 66 | Thiocolchicoside (4 mg) + Diclofenac (75 mg) IM injection | Diclofenac (75 mg) IM injection | 3 h VAS: MD = −1.30 [−1.87, −0.72] |
| 1 h VAS: MD = −0.36 [−0.98, 0.26] | ||||||||||
| 1 h, > 30% reduction VAS: RR = 1.50 [0.86, 2.65] | ||||||||||
| 3 h, > 30% reduction VAS: RR = 1.60 [1.03, 2.52] | ||||||||||
| 1 h HTF distance: MD = −0.92 [−6.79, 4.95] | ||||||||||
| 3 h HTF distance: MD = −4.55 [−9.66, 0.56] | ||||||||||
| Ketenci [25] | 2022 | Turkey | Multiple sponsors C | Adults (18–64 y) with acute LBP with muscle spasms | 292 ITT, 276 PP | 39 ± 11 y | 64 | Thiocolchicoside oinment (0.25%) | Placebo | Day 3 PPT: MD = 0.10 [−0.29, 0.49] |
| Day 7 PPT: MD = −0.20 [−0.65, 0.25] | ||||||||||
| Day 3 VAS (patient-reported): MD = −0.10 [−0.51, 0.31] | ||||||||||
| Day 7 VAS (patient-reported): MD = −0.10 [−0.62, 0.42] | ||||||||||
| Day 3 VAS (physician-reported): MD = −0.10 [−0.49, 0.29] | ||||||||||
| Day 7 VAS (physician-reported): MD = −0.10 [−0.60, 0.40] | ||||||||||
| Use of paracetamol as rescue drug: RR = 0.77 [0.48, 1.23] | ||||||||||
| Marcel [8] | 1990 | France | Not reported | Patients (range not reported) with acute LBP | 98 ITT, 94 PP | 38 ± 10 y | 38 | Thiocolchicoside tablets (8 mg), twice daily | Placebo | Day 2 VAS: MD = −0.52 [−1.26, 0.22] |
| Day 5 VAS: MD = −1.20 [−2.05, −0.35] | ||||||||||
| Day 2 HTF distance: MD = −4.10 [−10.51, 2.31] | ||||||||||
| Day 5 HTF distance: MD = −8.80 [−15.92, −1.68] | ||||||||||
| Day 2 Schober Index: MD = −0.20 [−0.64, 0.24] | ||||||||||
| Day 5 Schober Index: MD = −0.50 [−1.02, 0.02] | ||||||||||
| Use paracetamol as rescue drug: MD = −3.70 [−7.07, −0.33] | ||||||||||
| Patients with very good/good global evolution score: RR = 2.13 [1.36, 3.31] | ||||||||||
| Tüzün [9] | 2003 | Turkey | Not clearly reported D | Adults (18–65 y) with acute LBP | 143 ITT, 137 PP | 41 ± 11 y | 54 | Thiocolchicoside IM injection (4 mg), twice daily | Placebo | Day 3 VAS: MD = −1.09 [−1.69, −0.49] |
| Day 5 VAS: MD = −2.23 [−2.90, −1.56] | ||||||||||
| Patients with no spasms at day 5: RR = 1.92 [1.19, 3.09] | ||||||||||
| Use of paracetamol as rescue drug: MD = −2.5 B | ||||||||||
| Patients with very good/good global evolution score: RR = 2.89 [1.89, 4.42] | ||||||||||
| Ventura [26] | 1983 | Italy | Not clearly reported E | Patients with coxarthrosis, gonarthrosis, scapulohumeral periarthritis | 30 ITT | Not reported | Not reported | Thiocolchicoside capsules (8 mg), twice daily | Placebo | Day 5 reduction ASM: MD = −10.24 [−18.90, −1.58] |
| Day 10 reduction in ASM: MD = −18.92 [−27.20, −10.64] | ||||||||||
| Day 5 reduction in PSM: MD = −10.74 [−19.53, −1.95] | ||||||||||
| Day 10 reduction in PSM: MD = −17.86 [−25.70, −10.02] |
| Certainty Assessment | № of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Thiocolchicoside | Placebo/No Treatment | Relative (95% CI) | Absolute (95% CI) | ||
| Low Back Pain—Intensity (follow-up ranging from 2 days to 3 days, assessed with Visual Analogue Scale from 0 to 10) | ||||||||||||
| 4 | Randomized trials | Very serious a | Serious b | Not serious | Serious c | None | 403 | 398 | - | MD 0.49 lower (0.9 lower to 0.09 lower) | ⨁◯◯◯ Very low | |
| Low Back Pain—Intensity (follow-up ranging from 5 days to 7 days, assessed with Visual Analogue Scale from 0 to 10) | ||||||||||||
| 6 | Randomized trials | Very serious a | Serious b | Not serious | Serious c | None | 599 | 573 | - | MD 0.82 lower (1.46 lower to 0.18 lower) | ⨁◯◯◯ Very low | |
| Low Back Pain—Intensity (assessed with other measures, including Visual Analogue Scale for Pain at 1 h and 3 h; Visual Analogue Scale scores at day 31; pain pressure threshold; the use of paracetamol as a rescue drug, physician-reported Visual Analogue Scale for pain scores at Day 3 and Day 7; the presence of muscle spasms) | ||||||||||||
| 5 | Randomized trials | Very serious a | Not serious | Not serious | Serious d | None | Two studies found favourable effects of thiocolchicoside on other pain intensity outcomes (the presence of muscle spasms and the mean use of paracetamol as a rescue drug), two studies found null effects of thiocolchicoside on other pain intensity outcomes (Visual Analogue Scale scores at day 31, pain pressure threshold, the use of paracetamol as a rescue drug, and physician-reported Visual Analogue Scale for pain scores at Day 3 and Day 7). One study found null effects from a single administration of thiocolchicoside on Visual Analogue Scale scores after 1 h, and statistically significant, but very small, effects on Visual Analogue Scale scores after 3 h (upper limits of the 95% CIs were above the minimally important difference threshold). | ⨁◯◯◯ Very low | ||||
| Low Back Pain—Functional impairment (assessed with hand-to-floor distance; Schober’s test score; patient-reported global evaluation scores) | ||||||||||||
| 4 | Randomized trials | Very serious a | Serious e | Not serious | Serious d | None | Two studies found favourable effects on functional impairment outcomes (ratio of patients with very good/good global evolution score, and hand-to-floor distance at days 3 and 7). One study found mixed favourable effects on functional impairment outcomes (hand-to-floor distance at day 5) and null effects on functional impairment outcomes (hand-to-floor distance at day 2, Schober Index at days 2 and 5, and ratio of patients with very good/good global evolution score). One study found null effects on functional impairment outcomes (hand-to-floor distance at 1 and 3 h after a single administration). | ⨁◯◯◯ Very low | ||||
| Osteoarthritis—Functional impairment (assessed with a reduction in active segmental mobility score; reduction in passive mobility score) | ||||||||||||
| 1 | Randomized trials | Very serious a | Not serious | Serious f | Not serious | None | One study found favourable effects on functional impairment outcomes (reduction in active segmental mobility at days 5 and 10, and reduction in passive segmental mobility at days 5 and 10). | ⨁◯◯◯ Very low | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianconi, A.; Fiore, M.; Rosso, A.; Acuti Martellucci, C.; Calò, G.L.; Cioni, G.; Imperiali, G.; Orazi, V.; Tiseo, M.; Troia, A.; et al. Efficacy of Thiocolchicoside for Musculoskeletal Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2024, 13, 6133. https://doi.org/10.3390/jcm13206133
Bianconi A, Fiore M, Rosso A, Acuti Martellucci C, Calò GL, Cioni G, Imperiali G, Orazi V, Tiseo M, Troia A, et al. Efficacy of Thiocolchicoside for Musculoskeletal Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine. 2024; 13(20):6133. https://doi.org/10.3390/jcm13206133
Chicago/Turabian StyleBianconi, Alessandro, Matteo Fiore, Annalisa Rosso, Cecilia Acuti Martellucci, Giovanna Letizia Calò, Giovanni Cioni, Gianmarco Imperiali, Vittorio Orazi, Marco Tiseo, Anastasia Troia, and et al. 2024. "Efficacy of Thiocolchicoside for Musculoskeletal Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Journal of Clinical Medicine 13, no. 20: 6133. https://doi.org/10.3390/jcm13206133
APA StyleBianconi, A., Fiore, M., Rosso, A., Acuti Martellucci, C., Calò, G. L., Cioni, G., Imperiali, G., Orazi, V., Tiseo, M., Troia, A., & Zauli, E. (2024). Efficacy of Thiocolchicoside for Musculoskeletal Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine, 13(20), 6133. https://doi.org/10.3390/jcm13206133