Abstract
Background: We aimed to assess the status of the optic nerve and retina by optical coherence tomography (OCT) in a group of patients with idiopathic intracranial hypertension (IIH) on the basis of dynamic changes in intracranial pressure. Methods: This observational and cross-sectional study included patients affected by idiopathic intracranial hypertension with papilledema (IIHWP) and patients with idiopathic intracranial hypertension without papilledema (IIHWOP). All participants underwent an OCT examination of the macula and optic nerve head. Parameters related to intracranial pressure, including cerebrospinal fluid (CSF) opening pressure (oCSFp), CSF mean pressure (mCSFp), and pulse wave amplitude (PWA), were included in the analysis. Results: Out of the 22 subjects enlisted for the study, a total of 16 patients suggestive of IIH were finally enrolled. Papilledema was detected in nine subjects (56.2%) and seven patients were affected by IIHWOP (43.7%). The OCT examination showed a higher mean RNFL thickness in IIHWP patients in comparison to IIHWOP in both eyes (p < 0.05 and p < 0.01, respectively). Intracranial pressure (ICP) measurements showed that IIHWP had higher values of oCSFp, mCSFp, and PWA compared to IIHWOP (p = 0.0001, p = 0.0001, and p = 0.0001, respectively). In addition, ICP parameters significantly correlated with RNFL. Conclusions: Clinical parameters suggestive of idiopathic intracranial hypertension are associated with retina and optic nerve OCT parameters. OCT is a useful tool to detect these alterations in a non-invasive fashion.
1. Introduction
Idiopathic intracranial hypertension (IIH) syndrome is characterized by elevated intracranial pressure (ICP) in the absence of a mass lesion, venous sinus thrombosis, or hydrocephalus [1,2]. IIH typically presents with several symptoms, including high-frequency headaches, transient visual obscuration, pulsatile tinnitus, and blurred or double vision. IIH is usually observed in young women of childbearing age [3,4], and its frequency reflects the increased prevalence of obesity [5]. Magnetic resonance venography (MRV) usually depicts bilateral cerebral transverse venous sinus stenosis [6,7]. Papilledema is a common sign and, if not treated, can result in insidious and slowly progressive visual loss, leading to the development of retinal atrophy and permanent visual impairment [8,9]. Additionally, IIH without papilledema (IIHWOP) has been identified as a variant of the form with papilledema (IIHWP) [10,11,12,13,14]. According to recent studies, IIH with and without papilledema share similar features, including refractory headaches and radiological signs such as cerebral transverse venous sinus stenosis [15], but the reason why some IIH patients develop papilledema, while others do not, remains controversial. It has been proposed that the development of IIHWOP could be explained by (1) anatomical variants in the optic nerve sheaths or in its trabeculations protecting from elevated ICP in the optic disc (OP); (2) the fluctuation in ICP values below the threshold responsible for papilledema; (3) considering IIHWOP as a presymptomatic form of IIH which improves or disappears prior to developing papilledema [13,16].
It is known that a chronic elevation in ICP leads to several biomechanical alterations in the optic nerve head (ONH) [17,18,19,20]. Some studies have demonstrated that retinal and ONH parameters, measured by optical coherence tomography (OCT), are correlated with increased ICP in papilledema due to IIH [17,21,22,23]. OCT is a widely employed imaging technique that allows us to assess neuroretinal changes in a non-invasive fashion.
According to several studies, the lumbar subarachnoid space and ONH regions are directly connected and, thus, OCT may represent a suitable non-invasive tool for monitoring ICP and for determining objective changes in the ONH after an acute decrease in lumbar cerebrospinal fluid (CSF) pressure [20,24]. Even though the literature reporting the effects of ICP on diseases causing papilledema is rapidly growing, research on the effects of dynamic ICP on optic nerve structures in IIHWP and IIHWOP is lacking.
In order to better understand the impact of papilledema, we segregated patients with papilledema and without papilledema into distinct groups. Thus, we designed an observational and cross-sectional study to investigate the status of the optic nerve head and retina in patients affected by IIH using OCT and the association with parameters suggestive of ICP.
2. Materials and Methods
This observational and cross-sectional study screened patients with high-frequency or daily persistent headaches, consecutively admitted to the Department of General Ophthalmology of the Medical University of Lublin (Lublin, Poland) from December 2020 to December 2021. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the Medical University of Lublin (ethical committee number KE-0254/123/2020). Overall, 22 patients were selected for the study, including those with clinical and radiological evidence suggestive of IIH with or without papilledema. All patients underwent brain MRI and MRI-venography (MRV).
2.1. Inclusion Criteria
- Clinical evidence suggestive of IIH with or without papilledema:
- -
- High-frequency headache attacks persisting for at least one year refractory to prophylactic therapy;
- -
- High-frequency headache attacks with papilledema, or transient or persistent visual obscuration, and/or blurred vision, or diplopia.
- Normal neurological examination.
- Evidence of bilateral stenosis of the cerebral transverse sinus at MRV [25].
2.2. Exclusion Criteria
- Evidence of central nervous system disease at brain MRI.
- Evidence of current or previous cerebral venous thrombosis revealed by cerebral MRV.
- Treatment with psychoactive, antihypertensive, cardiac, or other drugs interfering with the CSF pressure.
- Comorbidities such as diabetes mellitus, hypertension, cardiovascular and renal diseases, neurological diseases (i.e., tumors, ictus, infectious diseases, epilepsy, multiple sclerosis, previous head or eye injury).
- Eyes with optic disc and retinal pathology (including anterior ischemic optic neuropathy, posterior uveitis, and central retinal vein occlusion), which could bias measurements, and eyes affected by corneal opacity or cataract for which OCT imaging could not be obtained were ruled out from the analysis.
Approval from the local committee for the study protocol and consent for participation from the enrolled patients were both obtained before commencing the study.
Lumbar puncture and short CSF dynamic monitoring were performed to evaluate those patients with evidence of cerebral venous outflow disturbances at MRV documented by the presence of bilateral cerebral transverse sinus stenosis. The ophthalmological evaluation and the OCT were performed in all enrolled patients, 24 h before the ICP measurement.
Ophthalmological evaluation: all the participants were subjected to a comprehensive ophthalmological assessment, including best corrected visual acuity (BCVA) testing with slit lamp biomicroscopy, dilated fundus examination, Snellen charts, and intraocular pressure measurement by applanation tonometry.
OCT imaging: The Stratus OCT (Cirrus OCT 5000, Carl Zeiss Meditec, Dublin, CA, USA) was used to perform the OCT. A single operator masked to the patients’ diagnosis recorded an average of three sequential circular scans of diameter 3.46 mm centered on the optic disc. All scans were collected after pupil dilation with 1% tropicamide. The optic disc cube 200 × 200 protocol for the ONH (Figure 1) and the Macular Cube 512 × 128 protocol centered on the fovea were carried out in order to obtain peripapillary and macular data, respectively. Scans with a signal strength less than 7/10 were not considered for further data analyses. For each eye, we analyzed the mean retinal nerve fiber layer (RNFL) thickness and single-quadrant thickness, both calculated automatically by OCT using the existing software. We also evaluated the ganglion cell layer (GCL), foveal thickness (FT), and macular volume (MV) of all the patients. Neuroretinal rim area (NRRA, µm2) and thickness (NRRT, µm), cup volume (CV, µm3), and cup-to-disc ratio (C/D ratio) were also calculated automatically. The OCT automated segmentation was validated by two independent expert OCT examiners (T.M.D. and R.M.). Quality control and APOSTEL guidelines according to published criteria were applied [26,27,28].
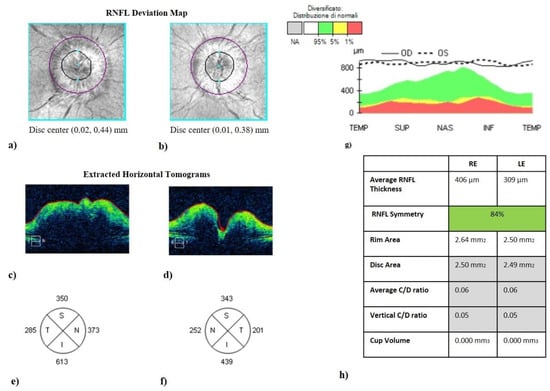
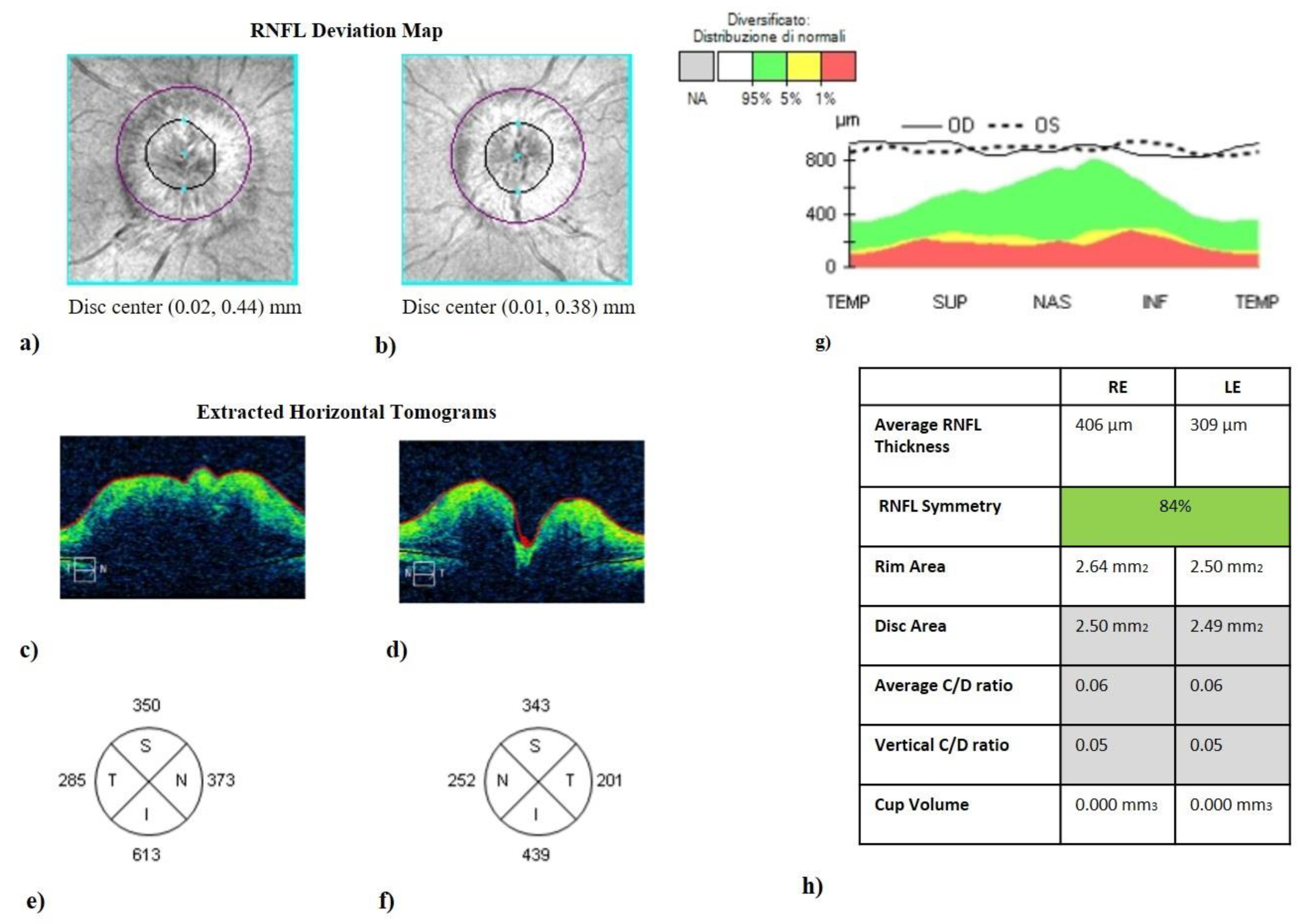
Figure 1.
Optic disc cube (200 × 200 protocol) centered on the optic nerve head (ONH). It represents the optical coherence tomography of a patient with idiopathic intracranial hypertension without papilledema (IIHWP), demonstrating the presence of bilateral papilledema, more prominent in the right eye. (a,b) Images of ONH (bordered by a dark line). ONH swelling and consequent increased thickness in the right eye (c) and in the left eye (d) are discernible looking at horizontal B-scans. Thickening of RNFL of all quadrants can be observed in (e–g). An overview of OCT parameters is represented in (h). IIHWP: idiopathic intracranial hypertension with papilledema; OCT: optical coherence tomography; ONH: optic nerve head; RNFL: retinal nerve fiber layer; RE: right eye; LE: left eye.
ICP monitoring: The monitoring was performed for 1 h in a silent room. After a pre-treatment with local anesthesia (subcutaneous lidocaine), the lumbar puncture was conducted at the L3–L4 level in the lateral decubitus position using a Quincke 20-gauge needle with a three-way stopcock. We measured the CSF opening pressure (oCSFp), CSF mean pressure (mCSFp), and pulse wave amplitude (PWA). IIH was diagnosed when the CSF opening pressure or CSF mean pressure was >250 mmH2O confirmed by lumbar ICP monitoring [25].
The recording of oCSFp was commenced four minutes after the CSF pressure monitoring started (the opening pressure phase) [29]. Both the mCSFp and the CSF mean PWA were recorded from the culmination of the opening phase to the sixteenth minute (short-term monitoring). We also calculated the CSF PWA as the peak-to-peak amplitude, where the systolic peak was the highest and the diastolic peak of the CSF pressure was the lowest. The upper normal limit of CSF PWA was 54.8 mmH2O [30,31].
We excluded from the analysis the values of CSF pressure and PWA collected during artifact period (i.e., movements of the arms or of the head, coughing, etc.).
Statistical analysis: The normality of all the values included in the analysis was assessed using the Shapiro–Wilk test. The differences between subgroups were evaluated with Student’s t-tests and Mann–Whitney tests for parametric and for non-parametric variables, respectively. For correlation analysis, the Pearson correlation coefficient (r) was used. Statistical significance was indicated by a probability value (p) of 0.05. STATA 11 was used for all statistical analyses (Boston RC, Sumner AE. 2003) [32].
3. Results
Out of the 22 subjects recruited for the study, a total of 16 patients suggestive of IIH were finally enrolled. Six patients were excluded on the basis of ICP lumbar monitoring, which resulted in the range of normal limits. None of the selected patients presented systemic hypertension at the moment of the ICP monitoring. Only three patients abused symptomatic treatment (two patients of triptans and one of non-steroid anti-inflammatory drugs). All selected patients were divided into two groups according to the finding of papilledema: nine IIHWP (56.2%) and seven IIHWOP (43.7%). Clinical and demographical data are shown in Table 1. Visual acuity (VA) was markedly reduced in patients with papilledema.

Table 1.
Clinical and demographic characteristics.
ICP measurement showed that IIHWP had higher values of oCSFp, mCSFp, and PWA compared to IIHWOP (Table 2). The OCT examination showed that a higher mean RNFL thickness in IIHWP patients was detected in comparison to IIHWOP in both eyes (209.4 vs. 98.5, p < 0.05; 166.6 vs. 98.6, p < 0.01 in right- and left-eye groups, respectively). IIHWP showed lower values of CV and C/D ratio compared to IIHWOP; the complete data are reported in Table 3.

Table 2.
CSF pressure monitoring data a in IIH with and without papilledema.

Table 3.
Retinal characteristics a in IIH with and without papilledema.
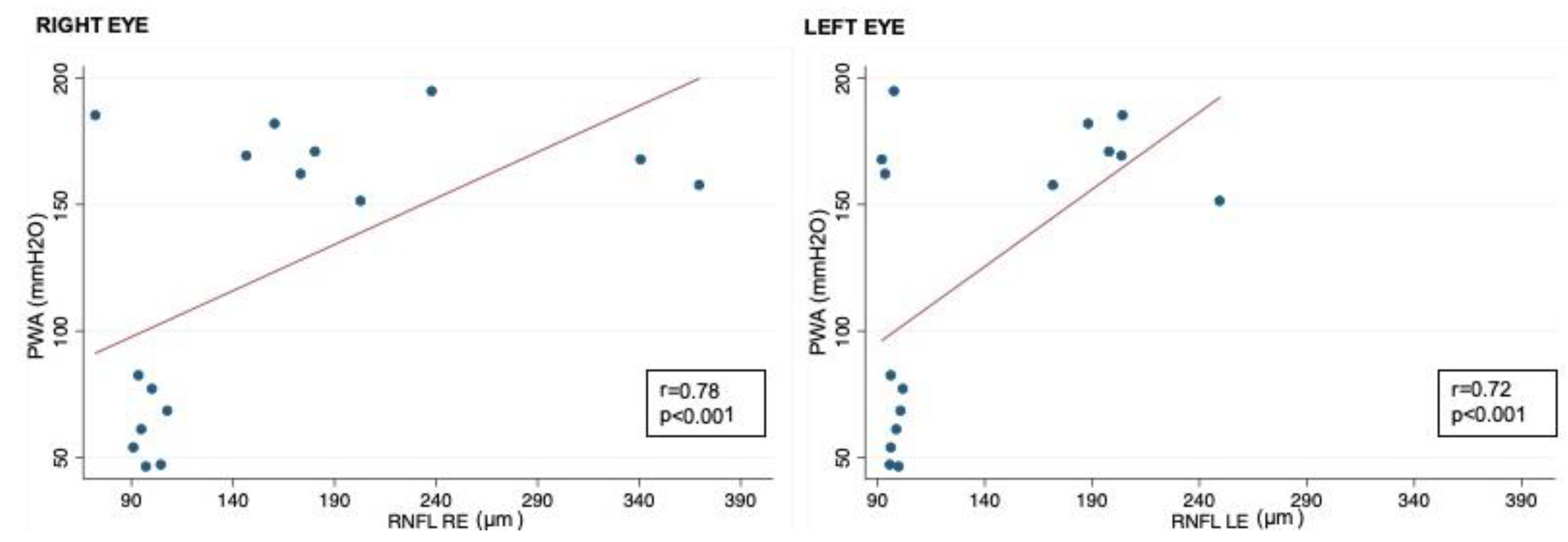
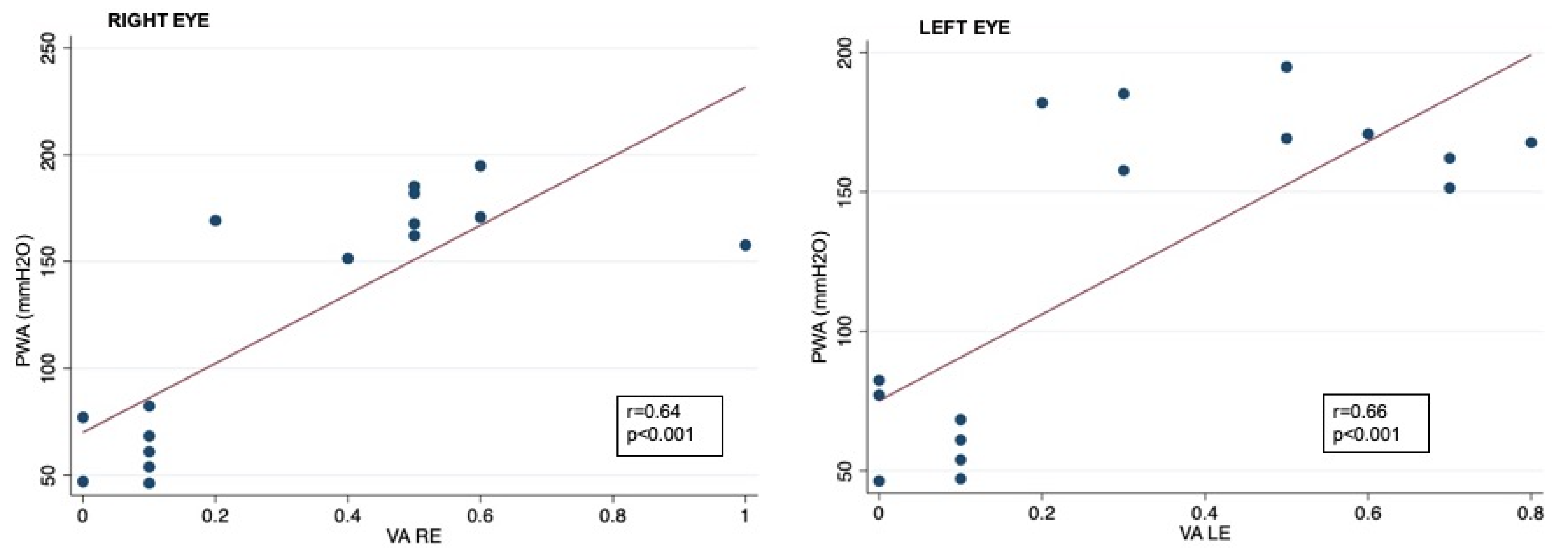
Correlation analysis showed that RNFL thickness was significantly associated with oCSFp, mCSFp, and PWA; in contrast, C/DR and CV inversely correlated with oCSFp, mCSFp, and PWA (Table 4, Figure 2). We also found a positive correlation between rim thickness and both mCSFp and PWA, but not oCSFp (Table 4). VA values showed a significant correlation with OCT parameters (Table 5). Moreover, VA correlated with mCSf and PWA (Table 6, Figure 3).

Table 4.
Correlation analysis between retinal parameters and CSF pressure monitoring data.
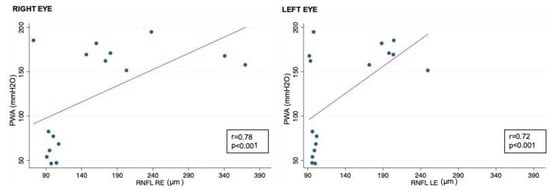
Figure 2.
Correlation between CSF PWA and RNFL in right and left eyes; RNFL: retinal nerve fiber layer; CSF PWA: cerebrospinal fluid pressure pulse wave amplitude; r: Spearman correlation coefficient.

Table 5.
Correlation analysis between retinal parameters and visual acuity data.

Table 6.
Correlation analysis between CSF pressure monitoring and visual acuity data.
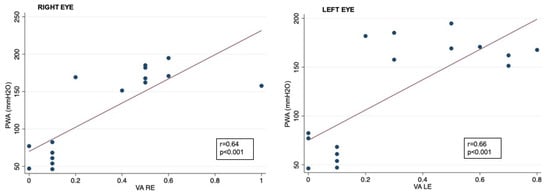
Figure 3.
Correlation between CSF PWA and visual acuity data in right and left eyes; VA: visual acuity expressed as logarithm of minimal angle of resolution (LogMAR); CSF PWA: cerebrospinal fluid pressure pulse wave amplitude; r: Spearman correlation coefficient.
4. Discussion
Our study confirmed that a chronic elevation in ICP induces biomechanical changes in the ONH, which can result in papilledema. The one-hour ICP lumbar monitoring demonstrated that dynamic changes in ICP correlate to structural optic disc damage in IIH patients, as shown in several studies [18,19,33,34,35]. More interestingly, we demonstrated that a higher PWA is associated with increased visual acuity loss and optic disk oedema.
Papilledema can be detected clinically by fundus examination even without pupil dilation; however, OCT allows the quantification of the extent of morphological alteration of the optic nerve associated with papilledema with a topographical analysis.
In our study, the RNFL thicknesses of the four sectors were all increased, especially in the inferior sector. Indeed, several studies have demonstrated the efficiency of OCT in identifying and measuring RNFL edema and that a significant increase in the mean RNFL thickness in all four quadrants is evident in eyes with papilledema [21,36,37,38].
As already reported [14,18], all measurements of ONH in patients with IIHWP were abnormal compared to IIHWOP. In particular, NRRT and NRRA were significantly increased, and optic CV was significantly reduced. IIHWP patients also showed significantly reduced CV and C/D. In another study, a short-term increase in CSF pressure associated with significant changes in ONH morphology was reported following the Valsalva maneuver. These findings suggested that ONH parameters may undergo sensitive changes in response to increased ICP and may also support our results [16].
Our study demonstrated that all ONH parameters are associated with CSF pressure monitoring data and visual acuity outcomes, presenting a correlation between edema severity and ICP in IIH, as corroborated in other studies [39]. Moreover, in line with our results, a positive correlation between the volumetric measurements of retinal surface elevation estimated from stereo fundus photographs and OCT and the severity of papilledema measured by grade of Frisén was reported [40,41,42]. Furthermore, papilledema can be regarded as a result of a compartmentation process involving the subarachnoid space of the optic nerve and causing, in the end, a toxic milieu around the nerve. For these reasons, several studies have proposed OCT parameters, such as the total retinal thickness and the peripapillary RNFL thickness, as useful markers for the quantification of papilledema severity [18,41].
It is well established that the optic nerve is under the influence of hydrostatic pressure along its length, and an alteration in this pressure may play a role in the etiology of papilledema [19,20]. Experimental studies designed to measure the eye lamina cribrosa pressure gradient have demonstrated a relationship between retrolaminar tissue pressure, lateral ventricle CSF pressure, and the optic nerve subarachnoid space [17]. It has been shown that CSF pressure influences retrolaminar tissue pressure; thus, CSF pressure plays a dominant role in the eye translaminar optic nerve tissue pressure gradient. Moreover, the existence of hydrostatic continuity between the lateral ventricle and optic nerve subarachnoid space has also been established [17,18].
More interestingly, PWA seems to strongly correlate with RNFL, CV, C/D ratio, and rim thickness. This finding, though limited by the small size of the patient population studied, could be interpreted assuming that PWA rises in conjunction with intraocular pulse amplitude; it is conceivable that an increase in both CSF and intraocular pressure may determine the elevation in the eye’s venous pressure and orbit-lowering venous blood flow in the retina, disc, and choroid, resulting in papilledema [43]. Our data suggest that the degree of PWA increase contributes more to neuroretinal tissue damage than the opening pressure level, inducing structural and metabolic alterations in the optic nerve in IIH patients. CSF PWA can be considered as a measure of the intracranial pressure pulsation strongly associated with the systolic and diastolic components of arterial pressure [44], thus representing a valuable CSF parameter to estimate intracranial compliance [31]. CSF PWA is a result of intracranial blood circulation and is strongly associated with the R-wave of an electrocardiogram [45]. Consequently, any cerebral volume alteration acting on the lateral ventricles, such as increased ICP or brain–water imbalance, potentially induce an increase in the CSF PWA [45]. Similarly, in IIH, the increased level of ICP may lead to the compression of cerebral bridging veins and, as a consequence, to a raised venous resistance to outflow [46]. Since it is closely related with the pressure–volume curve, an increase in the CSF PWA proceeds linearly with the intracranial CSF pressure. Indeed, as per the model of pressure–volume compensatory reserve, a decrease in intracranial compliance raises the CSF PWA linearly with the increment in ICP. Particularly, the rise in the CSF PWA curve from the flat to the exponential zone may represent an expression of the transition from good to poor compensatory reserve status [47]. To confirm this, abnormal levels of CSF PWA are usually tracked in patients with idiopathic normal pressure hydrocephalus, in which intracranial compliance is typically impaired [31,48].
This study has several limitations: the small sample size of our study and the absence of a control group may have limited the statistical power of our results. In addition, a physiological time-lapse existed between the OCT assessment and the ICP monitoring (approximately 24 h). Larger prospective studies are needed to confirm our findings. However, it should be noted that IIH being a rare disease made it difficult to find patients with the condition to perform the CSF monitoring; hence, the sample size may be considered adequate in such conditions. We also found that retinal measurements in the group of patients with papilledema were different between the right eye and left eye. There are several pieces of evidence that the severity of papilledema may be asymmetric, although it is uncommon [49,50]. The finding of worse papilledema in the right eye compared to left eye in some patients of our small cohort may have contributed to such differences. Moreover, in our study, patient groups showed differences in BMI and age. Although data on the correlation between BMI and OCT macular thickness are controversial [51], several studies have shown that BMI and OCT-measured retinal thickness are related [52,53]. Nevertheless, the effect of weight on peripapillary thicknesses is still poorly understood. Lastly, the cross-sectional design of this study did not allow us to define the prognostic value of the CSF dynamic parameters on ONH structure in IIH and to understand the differences in retinal and ONH profiles between IIHWP and IIHWOP. Thus, further studies with a longitudinal design are warranted.
5. Conclusions
Our data demonstrated that, during CSF short-term monitoring, the increased mean ICP and resulting mean PWA correlated with the OCT changes, indicating a reduced intracranial compliance. The combined assessment of OCT and CSF monitoring seemed to present reliable and sensitive measurements of elevated ICP in IIH. In particular, PWA, as an index of vascular dynamic changes, seemed to better correlate with all neuroretinal parameters, especially to C/DR, a mechanical and structural risk factor that leads to disc oedema in a self-perpetual cycle. The clinical value of PWA in monitoring the course of IIH needs to be evaluated in longitudinal studies.
Author Contributions
Conceptualization, C.G.C. and M.D.T.; methodology, T.A., V.C. and R.R.; data curation and analysis, M.D.T., C.G.C., M.R. and A.C.; writing—original draft preparation, M.D.T., C.G.C. and N.C.; review and editing, D.S., C.C. and A.R. All authors have read and agreed to the published version of the manuscript.
Funding
The authors did not receive support from any organization for the submitted work.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the Medical University of Lublin (ethical committee number KE-0254/123/2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013, 33, 629–808. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.I.; Jacobson, D.M. Diagnostic criteria for idiopathic intracranial hypertension. Neurology 2002, 59, 1492–1495. [Google Scholar] [CrossRef]
- Wang, M.T.; Bhatti, M.T.; Danesh-Meyer, H.V. Idiopathic intracranial hypertension: Pathophysiology, diagnosis and management. J. Clin. Neurosci. 2022, 95, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Raoof, N.; Hoffmann, J. Diagnosis and treatment of idiopathic intracranial hypertension. Cephalalgia 2021, 41, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Caballero, B. The global epidemic of obesity: An overview. Epidemiol. Rev. 2007, 29, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.N.P.; Gillard, J.H.; Owler, B.K.; Harkness, K.; Pickard, J.D. MR venography in idiopathic intracranial hypertension: Unappreciated and misunderstood. J. Neurol. Neurosurg. Psychiatry 2004, 75, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Fera, F.; Bono, F.; Messina, D.; Gallo, O.; Lanza, P.L.; Auteri, W.; Nicoletti, G.; Santoro, G.; Quattrone, A. Comparison of different MR venography techniques for detecting transverse sinus stenosis in idiopathic intracranial hypertension. J. Neurol. 2005, 252, 1021–1025. [Google Scholar] [CrossRef]
- Digre, K.B. Not so benign intracranial hypertension. BMJ 2003, 326, 613–614. [Google Scholar] [CrossRef]
- Kanagalingam, S.; Subramanian, P.S. Update on Idiopathic Intracranial Hypertension. Curr. Treat. Options Neurol. 2018, 20, 24. [Google Scholar] [CrossRef]
- Wang, S.J.; Silberstein, S.D.; Patterson, S.; Young, W.B. Idiopathic intracranial hypertension without papilledema: A case-control study in a headache center. Neurology 1998, 51, 245–249. [Google Scholar]
- Mathew, N.T.; Ravishankar, K.; Sanin, L.C. Coexistence of migraine and idiopathic intracranial hypertension without papilledema. Neurology 1996, 46, 1226–1230. [Google Scholar] [CrossRef]
- Favoni, V.; Pierangeli, G.; Toni, F.; Cirillo, L.; La Morgia, C.; Abu-Rumeileh, S.; Messia, M.; Agati, R.; Cortelli, P.; Cevoli, S. Idiopathic Intracranial Hypertension Without Papilledema (IIHWOP) in Chronic Refractory Headache. Front. Neurol. 2018, 9, 503. [Google Scholar] [CrossRef] [PubMed]
- Mollan, S.P.; Chong, Y.J.; Grech, O.; Sinclair, A.J.; Wakerley, B.R. Current Perspectives on Idiopathic Intracranial Hypertension without Papilloedema. Life 2021, 11, 472. [Google Scholar] [CrossRef]
- Toscano, S.; Fermo, S.L.; Reggio, E.; Chisari, C.G.; Patti, F.; Zappia, M. An update on idiopathic intracranial hypertension in adults: A look at pathophysiology, diagnostic approach and management. J. Neurol. 2021, 268, 3249–3268. [Google Scholar] [CrossRef] [PubMed]
- Liguori, C.; Romigi, A.; Albanese, M.; Marciani, M.G.; Placidi, F.; Friedman, D.; Digre, K.; Liu, G. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology 2014, 82, 1752–1753. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.S.; Donaldson, L.; Margolin, E.A. Swelling of Atrophic Optic Discs in Idiopathic Intracranial Hypertension. J. Neuroophthalmol. 2023. [Google Scholar] [CrossRef]
- Albrecht, P.; Blasberg, C.; Ringelstein, M.; Müller, A.-K.; Finis, D.; Guthoff, R.; Kadas, E.-M.; Lagreze, W.; Aktas, O.; Hartung, H.-P.; et al. Optical coherence tomography for the diagnosis and monitoring of idiopathic intracranial hypertension. J. Neurol. 2017, 264, 1370–1380. [Google Scholar] [CrossRef]
- Huang-Link, Y.-M.; Al-Hawasi, A.; Oberwahrenbrock, T.; Jin, Y.-P. OCT measurements of optic nerve head changes in idiopathic intracranial hypertension. Clin. Neurol. Neurosurg. 2015, 130, 122–127. [Google Scholar] [CrossRef]
- Kaufhold, F.; Kadas, E.M.; Schmidt, C.; Kunte, H.; Hoffmann, J.; Zimmermann, H.; Oberwahrenbrock, T.; Harms, L.; Polthier, K.; Brandt, A.U.; et al. Optic nerve head quantification in idiopathic intracranial hypertension by spectral domain OCT. PLoS ONE 2012, 7, e36965. [Google Scholar] [CrossRef]
- Anand, A.; Pass, A.; Urfy, M.Z.; Tang, R.; Cajavilca, C.; Calvillo, E.; Suarez, J.I.; Rao, C.P.V.; Bershad, E.M. Optical coherence tomography of the optic nerve head detects acute changes in intracranial pressure. J. Clin. Neurosci. 2016, 29, 73–76. [Google Scholar] [CrossRef]
- Ahuja, S.; Anand, D.; Dutta, T.; Kumar, V.R.; Kar, S.S. Retinal nerve fiber layer thickness analysis in cases of papilledema using optical coherence tomography—A case control study. Clin. Neurol. Neurosurg. 2015, 136, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Thaller, M.; Homer, V.; Hyder, Y.; Yiangou, A.; Liczkowski, A.; Fong, A.W.; Virdee, J.; Piccus, R.; Roque, M.; Mollan, S.P.; et al. The idiopathic intracranial hypertension prospective cohort study: Evaluation of prognostic factors and outcomes. J. Neurol. 2023, 270, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Nichani, P.; Micieli, J.A. Retinal Manifestations of Idiopathic Intracranial Hypertension. Ophthalmol. Retina 2021, 5, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, J.L.; Lam, K.V.B.; Wall, M.; Wilson, M.D.; Keltner, J.L. Perimetry, retinal nerve fiber layer thickness and papilledema grade after cerebrospinal fluid shunting in patients with idiopathic intracranial hypertension. J. Neuroophthalmol. 2015, 35, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Bono, F.; Cristiano, D.; Mastrandrea, C.; Latorre, V.; D’Asero, S.; Salvino, D.; Fera, F.; Lavano, A.; Quattrone, A. The upper limit of normal CSF opening pressure is related to bilateral transverse sinus stenosis in headache sufferers. Cephalalgia 2010, 30, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Tewarie, P.; Balk, L.; Costello, F.; Green, A.; Martin, R.; Schippling, S.; Petzold, A. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS ONE 2012, 7, e34823. [Google Scholar] [CrossRef] [PubMed]
- Schippling, S.; Balk, L.; Costello, F.; Albrecht, P.; Balcer, L.; Calabresi, P.; Frederiksen, J.; Frohman, E.; Green, A.; Klistorner, A.; et al. Quality control for retinal OCT in multiple sclerosis: Validation of the OSCAR-IB criteria. Mult. Scler. J. 2015, 21, 163–170. [Google Scholar] [CrossRef]
- Cruz-Herranz, A.; Balk, L.J.; Oberwahrenbrock, T.; Saidha, S.; Martinez-Lapiscina, E.H.; Lagreze, W.A.; Schuman, J.S.; Villoslada, P.; Calabresi, P.; Balcer, L.; et al. The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology 2016, 86, 2303–2309. [Google Scholar] [CrossRef]
- Bono, F.; Salvino, D.; Tallarico, T.; Cristiano, D.; Condino, F.; Fera, F.; Lanza, P.; Lavano, A.; Quattrone, A. Abnormal pressure waves in headache sufferers with bilateral transverse sinus stenosis. Cephalalgia 2010, 30, 1419–1425. [Google Scholar] [CrossRef]
- Eide, P.K.; Brean, A. Lumbar cerebrospinal fluid pressure waves versus intracranial pressure waves in idiopathic normal pressure hydrocephalus. Br. J. Neurosurg. 2006, 20, 407–414. [Google Scholar] [CrossRef]
- Eide, P.K.; Kerty, E. Static and pulsatile intracranial pressure in idiopathic intracranial hypertension. Clin. Neurol. Neurosurg. 2011, 113, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Boston, R.C.; Sumner, A.E. STATA: A statistical analysis system for examining biomedical data. Adv. Exp. Med. Biol. 2003, 537, 353–369. [Google Scholar] [PubMed]
- Skau, M.; Yri, H.; Sander, B.; Gerds, T.A.; Milea, D.; Jensen, R. Diagnostic value of optical coherence tomography for intracranial pressure in idiopathic intracranial hypertension. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Yri, H.M.; Wegener, M.; Sander, B.; Jensen, R. Idiopathic intracranial hypertension is not benign: A long-term outcome study. J. Neurol. 2012, 259, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.H.; Radojicic, A.; Yri, H. The diagnosis and management of idiopathic intracranial hypertension and the associated headache. Ther. Adv. Neurol. Disord. 2016, 9, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Tariq, Y.M.; Li, H.; Burlutsky, G.; Mitchell, P. Retinal nerve fiber layer and optic disc measurements by spectral domain OCT: Normative values and associations in young adults. Eye 2012, 26, 1563–1570. [Google Scholar] [CrossRef]
- Rebolleda, G.; Muñoz-Negrete, F.J. Munoz-Negrete, Follow-up of mild papilledema in idiopathic intracranial hypertension with optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5197–5200. [Google Scholar] [CrossRef]
- Savini, G.; Bellusci, C.; Carbonelli, M.; Zanini, M.; Carelli, V.; Sadun, A.A.; Barboni, P. Detection and quantification of retinal nerve fiber layer thickness in optic disc edema using stratus OCT. Arch. Ophthalmol. 2006, 124, 1111–1117. [Google Scholar] [CrossRef]
- Heckmann, J.; Weber, M.; Jünemann, A.; Neundörfer, B.; Mardin, C. Laser scanning tomography of the optic nerve vs CSF opening pressure in idiopathic intracranial hypertension. Neurology 2004, 62, 1221–1223. [Google Scholar] [CrossRef]
- Tang, L.; Kardon, R.H.; Wang, J.-K.; Garvin, M.K.; Lee, K.; Abràmoff, M.D. Quantitative evaluation of papilledema from stereoscopic color fundus photographs. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4490–4497. [Google Scholar] [CrossRef]
- Malhotra, K.; Padungkiatsagul, T.; Moss, H.E. Optical coherence tomography use in idiopathic intracranial hypertension. Ann. Eye Sci. 2020, 5, 7. [Google Scholar] [CrossRef]
- Carey, A.R.; Bosley, T.M.; Miller, N.R.; McCulley, T.J.; Henderson, A.D. Use of En Face Optical Coherence Tomography to Monitor Papilledema in Idiopathic Intracranial Hypertension: A Pilot Study. J. Neuroophthalmol. 2021, 41, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, S.J.; Subramanian, P.S. Relationship of intraocular pulse pressure and spontaneous venous pulsations. Am. J. Ophthalmol. 2009, 147, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Bering, E.A., Jr. Circulation of the cerebrospinal fluid. Demonstration of the choroid plexuses as the generator of the force for flow of fluid and ventricular enlargement. J. Neurosurg. 1962, 19, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Portnoy, H.D. The CSF pulse wave in hydrocephalus. Childs Nerv. Syst. 1986, 2, 107–108. [Google Scholar] [CrossRef]
- Yada, K.; Nakagawa, Y.; Tsuru, M. Circulatory disturbance of the venous system during experimental intracranial hypertension. J. Neurosurg. 1973, 39, 723–729. [Google Scholar] [CrossRef]
- Marmarou, A.; Shulman, K.; LaMorgese, J. Compartmental analysis of compliance and outflow resistance of the cerebrospinal fluid system. J. Neurosurg. 1975, 43, 523–534. [Google Scholar] [CrossRef]
- Giliberto, C.; Mostile, G.; Fermo, S.L.; Reggio, E.; Sciacca, G.; Nicoletti, A.; Zappia, M. Vascular parkinsonism or idiopathic NPH? New insights from CSF pressure analysis. Neurol. Sci. 2017, 38, 2209–2212. [Google Scholar] [CrossRef]
- Abegg, M.; Fleischhauer, J.; Landau, K. Unilateral papilledema after trabeculectomy in a patient with intracranial hypertension. Klin. Monbl Augenheilkd. 2008, 225, 441–442. [Google Scholar] [CrossRef]
- Killer, H.E.; Jaggi, G.P.; Flammer, J.; Miller, N.R.; Huber, A.R.; Mironov, A. Cerebrospinal fluid dynamics between the intracranial and the subarachnoid space of the optic nerve. Is. it always bidirectional? Brain 2007, 130 Pt 2, 514–520. [Google Scholar] [CrossRef]
- Von Hanno, T.; Hareide, L.L.; Småbrekke, L.; Morseth, B.; Sneve, M.; Erke, M.G.; Mathiesen, E.B.; Bertelsen, G. Macular Layer Thickness and Effect of BMI, Body Fat, and Traditional Cardiovascular Risk Factors: The Tromso Study. Investig. Ophthalmol. Vis. Sci. 2022, 63, 16. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.C.M.; Chan, C.W.N.; Hui, S.P. Relationship of gender, body mass index, and axial length with central retinal thickness using optical coherence tomography. Eye 2005, 19, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Szewka, A.J.; Bruce, B.B.; Newman, N.J.; Biousse, V. Idiopathic intracranial hypertension: Relation between obesity and visual outcomes. J. Neuroophthalmol. 2013, 33, 4–8. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).