Very Early-Onset IBD-Associated IL-18opathy Treated with an Anti-IL-18 Antibody
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Medication
2.2. Ethics, Consent, and Drug Approval
2.3. Measurement of Serum Cytokines
3. Results
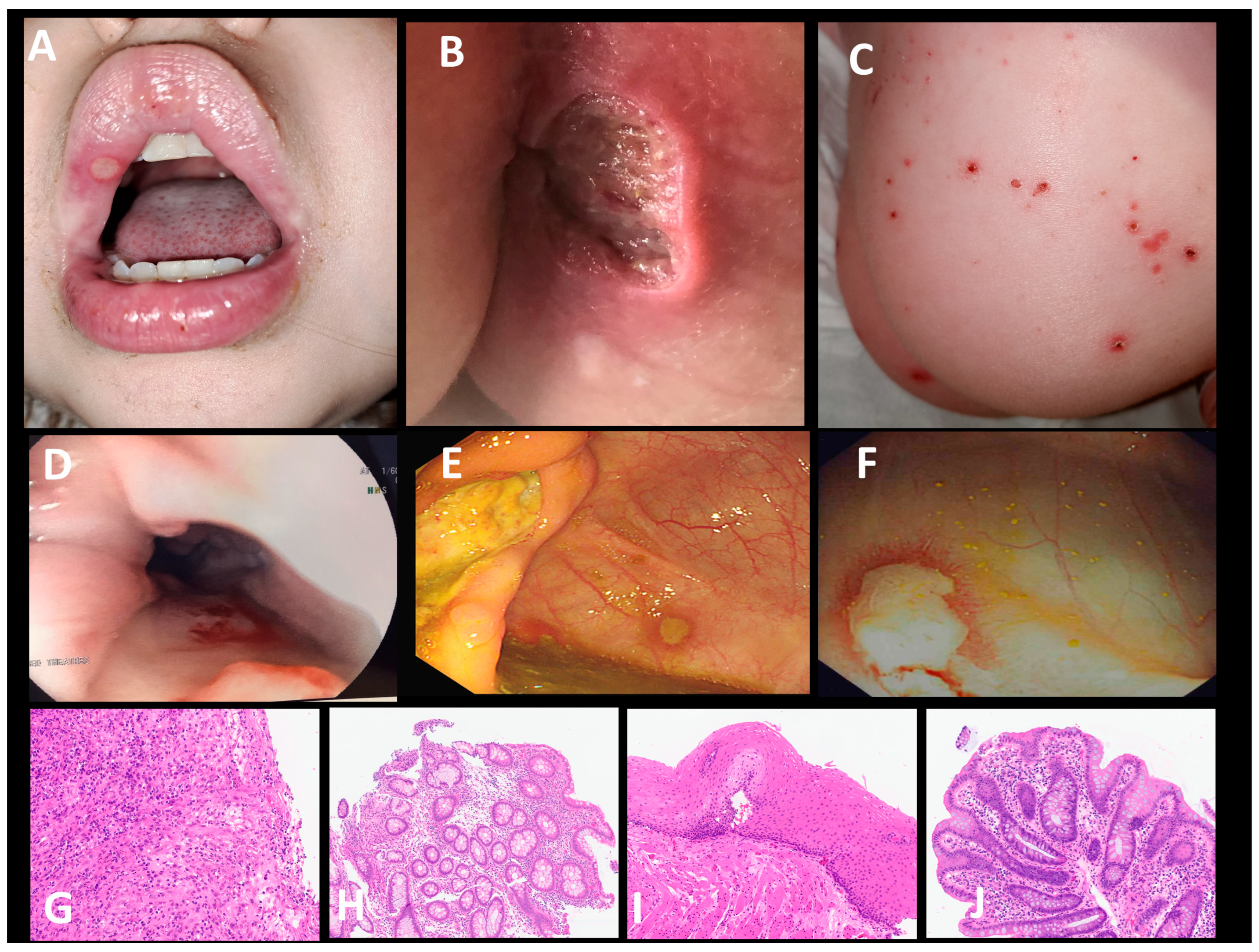
3.1. Case Presentation
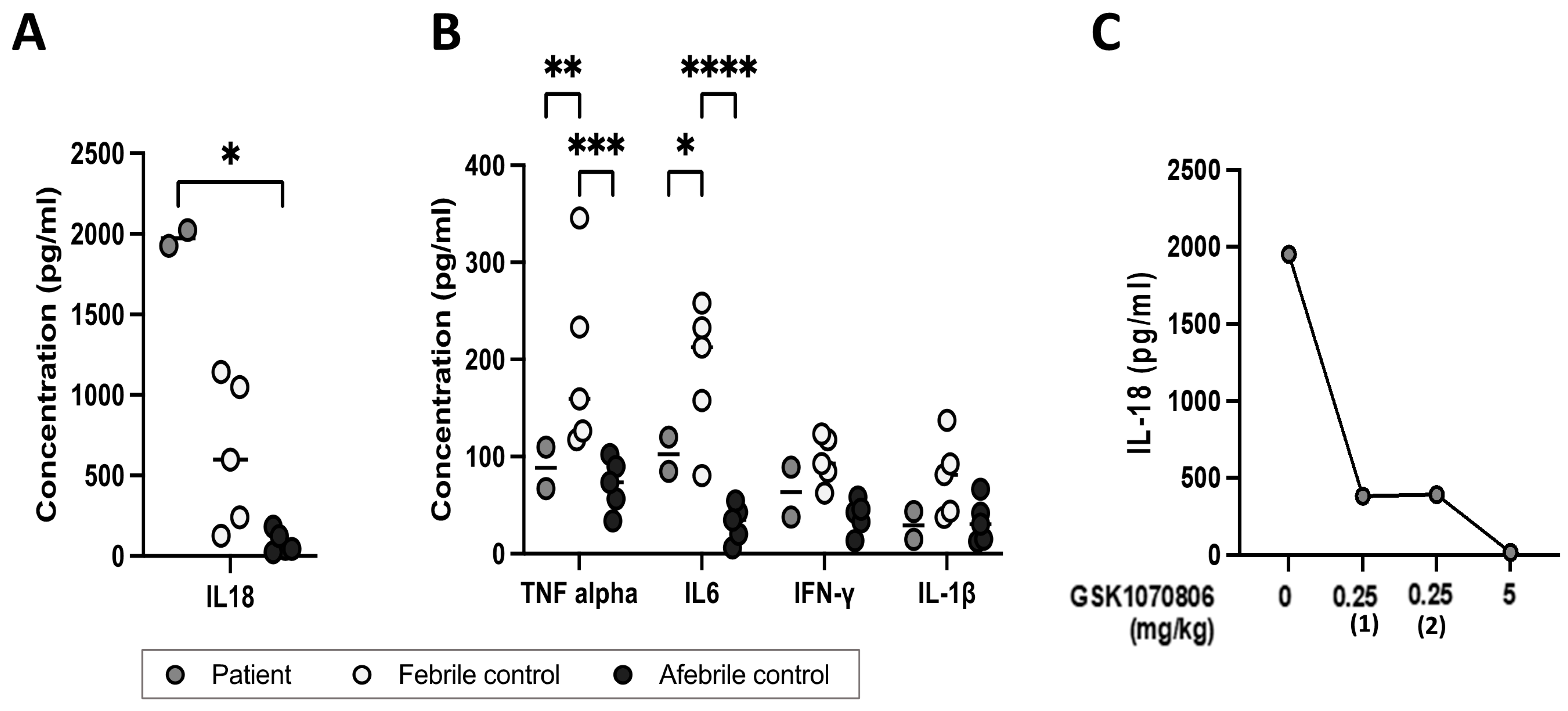
3.2. Cytokine Profiling
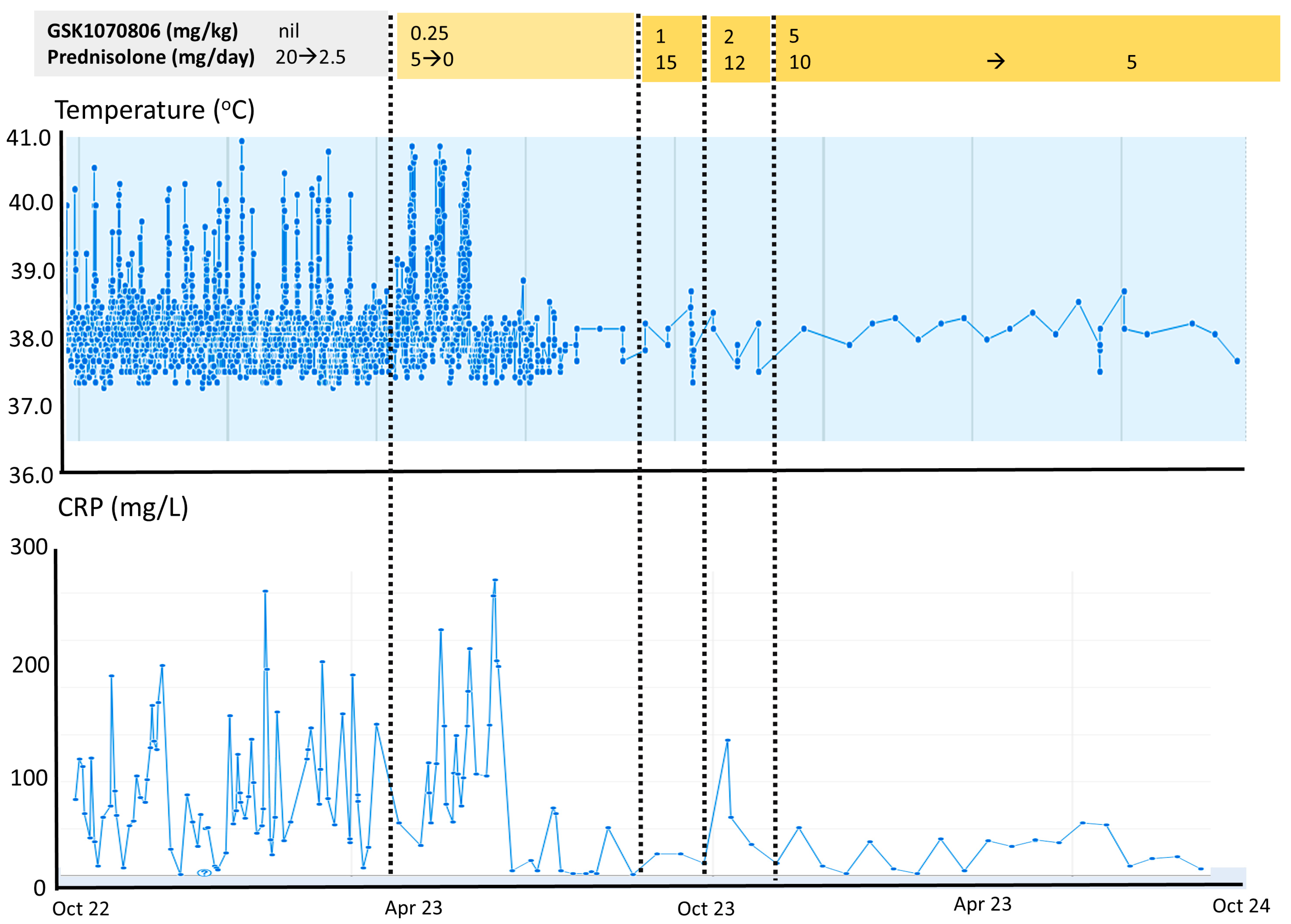
3.3. Impact of Anti IL-18 Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ouahed, J.D. Understanding inborn errors of immunity: A lens into the pathophysiology of monogenic inflammatory bowel disease. Front. Immunol. 2022, 13, 1026511. [Google Scholar] [CrossRef] [PubMed]
- Bolton, C.; Smillie, C.S.; Pandey, S.; Elmentaite, R.; Wei, G.; Argmann, C.; Aschenbrenner, D.; James, K.R.; McGovern, D.P.; Macchi, M.; et al. An integrated taxonomy for monogenic inflammatory bowel disease. Gastroenterology 2022, 162, 859–876. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.H.T.; de Zoeten, E.F. Understanding very early onset inflammatory bowel disease (VEOIBD) in relation to inborn errors of immunity. Immunol. Rev. 2024, 322, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Landy, E.; Carol, H.; Ring, A.; Canna, S. Biological and clinical roles of IL-18 in inflammatory diseases. Nat. Rev. Rheumatol. 2024, 20, 33–47. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X.; Wu, D.; Jin, O.; Gu, J. Evaluation of the causal associations between interleukin-18 levels and immune-mediated inflammatory diseases: A Mendelian randomization study. BMC Med. Genom. 2023, 16, 306. [Google Scholar] [CrossRef] [PubMed]
- Leach, S.T.; Messina, I.; Lemberg, D.A.; Novick, D.; Rubenstein, M.; Day, A.S. Local and systemic interleukin-18 and interleukin-18-binding protein in children with inflammatory bowel disease. Inflamm. Bowel Dis. 2008, 14, 68–74. [Google Scholar] [CrossRef]
- Bank, S.; Andersen, P.S.; Burisch, J.; Pedersen, N.; Roug, S.; Galsgaard, J.; Turino, S.Y.; Brodersen, J.B.; Rashid, S.; Rasmussen, B.K.; et al. Genetically determined high activity of IL-12 and IL-18 in ulcerative colitis and TLR5 in Crohns disease were associated with non-response to anti-RNF therapy. Pharmacogenom. J. 2018, 18, 87–97. [Google Scholar] [CrossRef]
- Mu, J.; Maeda, K.; Ohashi, A.; Urano, T.; Nariai, Y.; Kamino, H.; Nakamura, M.; Yamamura, T.; Sawada, T.; Ishikawa, E.; et al. Monclonal antibodies against mature interleukin-18 ameliorate colitis and repair goblet cell function. Dig. Dis. Sci. 2024, 69, 2573–2585. [Google Scholar] [CrossRef]
- Canna, S.W.; Girard, C.; Malle, L.; de Jesus, A.; Romberg, N.; Kelsen, J.; Surrey, L.F.; Russo, P.; Sleight, A.; Schiffrin, E.; et al. Life-threatening NLRC4-associated hyperinflammation successfully treated with interleukin-18 inhibition. J. Allergy Clin. Immunol. 2017, 139, 1698–1701. [Google Scholar] [CrossRef]
- Mistry, P.; Reid, J.; Pouliquen, I.; McHugh, S.; Abberley, L.; DeWall, S.; Taylor, A.; Tong, X.; del Cura, M.R.; McKie, E. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single-dose antiinterleukin-18 mAb GSK1070806 in healthy and obese subjects. Int. J. Clin. Pharmacol. Ther. 2014, 52, 867–879. [Google Scholar] [CrossRef]
- McKie, E.A.; Reid, J.L.; Mistry, P.C.; DeWall, S.L.; Abberley, L.; Ambery, P.D.; Gil-Extremera, B. A study to investigate the efficacy and safety of an anti-interleukin-18 monoclonal antibody in the treatment of type 2 diabetes mellitus. PLoS ONE 2016, 11, e0150018. [Google Scholar] [CrossRef] [PubMed]
- Genomics England. Primary Immunodeficiency or Monogenic Inflammatory Bowel Disease (Version 5.10). Available online: https://panelapp.genomicsengland.co.uk/panels/398/ (accessed on 6 October 2024).
- Crowley, E.; Warner, N.; Pan, J.; Khalouei, S.; Elkadri, A.; Fiedler, K.; Foong, J.; Turinsky, A.L.; Bronte-Tinkew, D.; Zhang, S.; et al. Prevalence and clinical features of inflammatory bowel diseases associated with monogenic variants, identified by whole-exome sequencing in 1000 children at a single center. Gastroenterology 2020, 158, 2208–2220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; Zhang, Y.; He, T.; Sweeney, C.L.; Baris, S.; Karakoc-Aydiner, E.; Yao, Y.; Ertem, D.; Matthews, H.F.; Gonzaga-Jauregui, C.; et al. Homozygous IL37 mutation associated with infantile inflammatory bowel disease. Proc. Natl. Acad. Sci. USA 2021, 118, e2009217118. [Google Scholar] [CrossRef]
- Shimizu, M.; Takei, S.; Mori, M.; Yachie, A. Pathogenic roles and diagnostic utility of interleukin-18 in autoimmune diseases. Front. Immunol. 2022, 13, 951535. [Google Scholar] [CrossRef]
- Okamura, H.; Kashiwamura, S.-I.; Tsutsui, H.; Yoshimoto, T.; Nakanishi, K. Regulation of interferon-gamma production by IL-12 and IL-18. Curr. Opin. Immunol. 1998, 10, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Fautrel, B.; Rech, J.; Spertini, F.; Feist, E.; Kötter, I.; Hachulla, E.; Morel, J.; Schaeverbeke, T.; A Hamidou, M.; et al. Open-label, multicentre, dose-escalating phase II trial on the safety and efficacy of tadekinig alfa (IL-18BP) in adult-onset Still’s disease. Ann. Rheum. Dis. 2018, 77, 840–847. [Google Scholar] [CrossRef]
- Kiltz, U.; Kiefer, D.; Braun, J.; Schiffrin, E.J.; Girard-Gard-Guyonvarc’h, C.; Gabay, C. Prolonged treatment with Tadekinig alfa in adult-onset Still’s disease. Ann. Rheum. Dis. 2020, 79, e10. [Google Scholar] [CrossRef]
- Geerlinks, A.V.; Dvorak, A.M. XIAP Deficiency Treatment Consortium. A case of XIAP deficiency successfully managed with tadekinig alfa (rhIL-18BP). J. Clin. Immunol. 2022, 42, 901–903. [Google Scholar] [CrossRef] [PubMed]
- Suryaprakash, S.; El-Baba, M.; Walkovich, K.L.; Savsan, S. Expanding clinical spectrum of female X-linked lymphoproliferative syndrome 2. Pediatr. Blood Cancer 2021, 68, e28592. [Google Scholar] [CrossRef]
- Tapia, V.S.; Daniels, M.J.; Palazon-Riquelme, P.; Dewhurst, M.; Luheshi, N.M.; Rivers-Auty, J.; Green, J.; Redondo-Castro, E.; Kaldis, P.; Lopez-Castejon, G.; et al. The three cytokines IL-1β, IL-18, and IL-1α share related but distinct secretory routes. J. Biol. Chem. 2019, 294, 8325–8335. [Google Scholar] [CrossRef]
- Nowarski, R.; Jackson, R.; Gagliani, N.; De Zoete, M.R.; Palm, N.W.; Bailis, W.; Low, J.S.; Harman, C.C.D.; Graham, M.; Elinav, E.; et al. Epithelial IL-18 equilibrium controls barrier function in collitis. Cell 2015, 163, 1444–1456. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Cao, M.; Yao, Y.; Hu, W.; Sun, H.; Zhang, Y.; Zeng, C.; Tang, J.; Luan, S.; Chen, P. Dysregulated microbiota-driven gasdermin D activation promotes colitis development by mediating IL-18 release. Front. Immunol. 2021, 12, 750841. [Google Scholar] [CrossRef] [PubMed]
- Mazodier, K.; Marin, V.; Novick, D.; Farnarier, C.; Robitail, S.; Schleinitz, N.; Veit, V.; Paul, P.; Rubinstein, M.; Dinarello, C.A.; et al. Severe imbalance of IL-18/IL-18BP in patients with secondary hemophagocytic syndrome. Blood 2005, 106, 3483–3489. [Google Scholar] [CrossRef] [PubMed]
- Canna, S.W.; De Benedetti, F. The 4th NextGen therapies of SJIA and MAS, part 4: It is time for IL-18 based trials in systemic juvenile idiopathic arthritis? Pediatr. Rheumatol. 2024, 21 (Suppl. S1), 79. [Google Scholar] [CrossRef]
| Gender | Age | Diagnosis | Cause of Fever |
|---|---|---|---|
| Female | 2 years | B-cell acute lymphoblastic leukaemia | Viral URTI |
| Female | 6 years | Ewings sarcoma | Viral URTI |
| Male | 6 years | Medulloblastoma | Viral URTI |
| Male | 4 years | Rhabdomyosarcoma | Line infection |
| Male | 20 months | Hepatoblastoma | Line infection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guha, A.; Diaz-Pino, R.; Fagbemi, A.; Hughes, S.M.; Wynn, R.F.; Lopez-Castejon, G.; Arkwright, P.D. Very Early-Onset IBD-Associated IL-18opathy Treated with an Anti-IL-18 Antibody. J. Clin. Med. 2024, 13, 6058. https://doi.org/10.3390/jcm13206058
Guha A, Diaz-Pino R, Fagbemi A, Hughes SM, Wynn RF, Lopez-Castejon G, Arkwright PD. Very Early-Onset IBD-Associated IL-18opathy Treated with an Anti-IL-18 Antibody. Journal of Clinical Medicine. 2024; 13(20):6058. https://doi.org/10.3390/jcm13206058
Chicago/Turabian StyleGuha, Anthea, Rodrigo Diaz-Pino, Andrew Fagbemi, Stephen M. Hughes, Robert F. Wynn, Gloria Lopez-Castejon, and Peter D. Arkwright. 2024. "Very Early-Onset IBD-Associated IL-18opathy Treated with an Anti-IL-18 Antibody" Journal of Clinical Medicine 13, no. 20: 6058. https://doi.org/10.3390/jcm13206058
APA StyleGuha, A., Diaz-Pino, R., Fagbemi, A., Hughes, S. M., Wynn, R. F., Lopez-Castejon, G., & Arkwright, P. D. (2024). Very Early-Onset IBD-Associated IL-18opathy Treated with an Anti-IL-18 Antibody. Journal of Clinical Medicine, 13(20), 6058. https://doi.org/10.3390/jcm13206058







