The Role of Automated Infrared Pupillometry in Traumatic Brain Injury: A Narrative Review
Abstract
1. Introduction
2. Methods
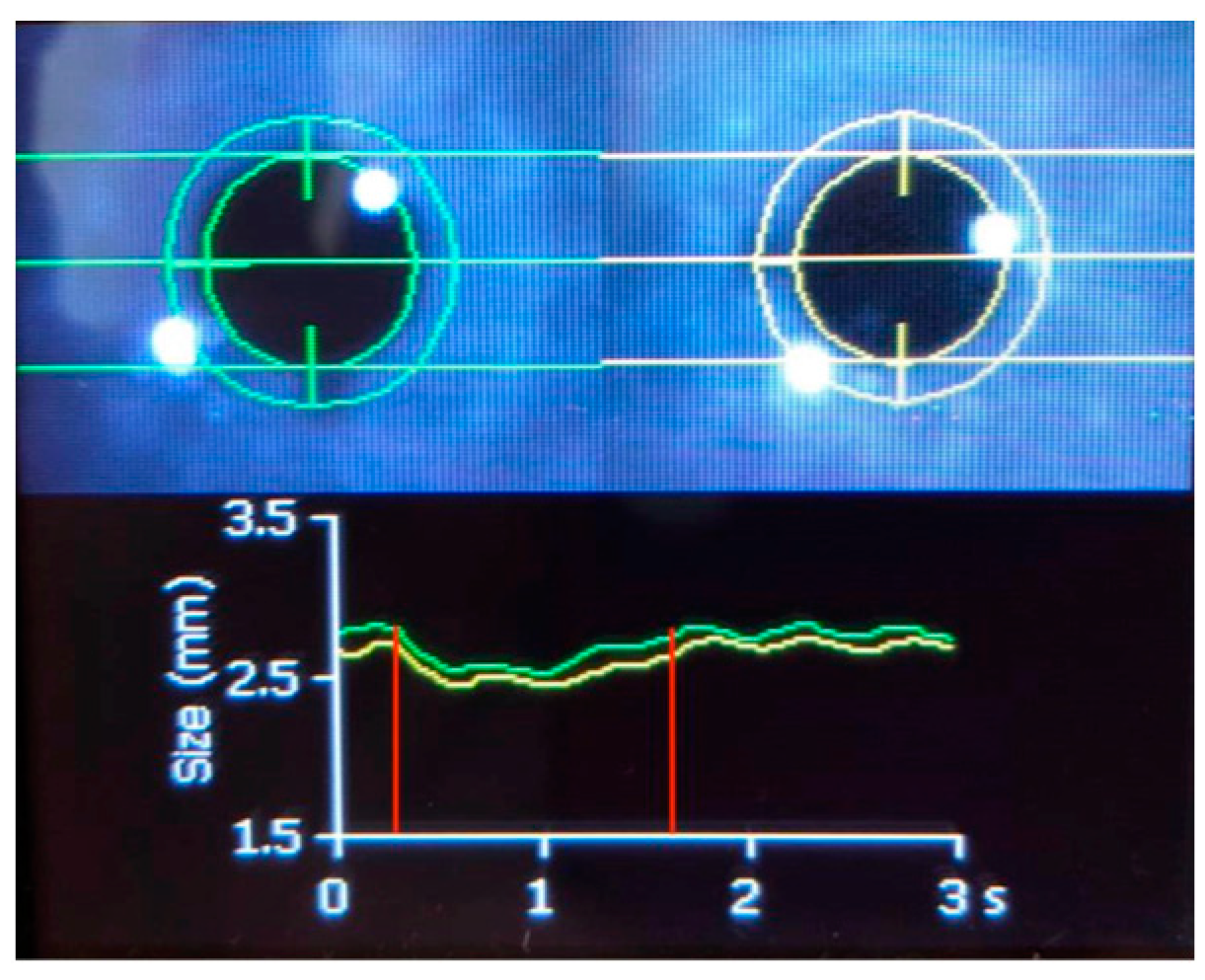
3. Technical Characteristics of PAIPs
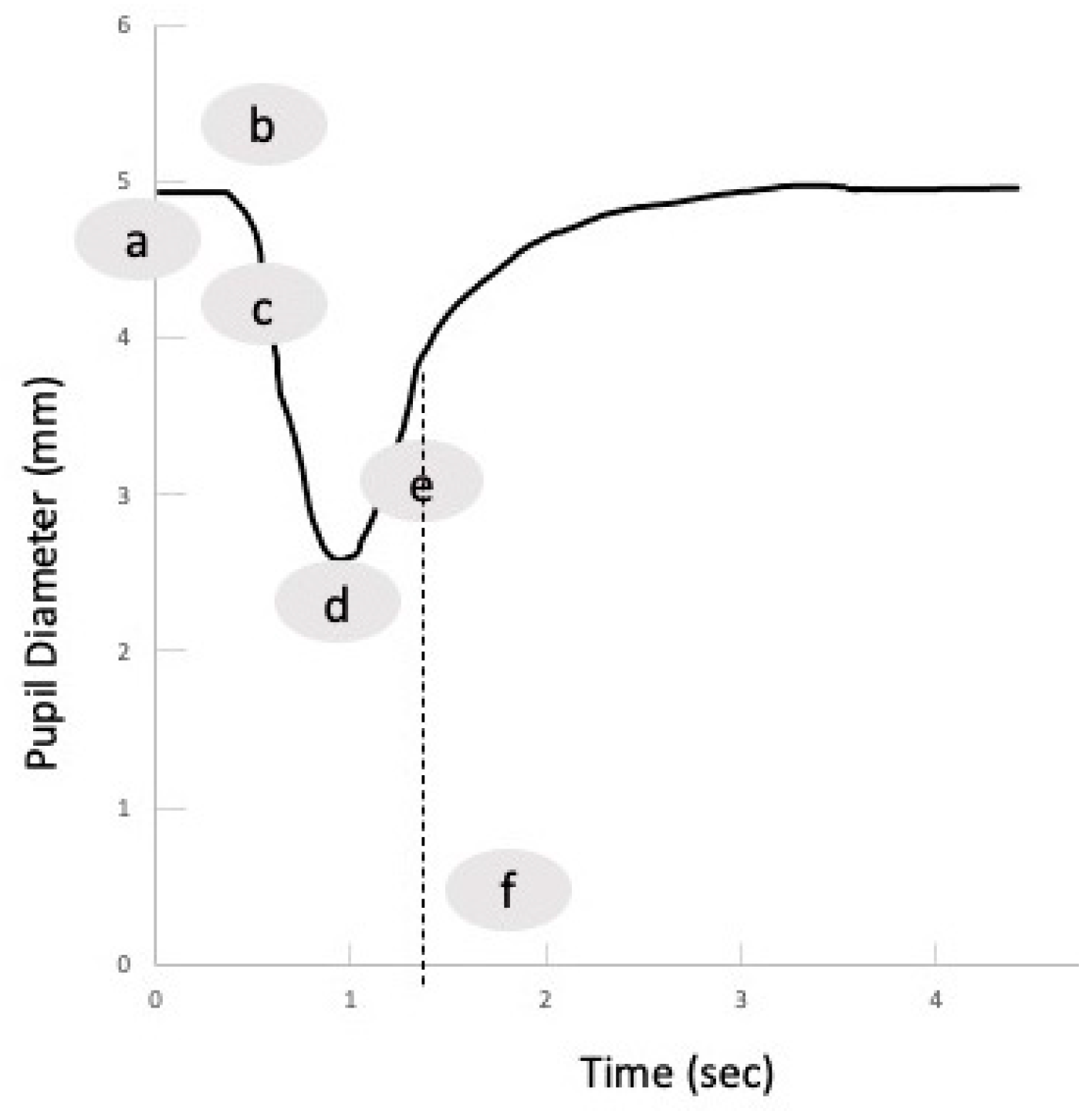
4. The Normal PLR Response
5. Evidence for PAIP Application in mTBI
6. Evidence for PAIP Application in Moderate and sTBI
7. Limitations of Pupillometry in TBI Assessment
8. Discussion
9. Conclusions
10. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dragoi, V. Section 3: Motor Systems Chapter 7: Ocular Motor System; The University of Texas, McGovern Medical School: Austin, TX, USA, 1997; Available online: https://nba.uth.tmc.edu/neuroscience/m/s3/chapter07.html (accessed on 1 January 2024).
- Hunyor, A.P. Reflexes and the Eye. Aust. N. Z. J. Ophthalmol. 1994, 22, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Andrefsky, J.C.; Frank, J.I.; Chyatte, D. The Ciliospinal Reflex in Pentobarbital Coma. J. Neurosurg. 1999, 90, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Havelius, U.; Heuck, M.; Milos, P.; Hindfelt, B. Ciliospinal Reflex Response in Cluster Headache. Headache 1996, 36, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Oddo, M.; Sandroni, C.; Citerio, G.; Miroz, J.P.; Horn, J.; Rundgren, M.; Cariou, A.; Payen, J.F.; Storm, C.; Stammet, P.; et al. Quantitative versus Standard Pupillary Light Reflex for Early Prognostication in Comatose Cardiac Arrest Patients: An International Prospective Multicenter Double-Blinded Study. Intensive Care Med. 2018, 44, 2102–2111. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.A.; Chilcott, R.P. Eyeing up the Future of the Pupillary Light Reflex in Neurodiagnostics. Diagnostics 2018, 8, 19. [Google Scholar] [CrossRef]
- Couret, D.; Boumaza, D.; Grisotto, C.; Triglia, T.; Pellegrini, L.; Ocquidant, P.; Bruder, N.J.; Velly, L.J. Reliability of Standard Pupillometry Practice in Neurocritical Care: An Observational, Double-Blinded Study. Crit. Care 2016, 20, 99. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Moro Salihovic, B.; Pozzebon, S.; Creteur, J.; Oddo, M.; Vincent, J.L.; Taccone, F.S. Comparison of 2 Automated Pupillometry Devices in Critically III Patients. J. Neurosurg. Anesthesiol. 2020, 32, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Patrick, S.D.; Rapport, L.J.; Kanser, R.J.; Hanks, R.A.; Bashem, J.R. Detecting Simulated versus Bona Fide Traumatic Brain Injury Using Pupillometry. Neuropsychology 2021, 35, 472–485. [Google Scholar] [CrossRef]
- Traylor, J.I.; El Ahmadieh, T.Y.; Bedros, N.M.; Al Adli, N.; Stutzman, S.E.; Venkatachalam, A.M.; Pernik, M.N.; Collum, C.M.; Douglas, P.M.; Aiyagari, V.; et al. Quantitative Pupillometry in Patients with Traumatic Brain Injury and Loss of Consciousness: A Prospective Pilot Study. J. Clin. Neurosci. 2021, 91, 88–92. [Google Scholar] [CrossRef]
- Boulter, J.H.; Shields, M.M.; Meister, M.R.; Murtha, G.; Curry, B.P.; Dengler, B.A. The Expanding Role of Quantitative Pupillometry in the Evaluation and Management of Traumatic Brain Injury. Front. Neurol. 2021, 12, 685313. [Google Scholar] [CrossRef]
- El Ahmadieh, T.Y.; Bedros, N.; Stutzman, S.E.; Nyancho, D.; Venkatachalam, A.M.; MacAllister, M.; Ban, V.S.; Dahdaleh, N.S.; Aiyagari, V.; Figueroa, S.; et al. Automated Pupillometry as a Triage and Assessment Tool in Patients with Traumatic Brain Injury. World Neurosurg. 2021, 145, e163–e169. [Google Scholar] [CrossRef] [PubMed]
- Ventura, R.E.; Balcer, L.J.; Galetta, S.L. The Concussion Toolbox: The Role of Vision in the Assessment of Concussion. Semin. Neurol. 2015, 35, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Master, C.L.; Podolak, O.E.; Ciuffreda, K.J.; Metzger, K.B.; Joshi, N.R.; McDonald, C.C.; Margulies, S.S.; Grady, M.F.; Arbogast, K.B. Utility of Pupillary Light Reflex Metrics as a Physiologic Biomarker for Adolescent Sport-Related Concussion. JAMA Ophthalmol. 2020, 138, 1135–1141. [Google Scholar] [CrossRef]
- Joseph, J.R.; Swallow, J.S.; Willsey, K.; Almeida, A.A.; Lorincz, M.T.; Fraumann, R.K.; Oppenlander, M.E.; Szerlip, N.J.; Broglio, S.P. Pupillary Changes after Clinically Asymptomatic High-Acceleration Head Impacts in High School Football Athletes. J. Neurosurg. 2020, 133, 1886–1891. [Google Scholar] [CrossRef]
- Vinciguerra, L.; Bösel, J. Noninvasive Neuromonitoring: Current Utility in Subarachnoid Hemorrhage, Traumatic Brain Injury, and Stroke. Neurocrit Care 2017, 27, 122–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Vakil-Gilani, K.; Williamson, K.L.; Cecil, S. Infrared Pupillometry, the Neurological Pupil Index and Unilateral Pupillary Dilation after Traumatic Brain Injury: Implications for Treatment Paradigms. J. Korean Phys. Soc. 2014, 3, 548. [Google Scholar] [CrossRef]
- Saliman, N.H.; Belli, A.; Blanch, R.J. Afferent Visual Manifestations of Traumatic Brain Injury. J. Neurotrauma 2021, 38, 2778–2789. [Google Scholar] [CrossRef] [PubMed]
- Oddo, M.; Taccone, F.; Galimberti, S.; Rebora, P.; Citerio, G. Outcome Prognostication of Acute Brain Injury Using the Neurological Pupil Index (ORANGE) Study: Protocol for a Prospective, Observational, Multicentre, International Cohort Study. BMJ Open 2021, 11, e046948. [Google Scholar] [CrossRef]
- Narayan, V.; Mohammed, N.; Savardekar, A.R.; Patra, D.P.; Notarianni, C.; Nanda, A. Noninvasive Intracranial Pressure Monitoring for Severe Traumatic Brain Injury in Children: A Concise Update on Current Methods. World Neurosurg. 2018, 114, 293–300. [Google Scholar] [CrossRef]
- Robba, C.; Pozzebon, S.; Moro, B.; Vincent, J.L.; Creteur, J.; Taccone, F.S. Multimodal Non-Invasive Assessment of Intracranial Hypertension: An Observational Study. Crit. Care 2020, 24, 379. [Google Scholar] [CrossRef]
- Stevens, A.R.; Su, Z.; Toman, E.; Belli, A.; Davies, D. Optical Pupillometry in Traumatic Brain Injury: Neurological Pupil Index and Its Relationship with Intracranial Pressure through Significant Event Analysis. Brain Inj. 2019, 33, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Namiki, J.; Sugawara, Y.; Sekine, K.; Yo, K.; Kanaya, T.; Yokobori, S.; Roberts, R.; Abe, T.; Yokota, H.; et al. Quantitative Assessment of Pupillary Light Reflex for Early Prediction of Outcomes after Out-of-Hospital Cardiac Arrest: A Multicentre Prospective Observational Study. Resuscitation 2018, 131, 108–113. [Google Scholar] [CrossRef]
- Riker, R.R.; Sawyer, M.E.; Fischman, V.G.; May, T.; Lord, C.; Eldridge, A.; Seder, D.B. Neurological Pupil Index and Pupillary Light Reflex by Pupillometry Predict Outcome Early After Cardiac Arrest. Neurocrit Care 2020, 32, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Obinata, H.; Yokobori, S.; Shibata, Y.; Takiguchi, T.; Nakae, R.; Igarashi, Y.; Shigeta, K.; Matsumoto, H.; Aiyagari, V.; Olson, D.W.M.; et al. Early Automated Infrared Pupillometry Is Superior to Auditory Brainstem Response in Predicting Neurological Outcome after Cardiac Arrest. Resuscitation 2020, 154, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Ellouze, O.; Constandache, T.; Berthoud, V.; Voizeux, P.; Bouhemad, B.; Guinot, P.G. Automated Infrared Pupillometry for Neurological Prognostication after Extracorporeal Cardiopulmonary Resuscitation. Intensive Care Med. 2020, 46, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Xu, J.; Wang, J.; Zhang, M.; Liu, F.; Khan, Z.U.; Liu, S.; Zhou, W.; Qian, A.; Zhang, J.; et al. Automated Pupillometry Helps Monitor the Efficacy of Cardiopulmonary Resuscitation and Predict Return of Spontaneous Circulation. Am. J. Emerg. Med. 2021, 49, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Muppidi, S.; Adams-Huet, B.; Tajzoy, E.; Scribner, M.; Blazek, P.; Spaeth, E.B.; Frohman, E.; Davis, S.; Vernino, S. Dynamic Pupillometry as an Autonomic Testing Tool. Clin. Auton. Res. 2013, 23, 297–303. [Google Scholar] [CrossRef]
- Park, K.W.; Choi, N.; Ryu, H.S.; Kim, M.S.; Lee, E.J.; Chung, S.J. Pupillary Dysfunction of Multiple System Atrophy: Dynamic Pupillometric Findings and Clinical Correlations. Park. Relat. Disord. 2019, 65, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.B.; Coppell, K.J.; Mitchell, L.V.; Ogbuehi, K.C. Dynamic Pupillometry in Type 2 Diabetes: Pupillary Autonomic Dysfunction and the Severity of Diabetic Retinopathy. Clin. Ophthalmol. 2020, 14, 3923–3930. [Google Scholar] [CrossRef]
- de Rodez Benavent, S.A.; Nygaard, G.O.; Harbo, H.F.; Tønnesen, S.; Sowa, P.; Landrø, N.I.; Wendel-Haga, M.; Etholm, L.; Nilsen, K.B.; Drolsum, L.; et al. Fatigue and Cognition: Pupillary Responses to Problem-Solving in Early Multiple Sclerosis Patients. Brain Behav. 2017, 7, e00717. [Google Scholar] [CrossRef]
- Oh, A.J.; Amore, G.; Sultan, W.; Asanad, S.; Park, J.C.; Romagnoli, M.; la Morgia, C.; Karanjia, R.; Harrington, M.G.; Sadun, A.A. Erratum: Pupillometry Evaluation of Melanopsin Retinal Ganglion Cell Function and Sleep-Wake Activity in Pre-Symptomatic Alzheimer’s Disease. PLOS ONE 2019, 14, e0226197. [Google Scholar] [CrossRef] [PubMed]
- Chougule, P.S.; Najjar, R.P.; Finkelstein, M.T.; Kandiah, N.; Milea, D. Light-Induced Pupillary Responses in Alzheimer’s Disease. Front. Neurol. 2019, 10, 360. [Google Scholar] [CrossRef]
- You, S.; Hong, J.H.; Yoo, J. Analysis of Pupillometer Results According to Disease Stage in Patients with Parkinson’s Disease. Sci. Rep. 2021, 11, 17880. [Google Scholar] [CrossRef] [PubMed]
- McGovern, J.E.; Reddy, L.F.; Reavis, E.A.; Green, M.F. Pupillary Change on a Cognitive Effort Task in Schizophrenia: Associations with Cognition and Motivation. Int. J. Psychophysiol. 2020, 155, 1–7. [Google Scholar] [CrossRef]
- Kvamme, T.L.; Pedersen, M.U.; Overgaard, M.; Rømer Thomsen, K.; Voon, V. Pupillary Reactivity to Alcohol Cues as a Predictive Biomarker of Alcohol Relapse Following Treatment in a Pilot Study. Psychopharmacology 2019, 236, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A Scale for the Quality Assessment of Narrative Review Articles. Res. Integr. Peer Rev. 2019, 26, 5. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.R.; Chen, J.W.; Meltzer, H.; Gennarelli, T.A.; Kelbch, C.; Knowlton, S.; Richardson, J.; Lutch, M.J.; Farin, A.; Hults, K.N.; et al. Quantitative Pupillometry, a New Technology: Normative Data and Preliminary Observations in Patients with Acute Head Injury—Technical Note. J. Neurosurg. 2003, 98, 205–213. [Google Scholar] [CrossRef]
- Lussier, B.L.; Stutzman, S.E.; Atem, F.; Venkatachalam, A.M.; Perera, A.C.; Barnes, A.; Aiyagari, V.; Olson, D.W.M. Distributions and Reference Ranges for Automated Pupillometer Values in Neurocritical Care Patients. J. Neurosci. Nurs. 2019, 51, 335–340. [Google Scholar] [CrossRef]
- Brennan, P.M.; Murray, G.D.; Teasdale, G.M. Simplifying the Use of Prognostic Information in Traumatic Brain Injury. Part 1: The GCS-Pupils Score: An Extended Index of Clinical Severity. J. Neurosurg. 2018, 128, 1612–1620. [Google Scholar] [CrossRef]
- Holm, L.; Cassidy, J.D.; Carroll, L.J.; Borg, J. Summary of the WHO Collaborating Centre for Neurotrauma Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 2005, 37, 137–141. [Google Scholar] [CrossRef]
- Silverberg, N.D.; Iaccarino, M.A.; Panenka, W.J.; Iverson, G.L.; McCulloch, K.L.; Dams-O’Connor, K.; Reed, N.; McCrea, M.; Cogan, A.M.; Park Graf, M.J.; et al. Management of Concussion and Mild Traumatic Brain Injury: A Synthesis of Practice Guidelines. Arch. Phys. Med. Rehabil. 2020, 101, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Isokuortti, H.; Iverson, G.L.; Silverberg, N.D.; Kataja, A.; Brander, A.; Öhman, J.; Luoto, T.M. Characterizing the Type and Location of Intracranial Abnormalities in Mild Traumatic Brain Injury. J. Neurosurg. 2018, 129, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Khong, E.; Odenwald, N.; Hashim, E.; Cusimano, M.D. Diffusion Tensor Imaging Findings in Post-Concussion Syndrome Patients after Mild Traumatic Brain Injury: A Systematic Review. Front. Neurol. 2016, 7, 156. [Google Scholar] [CrossRef] [PubMed]
- Asken, B.M.; DeKosky, S.T.; Clugston, J.R.; Jaffee, M.S.; Bauer, R.M. Diffusion Tensor Imaging (DTI) Findings in Adult Civilian, Military, and Sport-Related Mild Traumatic Brain Injury (MTBI): A Systematic Critical Review. Brain Imaging Behav. 2018, 12, 585–612. [Google Scholar] [CrossRef] [PubMed]
- Dasic, D.; Morgan, L.; Panezai, A.; Syrmos, N.; Ligarotti, G.K.I.; Zaed, I.; Chibbaro, S.; Khan, T.; Prisco, L.; Ganau, M. A Scoping Review on the Challenges, Improvement Programs, and Relevant Output Metrics for Neurotrauma Services in Major Trauma Centers. Surg. Neurol. Int. 2022, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Scheenen, M.E.; De Koning, M.E.; Van Der Horn, H.J.; Roks, G.; Yilmaz, T.; Van Der Naalt, J.; Spikman, J.M. Acute Alcohol Intoxication in Patients with Mild Traumatic Brain Injury: Characteristics, Recovery, and Outcome. J. Neurotrauma 2016, 33, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.K.; Schwab, K.; Wright, D.W.; Maas, A.I.; Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health. Position Statement: Definition of Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2010, 91, 1637–1640. [Google Scholar] [CrossRef]
- Capó-Aponte, J.E.; Urosevic, T.G.; Walsh, D.V.; Temme, L.A.; Tarbett, A.K. Pupillary Light Reflex as an Objective Biomarker for Early Identification of Blast-Induced MTBI. J. Spine 2013, 2, 1–4. [Google Scholar] [CrossRef]
- Thiagarajan, P.; Ciuffreda, K.J. Pupillary Responses to Light in Chronic Non-Blast-Induced MTBI. Brain Inj. 2015, 29, 1420–1425. [Google Scholar] [CrossRef]
- Truong, J.Q.; Ciuffreda, K.J. Comparison of Pupillary Dynamics to Light in the Mild Traumatic Brain Injury (MTBI) and Normal Populations. Brain Inj. 2016, 30, 1378–1389. [Google Scholar] [CrossRef]
- Truong, J.Q.; Ciuffreda, K.J. Quantifying Pupillary Asymmetry through Objective Binocular Pupillometry in the Normal and Mild Traumatic Brain Injury (MTBI) Populations. Brain Inj. 2016, 30, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Truong, J.Q.; Ciuffreda, K.J. Objective Pupillary Correlates of Photosensitivity in the Normal and Mild Traumatic Brain Injury Populations. Mil. Med. 2016, 181, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Hawryluk, G.W.J.; Aguilera, S.; Buki, A.; Bulger, E.; Citerio, G.; Cooper, D.J.; Arrastia, R.D.; Diringer, M.; Figaji, A.; Gao, G.; et al. A Management Algorithm for Patients with Intracranial Pressure Monitoring: The Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2019, 45, 1783–1794. [Google Scholar] [CrossRef] [PubMed]
- Chesnut, R.M.; Temkin, N.; Videtta, W.; Petroni, G.; Lujan, S.; Pridgeon, J.; Dikmen, S.; Chaddock, K.; Barber, J.; MacHamer, J.; et al. Consensus-Based Management Protocol (CREVICE Protocol) for the Treatment of Severe Traumatic Brain Injury Based on Imaging and Clinical Examination for Use When Intracranial Pressure Monitoring Is Not Employed. J. Neurotrauma 2020, 37, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Meyfroidt, G.; Bouzat, P.; Casaer, M.P.; Chesnut, R.; Hamada, S.R.; Helbok, R.; Hutchinson, P.; Maas, A.I.R.; Manley, G.; Menon, D.K.; et al. Management of Moderate to Severe Traumatic Brain Injury: An Update for the Intensivist. Intensive Care Med. 2022, 48, 649–666. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.; Joannides, A.; Adeleye, A.O.; Bajamal, A.H.; Bashford, T.; Biluts, H.; Budohoski, K.; Ercole, A.; Fernández-Méndez, R.; Figaji, A.; et al. Casemix, Management, and Mortality of Patients Receiving Emergency Neurosurgery for Traumatic Brain Injury in the Global Neurotrauma Outcomes Study: A Prospective Observational Cohort Study. Lancet Neurol. 2022, 21, 438–449. [Google Scholar] [CrossRef]
- Anderson, M.; Elmer, J.; Shutter, L.; Puccio, A.; Alexander, S. Integrating Quantitative Pupillometry into Regular Care in a Neurotrauma Intensive Care Unit. J. Neurosci. Nurs. 2018, 50, 30–36. [Google Scholar] [CrossRef]
- Morelli, P.; Oddo, M.; Ben-Hamouda, N. Role of Automated Pupillometry in Critically Ill Patients. Minerva Anestesiol. 2019, 85, 995–1002. [Google Scholar] [CrossRef]
- Martínez-Ricarte, F.; Castro, A.; Poca, M.A.; Sahuquillo, J.; Expósito, L.; Arribas, M.; Aparicio, J. Infrared Pupillometry. Basic Principles and Their Application in the Non-Invasive Monitoring of Neurocritical Patients. Neurología 2013, 28, 41–51. [Google Scholar] [CrossRef]
- Romagnosi, F.; Bernini, A.; Bongiovanni, F.; Iaquaniello, C.; Miroz, J.P.; Citerio, G.; Taccone, F.S.; Oddo, M. Neurological Pupil Index for the Early Prediction of Outcome in Severe Acute Brain Injury Patients. Brain Sci. 2022, 12, 609. [Google Scholar] [CrossRef]
- Jahns, F.P.; Miroz, J.P.; Messerer, M.; Daniel, R.T.; Taccone, F.S.; Eckert, P.; Oddo, M. Quantitative Pupillometry for the Monitoring of Intracranial Hypertension in Patients with Severe Traumatic Brain Injury. Crit. Care 2019, 23, 155. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.; Hutch, M.; Barra, M.; Kim, A.; Zafar, S.; Smirnakis, S. Effects of Osmotic Therapy on Pupil Reactivity: Quantification Using Pupillometry in Critically Ill Neurologic Patients. Neurocrit Care 2019, 30, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Papangelou, A.; Zink, E.K.; Chang, W.T.W.; Frattalone, A.; Gergen, D.; Gottschalk, A.; Geocadin, R.G. Automated Pupillometry and Detection of Clinical Transtentorial Brain Herniation: A Case Series. Mil. Med. 2018, 183, e113–e121. [Google Scholar] [CrossRef] [PubMed]
- Bower, M.M.; Sweidan, A.J.; Xu, J.C.; Stern-Neze, S.; Yu, W.; Groysman, L.I. Quantitative Pupillometry in the Intensive Care Unit. J. Intensive Care Med. 2021, 36, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Singer, K.E.; Wallen, T.E.; Jalbert, T.; Wakefield, D.; Spuzzillo, A.; Sharma, S.; Earnest, R.; Heh, V.; Foreman, B.; Goodman, M.D. Efficacy of Noninvasive Technologies in Triaging TBI and Correlating with ICP: A Prospective Study. J. Surg. Res. 2021, 262, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gombart, Z.; Rogers, S.; Gardiner, S.; Cecil, S.; Bullock, R. Pupillary Reactivity as an Early Indicator of Increased Intracranial Pressure: The Introduction of the Neurological Pupil Index. Surg. Neurol. Int. 2011, 2, 82. [Google Scholar] [CrossRef] [PubMed]
- Pansell, J.; Hack, R.; Rudberg, P.; Bell, M.; Cooray, C. Can Quantitative Pupillometry Be Used to Screen for Elevated Intracranial Pressure? A Retrospective Cohort Study. Neurocrit Care 2022, 37, 531–537. [Google Scholar] [CrossRef]
- Oddo, M.; Taccone, F.S.; Petrosino, M.; Badenes, R.; Blandino-Ortiz, A.; Bouzat, P.; Caricato, A.; Chesnut, R.M.; Feyling, A.C.; Ben-Hamouda, N.; et al. The Neurological Pupil Index for Outcome Prognostication in People with Acute Brain Injury (ORANGE): A Prospective, Observational, Multicentre Cohort Study. Lancet Neurol. 2023, 22, 925–933. [Google Scholar] [CrossRef]
- Larson, M.D.; Behrends, M. Portable Infrared Pupillometry: A Review. Anesth. Analg. 2015, 120, 1242–1253. [Google Scholar] [CrossRef]
- Couret, D.; Simeone, P.; Freppel, S.; Velly, L. The Effect of Ambient-Light Conditions on Quantitative Pupillometry: A History of Rubber Cup. Neurocrit Care 2019, 30, 492–493. [Google Scholar] [CrossRef]
- Ong, C.; Hutch, M.; Smirnakis, S. The Effect of Ambient Light Conditions on Quantitative Pupillometry. Neurocrit Care 2019, 30, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Kelbsch, C.; Strasser, T.; Chen, Y.; Feigl, B.; Gamlin, P.D.; Kardon, R.; Peters, T.; Roecklein, K.A.; Steinhauer, S.R.; Szabadi, E.; et al. Standards in Pupillography. Front. Neurol. 2019, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Rollins, M.D.; Feiner, J.R.; Lee, J.M.; Shah, S.; Larson, M. Pupillary Effects of High-Dose Opioid Quantified with Infrared Pupillometry. Anesthesiology 2014, 121, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Larson, M.D. Mechanism of Opioid-Induced Pupillary Effects. Clin. Neurophysiol. 2008, 119, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.T.; Krejci, S.T.; Larson, M.D. Neuromuscular Blocking Drugs Do Not Alter the Pupillary Light Reflex of Anesthetized Humans. Arch. Neurol. 1997, 54, 579–584. [Google Scholar] [CrossRef]
- Larson, M.D.; Muhiudeen, I. Pupillometric Analysis of the “Absent Light Reflex”. Arch. Neurol. 1995, 52, 369–372. [Google Scholar] [CrossRef]
- Meeker, M.; Du, R.; Bacchetti, P.; Privitera, C.M.; Larson, M.D.; Holland, M.C.; Manley, G. Pupil Examination: Validity and Clinical Utility of an Automated Pupillometer. J. Neurosci. Nurs. 2005, 37, 34–40. [Google Scholar] [CrossRef]
- Schmidt, F.A.; Ruprecht, K.; Connolly, F.; Maas, M.B.; Paul, F.; Hoffmann, J.; Harms, L.; Schreiber, S.J. B-Mode Ultrasound Assessment of Pupillary Function: Feasibility, Reliability and Normal Values. PLoS ONE 2017, 12, e0189016. [Google Scholar] [CrossRef]
- Ettinger, E.R.; Wyatt, H.J.; London, R. Anisocoria: Variation and Clinical Observation with Different Conditions of Illumination and Accommodation. Invest. Ophthalmol. Vis. Sci. 1991, 32, 501–509. [Google Scholar]
- McLaren, J.W.; Erie, J.C.; Brubaker, R.F. Computerized Analysis of Pupillograms in Studies of Alertness. Invest. Ophthalmol. Vis. Sci. 1992, 33, 671–676. [Google Scholar]
- Denny, J.C.; Arndt, F.V.; Dupont, W.D.; Neilson, E.G. Increased Hospital Mortality in Patients with Bedside Hippus. Am. J. Med. 2008, 121, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Vrettou, C.S.; Fragkou, P.C.; Trigkidis, K.; Charalampaki, E. Pupillary Light Response Abnormalities in Miller Fisher Syndrome. Intensive Care Med. 2023, 49, 1406–1407. [Google Scholar] [CrossRef] [PubMed]
- Ciuffreda, K.J.; Joshi, N.R.; Truong, J.Q. Understanding the Effects of Mild Traumatic Brain Injury on the Pupillary Light Reflex. Concussion 2017, 2, CNC36. [Google Scholar] [CrossRef] [PubMed]
- McAnany, J.J.; Smith, B.M.; Garland, A.; Kagen, S.L. IPhone-Based Pupillometry: A Novel Approach for Assessing the Pupillary Light Reflex. Optom. Vis. Sci. 2018, 95, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.Y.L.; Turuwhenua, J.; Qu, T.Y.; Black, J.M.; Acosta, M.L. Infrared Video Pupillography Coupled with Smart Phone Led for Measurement of Pupillary Light Reflex. Front. Integr. Neurosci. 2017, 11, 6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aissou, M.; Snauwaert, A.; Dupuis, C.; Atchabahian, A.; Aubrun, F.; Beaussier, M. Objective Assessment of the Immediate Postoperative Analgesia Using Pupillary Reflex Measurement A Prospective and Observational Study. Anesthesiology 2012, 116, 1006–1012. [Google Scholar] [CrossRef]
- Isnardon, S.; Vinclair, M.; Genty, C.; Hebrard, A.; Albaladejo, P.; Payen, J.F. Pupillometry to Detect Pain Response during General Anaesthesia Following Unilateral Popliteal Sciatic Nerve Block: A Prospective, Observational Study. Eur. J. Anaesthesiol. 2013, 30, 429–434. [Google Scholar] [CrossRef]
- Othman, M.H.; Møller, K.; Kjaergaard, J.; Kondziella, D. Detecting Signatures of Consciousness in Acute Brain Injury after Stimulation with Apomorphine and Methylphenidate: Protocol for a Placebo-Controlled, Randomized, Cross-over Study. BMJ Neurol. Open 2024, 6, e000584. [Google Scholar] [CrossRef]



| Pupillary Reflex | Function |
|---|---|
| Pupillary light reflex | Pupillary constriction to light |
| Pupillary dark reflex | Pupillary dilation in darkness |
| Ciliospinal reflex | Pupillary dilation in response to noxious stimuli to the face, neck, and upper trunk. |
| Near accommodation reflex | Pupil and lens accommodation and convergence of the eyes for near vision |
| Pupillometry Parameter * | Normal Volunteers | Neurocritical Care Patients |
|---|---|---|
| Maximum pupil size (mm) | 4.1 ± 0.34 | 3.5 ± 1.2 |
| Minimum pupil size (mm) | 2.7 ± 0.21 | - |
| Mean reduction in size % | 34 | - |
| Mean constriction velocity (mm/sec) | 1.48 ± 0.33 | 1.6 ± 0.9 |
| Mean latency duration (sec) | 0.24 ± 0.4 | 0.3 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrettou, C.S.; Fragkou, P.C.; Mallios, I.; Barba, C.; Giannopoulos, C.; Gavrielatou, E.; Dimopoulou, I. The Role of Automated Infrared Pupillometry in Traumatic Brain Injury: A Narrative Review. J. Clin. Med. 2024, 13, 614. https://doi.org/10.3390/jcm13020614
Vrettou CS, Fragkou PC, Mallios I, Barba C, Giannopoulos C, Gavrielatou E, Dimopoulou I. The Role of Automated Infrared Pupillometry in Traumatic Brain Injury: A Narrative Review. Journal of Clinical Medicine. 2024; 13(2):614. https://doi.org/10.3390/jcm13020614
Chicago/Turabian StyleVrettou, Charikleia S., Paraskevi C. Fragkou, Ioannis Mallios, Chrysanthi Barba, Charalambos Giannopoulos, Evdokia Gavrielatou, and Ioanna Dimopoulou. 2024. "The Role of Automated Infrared Pupillometry in Traumatic Brain Injury: A Narrative Review" Journal of Clinical Medicine 13, no. 2: 614. https://doi.org/10.3390/jcm13020614
APA StyleVrettou, C. S., Fragkou, P. C., Mallios, I., Barba, C., Giannopoulos, C., Gavrielatou, E., & Dimopoulou, I. (2024). The Role of Automated Infrared Pupillometry in Traumatic Brain Injury: A Narrative Review. Journal of Clinical Medicine, 13(2), 614. https://doi.org/10.3390/jcm13020614









