Survey on Nutrition in Neurological Intensive Care Units (SONNIC)—A Cross-Sectional Survey among German-Speaking Neurointensivists on Medical Nutritional Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey Design and Distribution
2.2. Questionnaire
2.3. Data Analysis
3. Results
3.1. Demographics of Participating Neurointensivists and Corresponding ICUs
3.2. Standardization and Multidisciplinarity of MNT
3.3. Assessment of Nutritional Status
3.4. Determination of Energy Expenditure
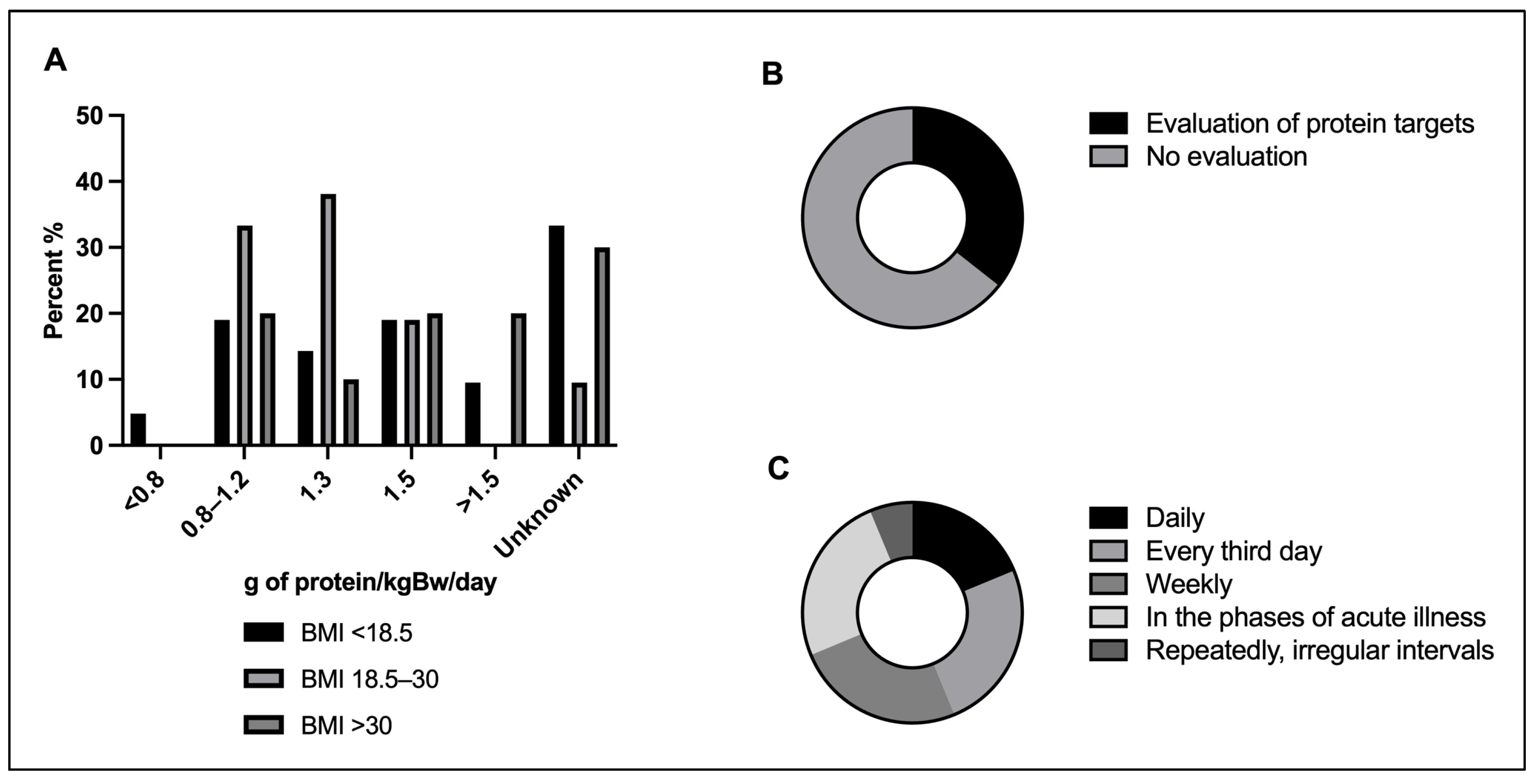
3.5. Protein Targets
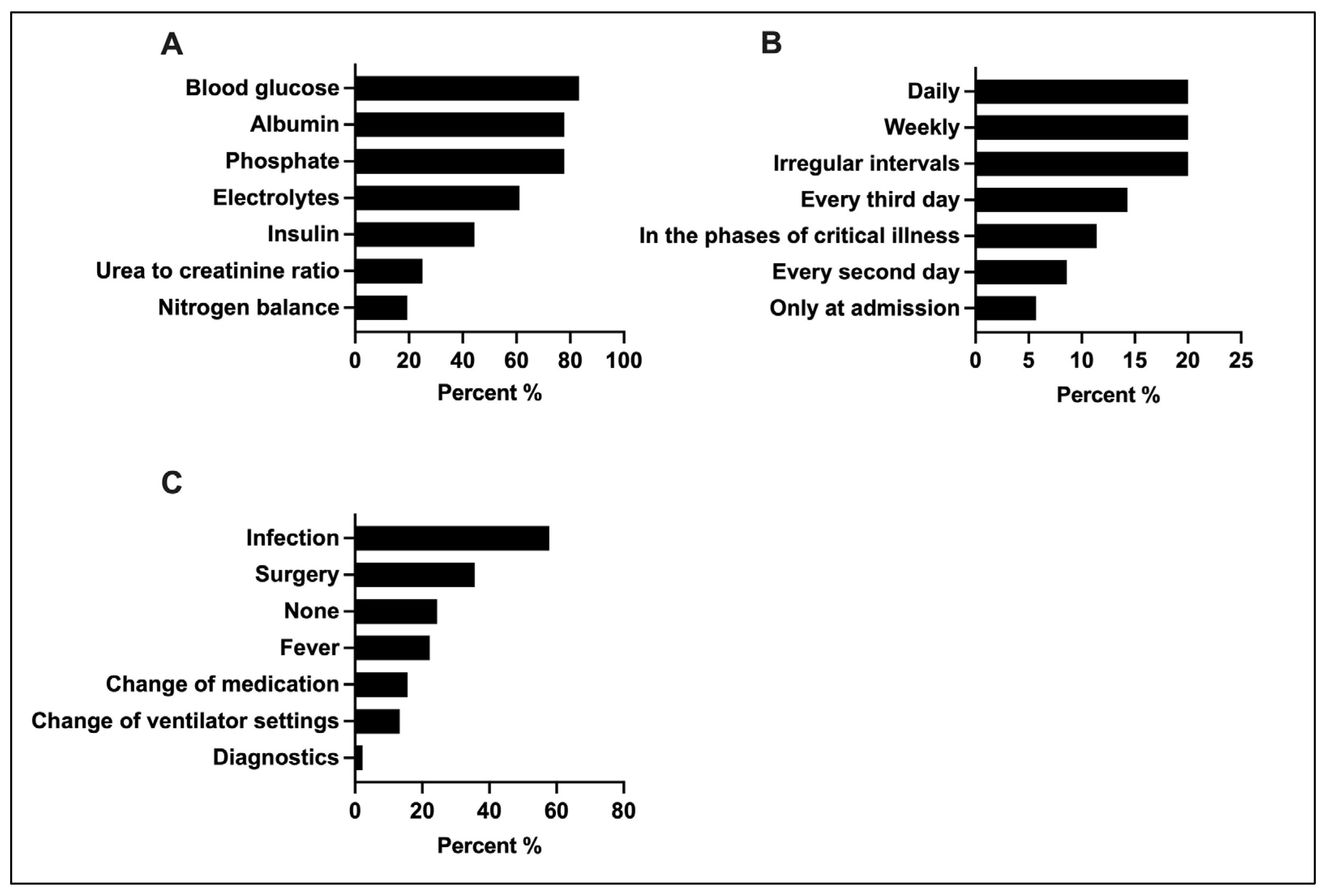
3.6. Monitoring of Metabolic Tolerance and Energy Expenditure
3.7. ICU-Acquired Weakness
3.8. Guideline Adherence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villet, S.; Chiolero, R.L.; Bollmann, M.D.; Revelly, J.P.; Cayeux Rn, M.C.; Delarue, J. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin. Nutr. 2005, 24, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Ndahimana, D.; Kim, E.K. Energy Requirements in Critically Ill Patients. Clin. Nutr. Res. 2018, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Compher, C.; Bingham, A.L.; McCall, M.; Patel, J.; Rice, T.W.; Braunschweig, C. Guidelines for the provision of nutrition support therapy in the adult critically ill patient: The American Society for Parenteral and Enteral Nutrition. J. Parenter. Enter. Nutr. 2022, 46, 12–41. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Calder, P.C.; Casaer, M.; Hiesmayr, M. ESPEN practical and partially revised guideline: Clinical nutrition in the intensive care unit. Clin. Nutr. 2023, 42, 1671–1689. [Google Scholar] [CrossRef] [PubMed]
- Elke, G.; Hartl, W.H.; Kreymann, K.G.; Adolph, M.; Felbinger, T.W.; Graf, T. Clinical Nutrition in Critical Care Medicine—Guideline of the German Society for Nutritional Medicine (DGEM). Clin. Nutr. ESPEN 2019, 33, 220–275. [Google Scholar] [CrossRef] [PubMed]
- ESICM Working Group on Gastrointestinal Function; Reintam Blaser, A.; Starkopf, J.; Alhazzani, W.; Berger, M.M.; Casaer, M.P. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. 2017, 43, 380–398. [Google Scholar] [CrossRef]
- Abdelmalik, P.A.; Dempsey, S.; Ziai, W. Nutritional and Bioenergetic Considerations in Critically Ill Patients with Acute Neurological Injury. Neurocrit. Care 2017, 27, 276–286. [Google Scholar] [CrossRef]
- Speyer, R.; Balaguer, M.; Cugy, E.; Devoucoux, C.; Morinière, S.; Soriano, G. Expert Consensus on Clinical Decision Making in the Disease Trajectory of Oropharyngeal Dysphagia in Adults: An International Delphi Study. J. Clin. Med. 2023, 12, 6572. [Google Scholar] [CrossRef]
- Zuercher, P.; Schenk, N.V.; Moret, C.; Berger, D.; Abegglen, R.; Schefold, J.C. Risk Factors for Dysphagia in ICU Patients after Invasive Mechanical Ventilation. Chest 2020, 158, 1983–1991. [Google Scholar] [CrossRef]
- Olkowski, B.F.; Shah, S.O. Early Mobilization in the Neuro-ICU: How Far Can We Go? Neurocrit. Care 2017, 27, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Cotoia, A.; Umbrello, M.; Ferrari, F.; Pota, V.; Alessandri, F.; Cortegiani, A. Nutritional support and prevention of post-intensive care syndrome: The Italian SIAARTI survey. J. Anesth. Analg. Crit. Care 2023, 3, 45. [Google Scholar] [CrossRef] [PubMed]
- Presser, S.; Couper, M.P.; Lessler, J.T.; Martin, E.; Martin, J.; Rothgeb, J.M. Methods For Testing And Evaluating Survey Questions. Public Opin. Q. 2004, 68, 109–130. [Google Scholar] [CrossRef]
- Collins, D. Pretesting survey instruments: An overview of cognitive methodso title found. Qual. Life Res. 2003, 12, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.M.; Doig, G.S.; Heyland, D.K.; Morrison, T.; Sibbald, W.J.; Southwestern Ontario Critical Care Research Network. Multicentre, cluster-randomized clinical trial of algorithms for critical-care enteral and parenteral therapy (ACCEPT). CMAJ Can. Med. Assoc. J. J. Assoc. Medicale Can. 2004, 170, 197–204. [Google Scholar] [CrossRef]
- Barr, J.; Hecht, M.; Flavin, K.E.; Khorana, A.; Gould, M.K. Outcomes in Critically Ill Patients Before and After the Implementation of an Evidence-Based Nutritional Management Protocol. Chest 2004, 125, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, K.M.; Robinson, M.K.; Casey, J.D.; Gunasekera, N.S.; Moromizato, T.; Rawn, J.D. Nutritional Status and Mortality in the Critically Ill*. Crit. Care Med. 2015, 43, 2605–2615. [Google Scholar] [CrossRef]
- Moisey, L.L.; Mourtzakis, M.; Cotton, B.A.; Premji, T.; Heyland, D.K.; Wade, C.E. Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit. Care 2013, 17, R206. [Google Scholar] [CrossRef]
- Sabbouh, T.; Torbey, M.T. Malnutrition in Stroke Patients: Risk Factors, Assessment, and Management. Neurocrit. Care 2018, 29, 374–384. [Google Scholar] [CrossRef]
- Zhang, P.; Bian, Y.; Tang, Z.; Wang, F. Use of Nutrition Risk in Critically Ill (NUTRIC) Scoring System for Nutrition Risk Assessment and Prognosis Prediction in Critically Ill Neurological Patients: A Prospective Observational Study. JPEN J. Parenter. Enter. Nutr. 2021, 45, 1032–1041. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, B.; Wang, L.; Qiu, Y.; Mao, E.; Sheng, H. Prognostic performance of the NRS2002, NUTRIC, and modified NUTRIC to identify high nutritional risk in severe acute pancreatitis patients. Front. Nutr. 2023, 10, 1101555. [Google Scholar] [CrossRef]
- Tatucu-Babet, O.A.; Fetterplace, K.; Lambell, K.; Miller, E.; Deane, A.M.; Ridley, E.J. Is Energy Delivery Guided by Indirect Calorimetry Associated With Improved Clinical Outcomes in Critically Ill Patients? A Systematic Review and Meta-analysis. Nutr. Metab. Insights 2020, 13, 1178638820903295. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.Y.; Zheng, W.H.; Zhou, H.; Xu, Y.; Huang, H.B. Energy delivery guided by indirect calorimetry in critically ill patients: A systematic review and meta-analysis. Crit. Care 2021, 25, 88. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; De Waele, E.; Sanchez, C.; Ruiz Santana, S.; Montejo, J.C.; Laterre, P.F. TICACOS international: A multi-center, randomized, prospective controlled study comparing tight calorie control versus Liberal calorie administration study. Clin. Nutr. 2021, 40, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Pertzov, B.; Bar-Yoseph, H.; Menndel, Y.; Bendavid, I.; Kagan, I.; Glass, Y.D. The effect of indirect calorimetry guided isocaloric nutrition on mortality in critically ill patients—A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2022, 76, 5–15. [Google Scholar] [CrossRef]
- Elke, G.; Hartl, W.H.; Adolph, M.; Angstwurm, M.; Brunkhorst, F.M.; Edel, A. Laborchemisches und kalorimetrisches Monitoring der medizinischen Ernährungstherapie auf der Intensiv- und Intermediate Care Station: Zweites Positionspapier der Sektion Metabolismus und Ernährung der Deutschen Interdisziplinären Vereinigung für Intensiv- und Notfallmedizin (DIVI). Med Klin—Intensivmed Notfallmedizin [Internet]. 17 April 2023. Available online: https://link.springer.com/10.1007/s00063-023-01001-2 (accessed on 2 November 2023).
- Delsoglio, M.; Achamrah, N.; Berger, M.M.; Pichard, C. Indirect Calorimetry in Clinical Practice. J. Clin. Med. 2019, 8, 1387. [Google Scholar] [CrossRef] [PubMed]
- Zusman, O.; Kagan, I.; Bendavid, I.; Theilla, M.; Cohen, J.; Singer, P. Predictive equations versus measured energy expenditure by indirect calorimetry: A retrospective validation. Clin. Nutr. 2019, 38, 1206–1210. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Iqbal, S.; Giroux, M.; Alam, N.; Campisi, J.; Razek, T. Penn State equation versus indirect calorimetry for nutritional assessment in patients with traumatic brain injury. Can. J. Surg. 2022, 65, E320–E325. [Google Scholar] [CrossRef]
- Morbitzer, K.A.; Wilson, W.S.; Chaben, A.C.; Darby, A.; Dehne, K.A.; Brown, E.R. Energy Expenditure in Critically Ill Adult Patients With Acute Brain Injury: Indirect Calorimetry vs. Predictive Equations. Front. Neurol. 2019, 10, 1426. [Google Scholar] [CrossRef]
- Frankenfield, D.C.; Ashcraft, C.M. Description and prediction of resting metabolic rate after stroke and traumatic brain injury. Nutrition 2012, 28, 906–911. [Google Scholar] [CrossRef]
- Koukiasa, P.; Bitzani, M.; Papaioannou, V.; Pnevmatikos, I. Resting Energy Expenditure in Critically Ill Patients With Spontaneous Intracranial Hemorrhage. J. Parenter. Enter. Nutr. 2015, 39, 917–921. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, C.T.; Cran, G.W.; Cooke, S.R.; Young, I.S. Resting energy expenditure in non-ventilated, non-sedated patients recovering from serious traumatic brain injury: Comparison of prediction equations with indirect calorimetry values. Clin. Nutr. 2009, 28, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Heyland, D.K.; Patel, J.; Compher, C.; Rice, T.W.; Bear, D.E.; Lee, Z.Y. The effect of higher protein dosing in critically ill patients with high nutritional risk (EFFORT Protein): An international, multicentre, pragmatic, registry-based randomised trial. Lancet 2023, 401, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Hurt, R.T.; McClave, S.A.; Martindale, R.G.; Ochoa Gautier, J.B.; Coss-Bu, J.A.; Dickerson, R.N. Summary Points and Consensus Recommendations From the International Protein Summit. Nutr. Clin. Pract. Off Pub. Am. Soc. Parenter. Enter. Nutr. 2017, 32 (Suppl. S1), 142S–151S. [Google Scholar] [CrossRef] [PubMed]
- Weijs, P.J.; Looijaard, W.G.; Beishuizen, A.; Girbes, A.R.; Oudemans-van Straaten, H.M. Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non-septic mechanically ventilated critically ill patients. Crit. Care 2014, 18, 701. [Google Scholar] [CrossRef] [PubMed]
- Deane, A.M.; Plummer, M.P.; Ali Abdelhamid, Y. Update on glucose control during and after critical illness. Curr. Opin. Crit. Care 2022, 28, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Hashmi, M.F.; Castro, D. Hypophosphatemia. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK493172/ (accessed on 6 December 2023).
- Bohé, J.; Abidi, H.; Brunot, V.; Klich, A.; Klouche, K.; Sedillot, N. Individualised versus conventional glucose control in critically-ill patients: The CONTROLING study-a randomized clinical trial. Intensive Care Med. 2021, 47, 1271–1283. [Google Scholar] [PubMed]
- Wang, W.; Xu, C.; Ma, X.; Zhang, X.; Xie, P. Intensive Care Unit-Acquired Weakness: A Review of Recent Progress With a Look Toward the Future. Front. Med. 2020, 7, 559789. [Google Scholar] [CrossRef]
- Watanabe, D.; Uranaka, K.; Asazawa, K.; Akimoto, T.; Ohnuma, H. Effects of interprofessional conferences on intensive care units: Comparing lengths of stay in the intensive care unit before and after the introduction of interprofessional conferences. J. Rural Med. 2023, 18, 133–142. [Google Scholar] [CrossRef]
- Arney, B.D.; Senter, S.A.; Schwartz, A.C.; Meily, T.; Pelekhaty, S. Effect of Registered Dietitian Nutritionist Order-Writing Privileges on Enteral Nutrition Administration in Selected Intensive Care Units. Nutr. Clin. Pract. 2019, 34, 899–905. [Google Scholar] [CrossRef]




| Demographics | Distribution |
|---|---|
| Age (years), mean (SD) | 46.0 (9.2) |
| Institution | |
| Academic hospitals, n (%) | 25/55 (45.5%) |
| Non-academic hospitals, n (%) | 30/55 (54.5%) |
| Subspeciality | |
| Neurology, n (%) | 43/55 (78.2%) |
| Neurosurgery, n (%) | 5/55 (9.1%) |
| Anesthesiology, n (%) | 6/55 (10.9%) |
| Internal medicine, n (%) | 1/55 (1.8%) |
| Others, n (%) | 0/55 (0.0%) |
| Structure of NICU | |
| Neurology led ICU, n (%) | 21/56 (37.5%) |
| Neurosurgery led ICU, n (%) | 5/56 (8.9%) |
| Interdisciplinary neurology/neurosurgery led ICU, n (%) | 3/56 (5.4%) |
| Interdisciplinary anesthesiology/neurology led ICU, n (%) | 6/56 (10.7%) |
| Interdisciplinary anesthesiology/neurosurgery led ICU, n (%) | 4/56 (7.1%) |
| Interdisciplinary internal medicine/neurology led ICU, n (%) | 7/56 (12.5%) |
| interdisciplinary internal medicine/neurosurgery led ICU, n (%) | 0/56 (0.0%) |
| Others, n (%) | 10/56 (17.9%) |
| Years of medical experience | |
| 0–5 years, n (%) | 0/55 (0.0%) |
| 5–10 years, n (%) | 7/55 (12.7%) |
| >10 years, n (%) | 48/55 (87.3%) |
| Years of experience in intensive care medicine | |
| 0–2 years, n (%) | 4/54 (7.4%) |
| 2–5 years, n (%) | 14/54 (25.9%) |
| >5 years, n (%) | 36/54 (66.7%) |
| Leadership position on ICU | |
| Yes, n (%) | 34/54 (63.0%) |
| No, n (%) | 20/54 (37.0%) |
| Number of intensive care beds on ICU | |
| 0–5, n (%) | 0/55 (0.0%) |
| 6–10, n (%) | 17/55 (30.9%) |
| 11–15, n (%) | 23/55 (41.8%) |
| >16, n (%) | 15/55 (27.3%) |
| Proportionate number of neurological/neurosurgical intensive care beds on ICU | |
| 0–5, n (%) | 14/54 (25.9%) |
| 6–10, n (%) | 21/54 (38.9%) |
| 11–15, n (%) | 9/54 (16.7%) |
| >16, n (%) | 0/54 (0.0%) |
| All, n (%) | 10/54 (18.5%) |
| Annual number of neurological/neurosurgical patients on ICU | |
| 0–149, n (%) | 12/53 (22.6%) |
| 150–299, n (%) | 10/53 (18.9%) |
| 300–449, n (%) | 10/53 (18.9%) |
| 450–599, n (%) | 5/53 (9.4%) |
| 600–750, n (%) | 1/53 (1.9%) |
| >750, n (%) | 7/53 (13.2%) |
| Unknown, n (%) | 8/53 (15.1%) |
| Survey-Topic | Guideline Adherence | DGEM Strength of Consensus | ESPEN Strength of Consensus | ESPEN Level of Evidence |
|---|---|---|---|---|
| Existence of SOP/feeding protocol | 80% (41/51) | Strong consensus (100%) | Proposed but not specified | - |
| Implementation of risk stratification at ICU admission | 36% (18/50) | Strong consensus (97%) | Strong consensus (100%) | GPP |
| Use of specific risk stratification scores | 20% (10/50) | Consensus (88%) | Not specified | - |
| Individualized determination of EE | 75% (36/48) | Implicit assumption | Implicit assumption | - |
| Use of indirect calorimetry to determine EE | 15% (7/48) | Strong consensus (100%) | Strong consensus (95%) | B |
| Use of actual body weight to determine EE (non-obese, non-cachectic patients) | 49% (18/37) | Strong consensus (94%) | Consensus (89%) | GPP |
| Hypocaloric energy target in the acute phase of disease (d 0–2) | 64% (23/36) | Strong consensus (94%) | Strong consensus (100%) | B |
| Isocaloric energy target in the post-acute phase (d 3–7) | 77% (28/36) | Strong consensus (94%) | Strong consensus (95%) | 0 |
| Individualized targets for protein intake | 57% (22/39) | Implicit assumption | Implicit assumption | Implicit assumption |
| Protein target during critical illness 1.0–1.2 g/kgBw/day (DGEM) or ESPEN (1.3 g) in non-obese patients | 39% (15/39) | Consensus (88%) | Strong consensus (91%) | 0 |
| Protein target 1.5 g (DGEM) or 1.3 g (ESPEN) in obese patients | 13% (6/48) | Strong consensus (94%) | Consensus (89%) | GPP |
| Evaluation of metabolic intolerance | 53% (24/45) | Strong consensus (97%) | Proposed but not specified | - |
| Re-evaluation of EE during critical illness | 38% (17/45) | Consensus (89%) | Not specified; note on phases of critical illness | - |
| Overall | 47% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gehri, L.; Schmidbauer, M.L.; Putz, T.; Ratkovic, L.; Maskos, A.; Zeisberger, C.; Zibold, J.; Dimitriadis, K.; on behalf of the IGNITE Study Group. Survey on Nutrition in Neurological Intensive Care Units (SONNIC)—A Cross-Sectional Survey among German-Speaking Neurointensivists on Medical Nutritional Therapy. J. Clin. Med. 2024, 13, 447. https://doi.org/10.3390/jcm13020447
Gehri L, Schmidbauer ML, Putz T, Ratkovic L, Maskos A, Zeisberger C, Zibold J, Dimitriadis K, on behalf of the IGNITE Study Group. Survey on Nutrition in Neurological Intensive Care Units (SONNIC)—A Cross-Sectional Survey among German-Speaking Neurointensivists on Medical Nutritional Therapy. Journal of Clinical Medicine. 2024; 13(2):447. https://doi.org/10.3390/jcm13020447
Chicago/Turabian StyleGehri, Leon, Moritz L. Schmidbauer, Timon Putz, Luka Ratkovic, Andreas Maskos, Cedric Zeisberger, Julia Zibold, Konstantinos Dimitriadis, and on behalf of the IGNITE Study Group. 2024. "Survey on Nutrition in Neurological Intensive Care Units (SONNIC)—A Cross-Sectional Survey among German-Speaking Neurointensivists on Medical Nutritional Therapy" Journal of Clinical Medicine 13, no. 2: 447. https://doi.org/10.3390/jcm13020447
APA StyleGehri, L., Schmidbauer, M. L., Putz, T., Ratkovic, L., Maskos, A., Zeisberger, C., Zibold, J., Dimitriadis, K., & on behalf of the IGNITE Study Group. (2024). Survey on Nutrition in Neurological Intensive Care Units (SONNIC)—A Cross-Sectional Survey among German-Speaking Neurointensivists on Medical Nutritional Therapy. Journal of Clinical Medicine, 13(2), 447. https://doi.org/10.3390/jcm13020447






