1. Introduction
Epithelial ovarian cancer (EOC) has been one of the leading causes of death in women worldwide over the past decade, with over 314,000 women being diagnosed in the year 2020 alone, and approximately 207,000 of them dying, making it the second most common cause of death from gynecologic cancers [
1]. In Taiwan, an incidence rate of 6.36 per 100,000 women in 2020 was documented, with a steady increase since the 1990s at an annual percentage change of 1.5% during the period 1995 to 2014 [
2]. Ovarian cancer is also one of the most common causes of death in women, with a standardized mortality rate of 3.3 per 100,000 women being seen in the year 2021. Approximately 70% of EOC patients present with advanced-stage disease, with the standard treatment consisting of cytoreductive surgery followed by platinum-based chemotherapy [
3]. Although platinum-based therapy is initially effective in most patients, the majority will experience disease recurrence within the first two years, and only a minority will achieve long-term remission. Furthermore, its platinum-free interval becomes shorter with every incidence of recurrence.
Recently, the use of poly-adenosine diphosphate–ribose polymerase (PARP) inhibitors and bevacizumab has become mainstream first-line maintenance management treatment of ovarian cancer, including even in a recurrent setting [
4,
5,
6,
7]. While many countries have incorporated the treatment into their routine method of care, there are still certain countries that do not offer sufficient insurance reimbursements for these agents, leaving many patients unable to personally afford the treatment due to financial limitations. Therefore, chemotherapy remains the key element in the recurrent setting in many countries.
The number of cycles required during first-line adjuvant chemotherapy has been previously evaluated [
8,
9,
10], however, the role of maintenance chemotherapy in first-line management remains controversial [
10,
11,
12]. Based on the results from several trials [
13,
14,
15], six cycles of first-line adjuvant chemotherapy were found to be adequate, with extended cycles not showing any survival benefits, but rather more adverse effects. However, in the platinum-sensitive recurrent setting, defined as when patients have a platinum-free interval (PFI) of more than 6 months after the completion of their last platinum-based regimen, there is still a lack of robust evidence on the number of cycles necessary during second-line platinum-based chemotherapy [
16,
17,
18]. Previous studies focused mainly on the chemotherapy agents instead of the relationship between the number of cycles and survival benefits, and most of them kept six to nine cycles without addressing the role of extended cycles.
According to the National Comprehensive Cancer Network (NCCN) guidelines, six cycles of platinum-based doublet chemotherapy is recommended, no matter whether the status of the disease differs between patients. Therefore, whether a complete or partial response is reached after six cycles poses the question: Do better survival outcomes result from maintenance chemotherapy?
In this retrospective study, we sought to validate the efficacy of maintenance chemotherapy in its role of prolonging progression-free survival, while also evaluating its adverse effects in patients with platinum-sensitive recurrent ovarian cancer in a tertiary medical center in Taiwan.
2. Materials and Methods
2.1. Study Population and Data Collection
This is a retrospective cohort study with data taken from a tertiary medical center in Taichung City, Taiwan. The study protocol was approved by our Institutional Review Board (IRB) (Number: CE21233B).
We identified patients who had experienced their first relapse of epithelial ovarian cancer (EOC) in a decade between the years 2011 and 2021 (
n = 134,
Figure 1), with or without having undergone secondary cytoreduction surgery or radiation therapy. We excluded patients who had not received either a primary debulking operation or first-line standard adjuvant therapy at our medical center (
n = 10), as well as patients with platinum-resistant disease (
n = 16) as a recurrent disease less than 6 months from their last first-line chemotherapy session. Patients who opted for maintenance therapy involving PARP inhibitors or immunotherapy (
n = 7) were excluded, however, those treated with bevacizumab were included. In addition, patients who declined standard chemotherapy treatment or were lost during follow-up (
n = 15), patients who experienced disease progression before their 6th cycle of second-line chemotherapy (
n = 6), and patients who were currently under chemotherapy before the evaluation of their 6th cycle of second-line chemotherapy as of the end date of this study on 2 May 2021 (
n = 8) were also excluded.
A total of 72 patients who had exhibited either complete or partial response after six cycles of second-line chemotherapy, based on the images from their CT scan and the incorporated criteria of RECIST 1.1 and CA-125 agreed by the Gynecological Cancer Intergroup (GCIG), were included in the study. The patients were then divided into two groups according to the number of cycles they received, which included both a standard group (6 cycles) and a maintenance group (more than 6 cycles). Patients who underwent secondary cytoreduction surgery were evaluated first in multidisciplinary team meetings and needed to fulfill the following criteria: single or countable tumor lesions that were resectable, minimal ascites, and no distant metastases seen on PET or CT scans.
The status of disease progression and survival was confirmed by the date of 2 May 2021. Either an increase in the volume of tumors, the presence of new lesions or more ascites on abdominal CT scans, or an elevation in CA-125 over twice the upper limit of the response range was defined as disease progression, which was 70 IU/mL at our institution. The primary outcome of this study was progression-free survival (PFS) and overall survival (OS) as a secondary endpoint in terms of maintenance therapy. To explore the role of maintenance chemotherapy, PFS was defined as the interval from the date of the sixth cycle of second-line chemotherapy to the date of disease progression or patient mortality. OS was defined as the interval from the date of the sixth cycle of second-line chemotherapy to the date of mortality.
2.2. Statistical Analysis
Differences in baseline characteristics between the two treatment groups were compared using the Mann–Whitney U test and chi-square test for continuous and categorical variables, respectively. The Kaplan–Meier method was used to generate survival curves for OS and PFS, while Cox proportional hazard analysis was performed to determine the association of maintenance chemotherapy treatment with OS and PFS after adjustments for relevant variables. Subgroup analysis was conducted to determine whether a significant variation existed in the aforementioned association. All statistical analyses were performed using the Statistical Package for Social Sciences (IBM SPSS version 22.0; International Business Machines Corp, Armonk, NY, USA) with a p value of <0.05 considered as being statistically significant.
4. Discussion
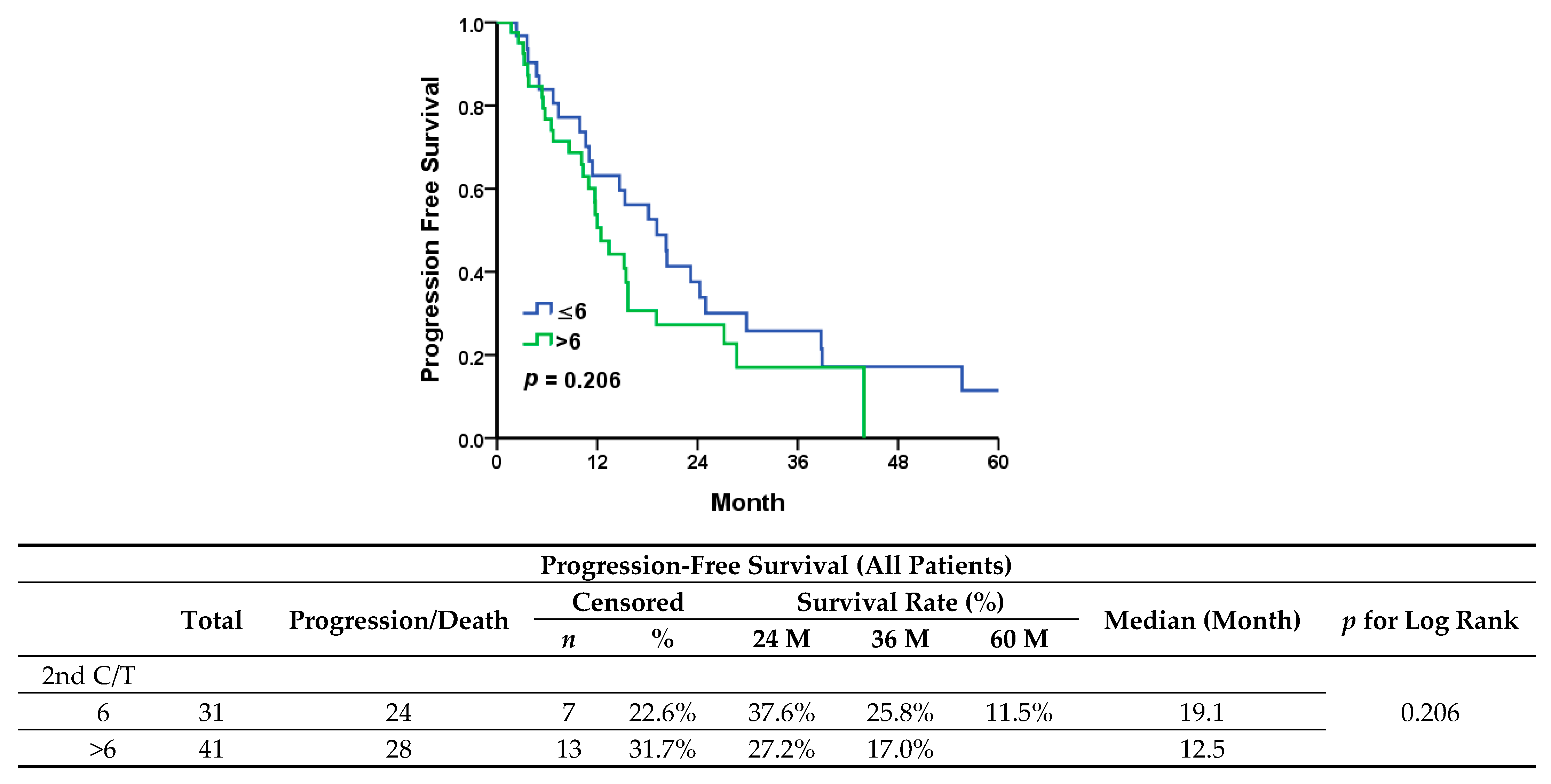
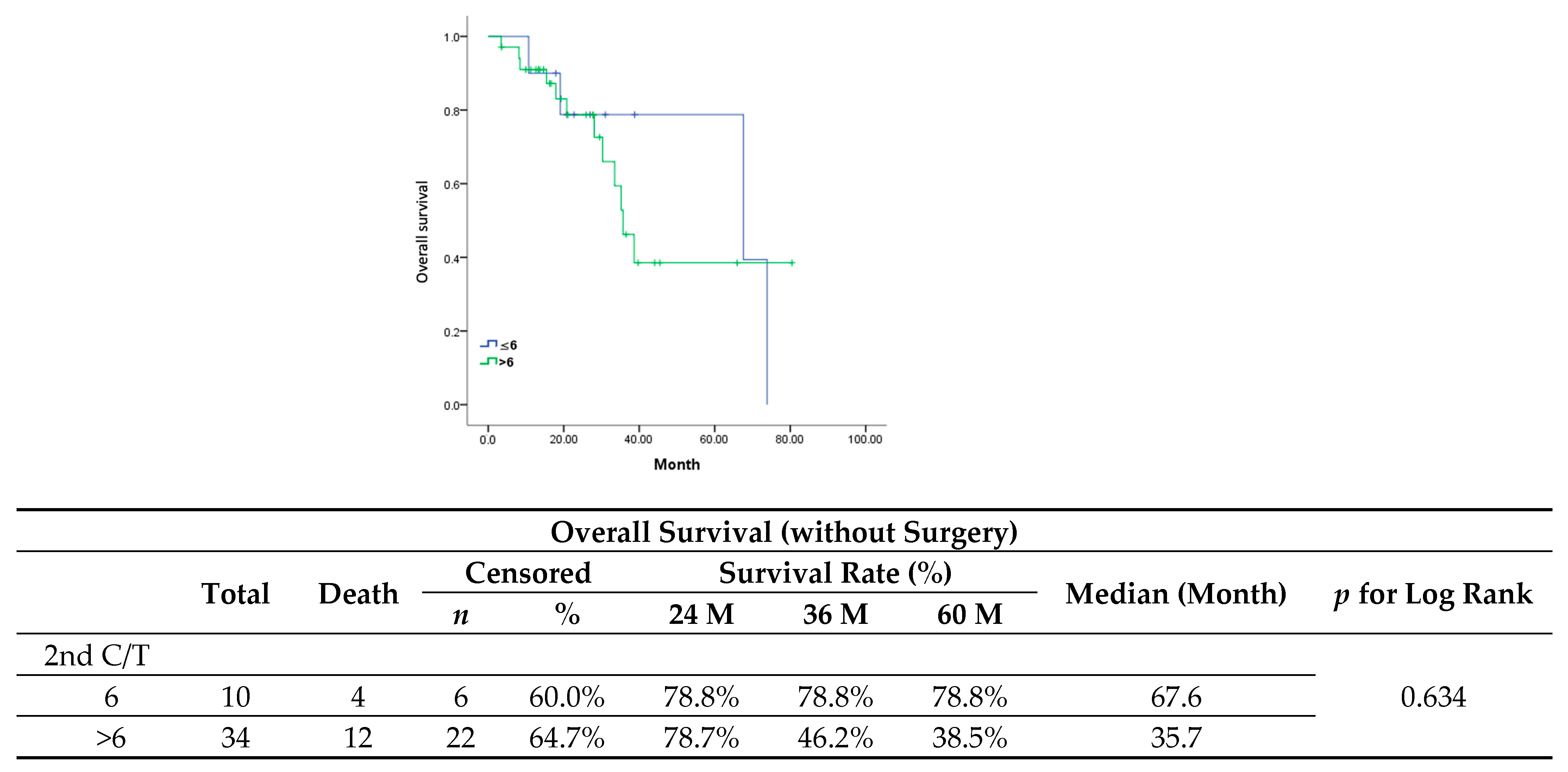
In our study, we evaluated maintenance chemotherapy in all patients diagnosed with platinum-sensitive recurrent ovarian cancer. In comparison to the standard chemotherapy group, the maintenance group presented a worse trend in progression-free survival (median, 12.5 vs. 19.1 months;
p = 0.206) and significantly shorter overall survival (median, 35.7 vs. 73.9 months;
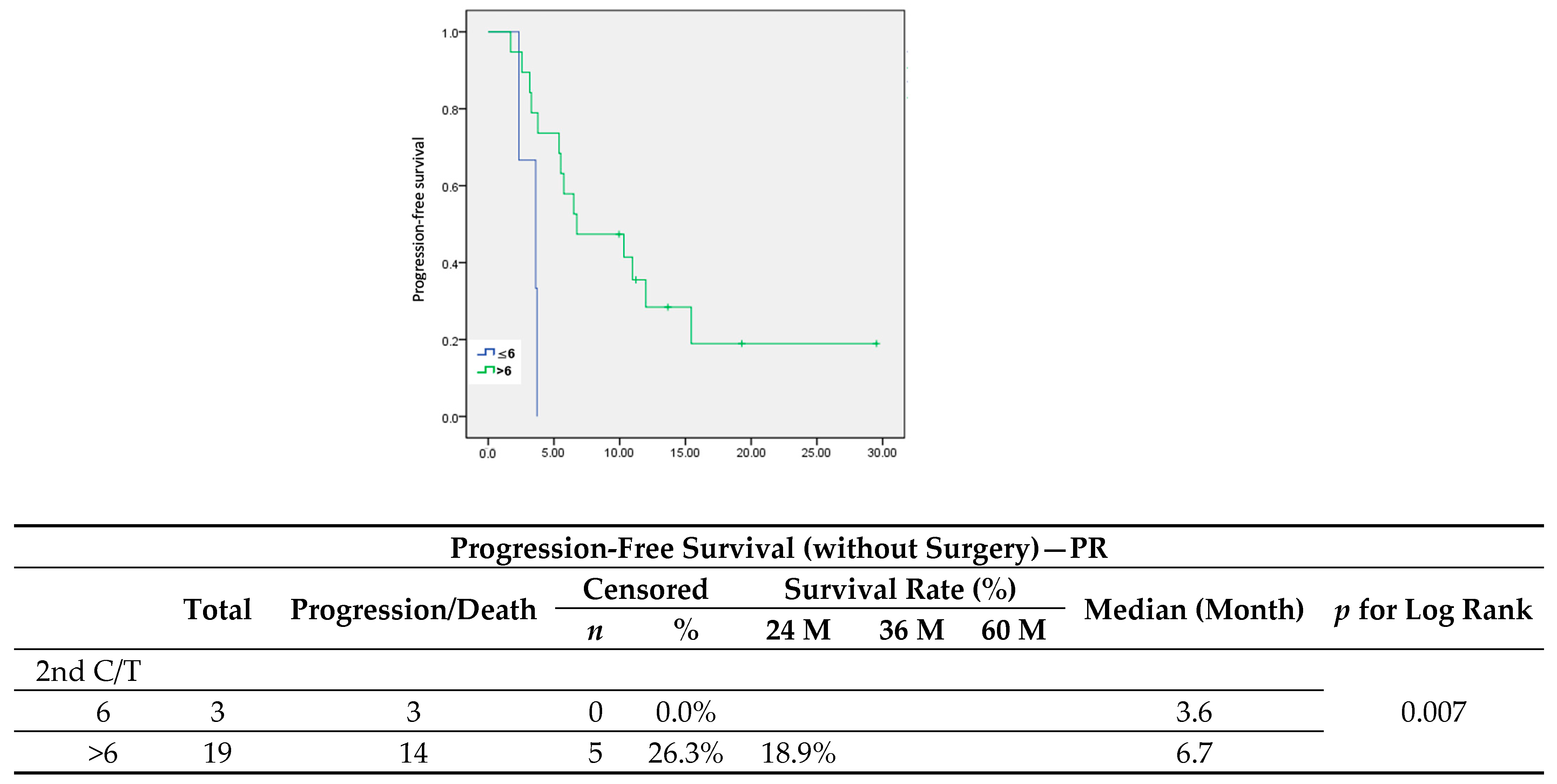
p = 0.031). Since patients diagnosed with less extensive recurrent disease are more prone to receiving either secondary cytoreduction surgery or radiotherapy, a better outcome in these patients would be expected, therefore they would then receive only six cycles of adjuvant chemotherapy. All patients in the standard group showed a longer PFI and a significantly lower serum level of CA-125 at first recurrence. However, when it came to patients with a partial response after six cycles of second-line chemotherapy who had received only chemotherapy after recurrence (
n = 21), maintenance chemotherapy led to a significant improvement in PFS (
Figure 6) (median, 3.6 vs. 6.7 months,
p = 0.007).
The essence of maintenance therapy lies in the prolonging of the platinum-free interval (PFI), which correlates to better survival outcomes and favorable use in platinum-based chemotherapy regimens. Maintenance chemotherapy has been studied with a single agent of paclitaxel, pegylated liposomal doxorubicin combined with carboplatin, or paclitaxel with carboplatin in first-line adjuvant therapy [
8,
10,
12]. Here, mixed results were found, as some showed improvement in PFS, although no significant improvement was seen in OS among those studies. As for other maintenance agents, bevacizumab in first-line maintenance therapy only offered a benefit in PFS to patients with advanced-stage disease [
19,
20]. In patients with BRCA mutations or homologous recombination deficiency (HRD), PARP inhibitors provided a significant improvement in PFS [
21,
22].
Regarding the recurrent setting, many studies have focused on patients with platinum-sensitive disease, with pegylated liposomal doxorubicin showing promising results [
23,
24], though there remains a lack of larger randomized trials. As long as the toxicities are tolerable, we expect survival benefits, particularly in patients with partial or complete response. Bevacizumab works as a maintenance agent according to the OCEANS study and GOG-0213 study, both of which showed there was an improvement in PFS and OS for patients with platinum-sensitive recurrence [
5,
25]. PARP inhibitors can also be candidates for maintenance therapy in platinum-sensitive recurrence [
6]. Maintenance chemotherapy still plays an important role, particularly in patients without BRCA mutations or homologous recombination deficiency (HRD), as well as patients in countries whose NHI does not cover the cost of the agents as mentioned above due to financial issues and the currently limited evidence among these populations [
7].
Upon reviewing previous studies [
24,
26,
27,
28,
29], controversies remain regarding the role of maintenance chemotherapy in patients with platinum-sensitive recurrent ovarian cancer. What we learned from previous studies validated the availability of platinum-based doublet chemotherapy regimens, which included platinum plus paclitaxel [
18], platinum plus gemcitabine [
30], and platinum plus pegylated liposomal doxorubicin [
16]. The adequate cycle number in second-line chemotherapy has yet to be determined due to the lack of convincing results in the aforementioned studies, which suggested no survival benefits after six cycles, particularly in patients with complete response [
8,
11,
31].
A recent retrospective study from Korea [
31] analyzed the use of extended chemotherapy in patients who experienced platinum-sensitive recurrence after primary management with a residual tumor ≥0.5 cm (on CT scans) after six cycles of second-line, platinum-based chemotherapy. Under the same circumstance without secondary cytoreduction surgery, the characteristics of their patients were closely similar to our patients with partial response after six cycles of chemotherapy. In our study, there was significantly better PFS in the maintenance group (median, 6.7 vs. 3.6 months;
p = 0.007). Compared to the data resulting from the Korean study, our results showed significantly worse PFS in the extended group (median, 13.9 vs. 14.8 months;
p = 0.036). However, in the Korean study, patients without surgery who received extended cycles of chemotherapy were inferior to the standard group in several factors: fewer patients achieved R0 (41.2% vs. 56.7%) after primary debulking surgery, the platinum-free interval was shorter (median, 11.0 vs. 12.7 months), and fewer patients received any maintenance therapy (11.8% vs. 32.8%). Even under multivariate analysis, all the above factors may still play a part in the reasons behind worse outcomes in the extended group. Additionally, the evaluations of CT scans in the Korean study showed no clear evidence of any response after second-line chemotherapy, which also raised the concern of whether smaller lesions would be neglected in CT scans. In conclusion, the reasons that no compatible results were found in the Korean study may lie not only in the fundamental inferior patient characteristics seen in the extended group but also in the incomplete evaluation of chemotherapy response in patients, some of whom may be identified as either platinum-resistant or experiencing progression of disease after six cycles of second-line chemotherapy.
Nevertheless, several limitations do exist in our study. First and foremost, the sample size of our study population was small, particularly concerning those patients who had not undergone secondary cytoreduction surgery or radiation (n = 44), which was our main focus here. Therefore, there exists the possibility of selection bias and statistical issues. When we focused on patients with partial response (n = 22), there were only 3 in the standard group and 19 in the maintenance group. Secondly, even though we performed multivariate analyses for identifying possible confounders and subgroup analyses such as PFI and secondary cytoreduction surgery, the heterogeneity of the whole study population was never resolved, thus possibly requiring further prospective RCTs. Notwithstanding, we used standardized criteria for selecting our patients with complete response and partial response to chemotherapy to identify the role of maintenance chemotherapy, which is seldom described in previous studies on the same topic. The results we came up with have helped shed light on the promising direction that maintenance chemotherapy will take in platinum-sensitive recurrent ovarian cancer.