Incidence and Risk Factors for Low Anterior Resection Syndrome following Trans-Anal Total Mesorectal Excision
Abstract
1. Introduction
2. Materials and Methods
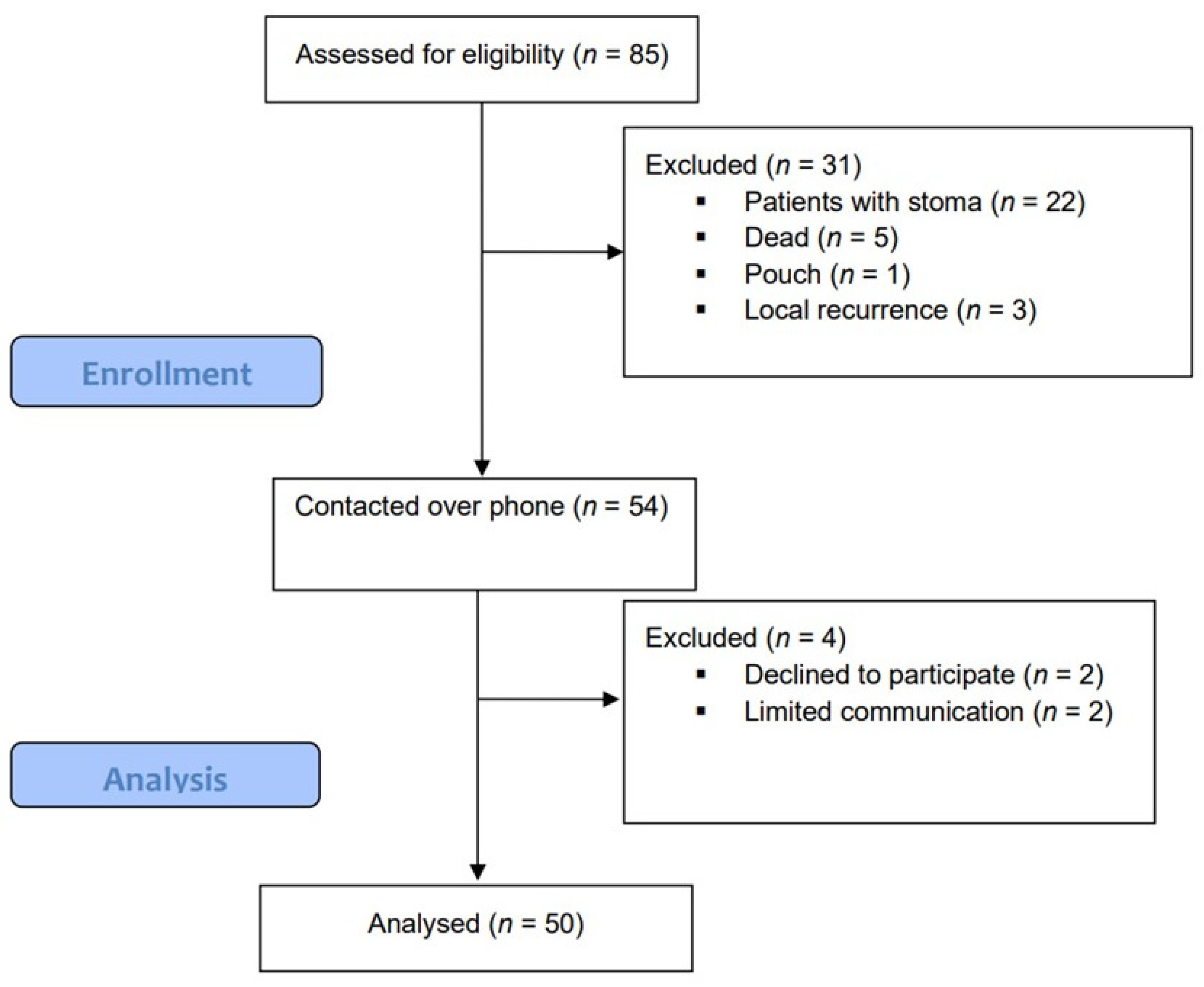
2.1. Study Design and Patient Inclusion
2.2. Perioperative Management and Surgical Technique
2.3. Baseline Data
2.4. Study Outcomes
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kitz, J.; Fokas, E.; Beissbarth, T.; Ströbel, P.; Wittekind, C.; Hartmann, A.; Rüschoff, J.; Papadopoulos, T.; Rösler, E.; Ortloff-Kittredge, P.; et al. Association of Plane of Total Mesorectal Excision with Prognosis of Rectal Cancer: Secondary Analysis of the CAO/ARO/AIO-04 Phase 3 Randomized Clinical Trial. JAMA Surg. 2018, 153, e181607. [Google Scholar] [CrossRef] [PubMed]
- Heald, R.J. A new approach to rectal cancer. Br. J. Hosp. Med. 1979, 22, 277–281. [Google Scholar] [PubMed]
- Quintana, J.M.; Anton-Ladislao, A.; Lázaro, S.; Gonzalez, N.; Bare, M.; de Larrea, N.F.; Redondo, M.; Briones, E.; Escobar, A.; Sarasqueta, C.; et al. Outcomes of open versus laparoscopic surgery in patients with rectal cancer. Int. J. Color. Dis. 2018, 33, 99–103. [Google Scholar] [CrossRef] [PubMed]
- van der Pas, M.H.; Haglind, E.; Cuesta, M.A.; Fürst, A.; Lacy, A.M.; Hop, W.C.; Bonjer, H.J.; COlorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group. Laparoscopic versus open surgery for rectal cancer (COLOR II): Short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013, 14, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Akiyoshi, T.; Kuroyanagi, H.; Oya, M.; Konishi, T.; Fukuda, M.; Fujimoto, Y.; Ueno, M.; Miyata, S.; Yamaguchi, T. Factors affecting the difficulty of laparoscopic total mesorectal excision with double stapling technique anastomosis for low rectal cancer. Surgery 2009, 146, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Wibe, A.; Rendedal, P.R.; Svensson, E.; Norstein, J.; Eide, T.J.; Myrvold, H.E.; Søreide, O. Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer. Br. J. Surg. 2002, 89, 327–334. [Google Scholar] [CrossRef]
- Lacy, A.M.; Adelsdorfer, C.; Delgado, S.; Sylla, P.; Rattner, D.W. Minilaparoscopy-assisted transrectal low anterior resection (LAR): A preliminary study. Surg. Endosc. 2013, 27, 339–346. [Google Scholar] [CrossRef]
- Velthuis, S.; van den Boezem, P.B.; van der Peet, D.L.; Cuesta, M.A.; Sietses, C. Feasibility study of transanal total mesorectal excision. Br. J. Surg. 2013, 100, 828–831. [Google Scholar] [CrossRef]
- Lacy, A.M.; Tasende, M.M.; Delgado, S.; Fernandez-Hevia, M.; Jimenez, M.; De Lacy, B.; Castells, A.; Bravo, R.; Wexner, S.D.; Heald, R.J. Transanal Total Mesorectal Excision for Rectal Cancer: Outcomes after 140 Patients. J. Am. Coll. Surg. 2015, 221, 415–423. [Google Scholar] [CrossRef]
- Veltcamp Helbach, M.; Deijen, C.L.; Velthuis, S.; Bonjer, H.J.; Tuynman, J.B.; Sietses, C. Transanal total mesorectal excision for rectal carcinoma: Short-term outcomes and experience after 80 cases. Surg. Endosc. 2016, 30, 464–470. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, W.; Chen, F.; Wang, W.; Feng, Y. Short-term outcomes of transanal versus laparoscopic total mesorectal excision: A systematic review and meta-analysis of cohort studies. J. Cancer 2019, 10, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Pieniowski, E.H.; Palmer, G.J.; Juul, T.; Lagergren, P.; Johar, A.; Emmertsen, K.J.; Nordenvall, C.; Abraham-Nordling, M. Low anterior resection syndrome and quality of life after sphincter-sparing rectal cancer surgery: A long-term longitudinal follow-up. Dis. Colon. Rectum. 2019, 62, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Rubinkiewicz, M.; Zarzycki, P.; Witowski, J.; Pisarska, M.; Gajewska, N.; Torbicz, G.; Nowakowski, M.; Major, P.; Budzyński, A.; Pędziwiatr, M. Functional outcomes after resections for low rectal tumors: Comparison of transanal with laparoscopic total mesorectal excision. BMC Surg. 2019, 19, 79. [Google Scholar] [CrossRef]
- Trenti, L.; Galvez, A.; Biondo, S.; Solis, A.; Vallribera-Valls, F.; Espin-Basany, E.; Garcia-Granero, A.; Kreisler, E. Quality of life and anterior resection syndrome after surgery for mid to low rectal cancer: A cross-sectional study. Eur. J. Surg. Oncol. 2018, 44, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Filips, A.; Haltmeier, T.; Kohler, A.; Candinas, D.; Brügger, L.; Studer, P. LARS is Associated with Lower Anastomoses, but not with the Transanal Approach in Patients Undergoing Rectal Cancer Resection. World J. Surg. 2021, 45, 873–879. [Google Scholar] [CrossRef]
- Heijden, J.A.G.; Koëter, T.; Smits, L.J.H.; Sietses, C.; Tuynman, J.B.; Maaskant-Braat, A.J.G.; Klarenbeek, B.R.; Wilt, J.H.W. Functional complaints and quality of life after transanal total mesorectal excision: A meta-analysis. Br. J. Surg. 2020, 107, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Bregendahl, S.; Emmertsen, K.J.; Lous, J.; Laurberg, S. Bowel dysfunction after low anterior resection with and without neoadjuvant therapy for rectal cancer: A population-based cross-sectional study. Color. Dis. 2013, 15, 1130–1139. [Google Scholar] [CrossRef]
- Emmertsen, K.J.; Laurberg, S. Low anterior resection syndrome score: Development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann. Surg. 2012, 255, 922–928. [Google Scholar] [CrossRef]
- Hughes, D.L.; Cornish, J.; Morris, C.; LARRIS Trial Management Group. Functional outcome following rectal surgery-predisposing factors for low anterior resection syndrome. Int. J. Color. Dis. 2017, 32, 691–697. [Google Scholar] [CrossRef]
- Desnoo, L.; Faithfull, S. A qualitative study of anterior resection syndrome: The experiences of cancer survivors who have undergone resection surgery. Eur. J. Cancer Care 2006, 15, 244–251. [Google Scholar] [CrossRef]
- Sturiale, A.; Martellucci, J.; Zurli, L.; Vaccaro, C.; Brusciano, L.; Limongelli, P.; Docimo, L.; Valeri, A. Long-term functional follow-up after anterior rectal resection for cancer. Int. J. Color. Dis. 2017, 32, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Kneist, W.; Wachter, N.; Paschold, M.; Kauff, D.W.; Rink, A.D.; Lang, H. Midterm functional results of ta-TME with neuromapping for low rectal cancer. Tech. Coloproctol. 2016, 20, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Pontallier, A.; Denost, Q.; Van Geluwe, B.; Adam, J.P.; Celerier, B.; Rullier, E. Potential sexual function improvement by using transanal mesorectal approach for laparoscopic low rectal cancer excision. Surg. Endosc. 2016, 30, 4924–4933. [Google Scholar] [CrossRef] [PubMed]
- Sarcher, T.; Dupont, B.; Alves, A.; Menahem, B. Anterior resection syndrome: What should we tell practitioners and patients in 2018? J. Visc. Surg. 2018, 155, 383–391. [Google Scholar] [CrossRef]
- Jimenez-Gomez, L.M.; Espin-Basany, E.; Trenti, L.; Martí-Gallostra, M.; Sánchez-García, J.L.; Vallribera-Valls, F.; Kreisler, E.; Biondo, S.; Armengol-Carrasco, M. Factors associated with low anterior resection syndrome after surgical treatment of rectal cancer. Color. Dis. 2018, 20, 195–200. [Google Scholar] [CrossRef]
- Nowakowski, M.M.; Rubinkiewicz, M.; Gajewska, N.; Torbicz, G.; Wysocki, M.; Małczak, P.; Major, P.; Wierdak, M.; Budzyński, A.; Pędziwiatr, M. Defunctioning ileostomy and mechanical bowel preparation may contribute to development of low anterior resection syndrome. Videosurg. Other Miniinvasive Tech. 2018, 13, 306–314. [Google Scholar] [CrossRef]
- Nesbakken, A.; Nygaard, K.; Lunde, O.C. Outcome and late functional results after anastomotic leakage following mesorectal excision for rectal cancer. Br. J. Surg. 2001, 88, 400–404. [Google Scholar] [CrossRef]
- Keller, D.S.; Reali, C.; Spinelli, A.; Penna, M.; Di Candido, F.; Cunningham, C.; Hompes, R. Patient-reported functional and quality-of-life outcomes after transanal total mesorectal excision. Br. J. Surg. 2019, 106, 364–366. [Google Scholar] [CrossRef]

| Factor | Total Population (n = 50) |
|---|---|
| Age, years, median (IQR) | 58.0 (49.3–66.3) |
| BMI, kg/m2, median (IQR) | 27.1 (24.7–29.6) |
| Sex, n (%) | |
| Male | 31 (62.0%) |
| Female | 19 (38.0%) |
| ASA score, n (%) | |
| 1 | 10 (20.0%) |
| 2 | 35 (70.0%) |
| 3 | 5 (10.0%) |
| Smoking, n (%) | 17 (34.0%) |
| Previous surgery, n (%) | |
| TAH | 3 (6.0%) |
| Bariatric | 1 (2.0%) |
| Anorectal | 2 (4.0%) |
| Tumor height from DL, cm, median (IQR) | 5.0 (3.0–7.0) |
| Preoperative staging, n (%) | |
| 1 | 6 (12.0%) |
| 2 | 11 (22.0%) |
| 3 | 31 (62.0%) |
| 4 | 1 (2.0%) |
| Other (NET) | 1 (2.0%) |
| Neo-adjuvant therapy, n (%) | |
| Short-course radiotherapy | 7 (14.0%) |
| Long-course chemoradiotherapy | 34 (68.0%) |
| Anastomosis distance from DL, cm, median (IQR) | 3.0 (1.0–3.8) |
| Type of anastomosis, n (%) | |
| Stapled | 30 (60.0%) |
| Hand-sewn | 20 (40.0%) |
| Operative time, hours, median (IQR) | 3.2(2.8–3.8) |
| Concomitant procedures, n (%) | |
| Right colectomy | 2 (4.0%) |
| TAH/Salpingo-oophorectomy | 1 (2.0%) |
| Wedge gastrectomy | 1 (2.0%) |
| Intraoperative complication, n (%) | |
| Conversion to open surgery | 3 (6.0%) |
| Hemorrhage | 2 (4.0%) |
| Postoperative complications, n (%) | |
| Anastomosis leakage | 3 (6.0%) |
| Other | 23 (46.0%) |
| Postoperative stay, days, median (IQR) | 8.0 (7.0–12.0) |
| Adjuvant chemotherapy, n (%) | 31 (62.0%) |
| Time to stoma reversal, months, median (IQR) | 6.0 (3.0–7.0) |
| Follow-up time after stoma reversal, months, median (IQR) | 7.0 (5.0–11.0) |
| No LARS n = 12 (24%) | Minor LARS n = 6 (12%) | Major LARS n = 32 (64%) | p-Value | |
|---|---|---|---|---|
| Age, years, median (IQR) | 48.5 (41.3–76.8) | 61.5 (54.0–71.3) | 58.5 (52.0–65.8) | 0.431 |
| Sex, n (%) | 0.388 | |||
| 6 (50.0%) | 5 (83.3%) | 20 (62.5%) | |
| 6 (50.0%) | 1 (16.7%) | 12 (37.5%) | |
| BMI, kg/m2, median (IQR) | 26.7 (24.3–29.1) | 31.9 (30.2–33.2) | 27.0 (24.4–29.1) | 0.053 |
| Previous surgery, n (%) | ||||
| 2 (16.7%) | 0 (0.0) | 1 (3.1%) | 0.195 |
| 0 (0.0) | 0 (0.0) | 1 (3.1%) | 0.762 |
| 1 (8.3%) | 0 (0.0) | 1 (3.1%) | 0.637 |
| Current smoking, n (%) | 4 (33.3%) | 4 (66.7%) | 9 (29.0%) | 0.208 |
| ASA, n (%) | 0.935 | |||
| 2 (16.6%) | 1 (16.7%) | 7 (21.9%) | |
| 9 (75.0%) | 4 (66.7%) | 22 (68.8%) | |
| 1 (8.3%) | 1 (16.7%) | 3 (9.4%) | |
| Preoperative staging, n (%) | 0.800 | |||
| 0 (0.0) | 2 (33.3%) | 4 (12.5%) | |
| 4 (33.3%) | 0 (0.0) | 7 (22.9%) | |
| 8 (66.7%) | 4 (66.7%) | 19 (59.4%) | |
| 0 (0.0) | 0 (0.0) | 1 (3.1%) | |
| 0 (0.0) | 0 (0.0) | 1 (3.1%) | |
| Neoadjuvant therapy, n (%) | 0.501 | |||
| 1 (9.1%) | 2 (33.3%) | 6 (18.8%) | |
| 3 (25.0%) | 0 (0.0) | 4 (12.5%) | |
| 8 (66.7%) | 4 (66.7%) | 22 (68.8%) | |
| Median tumor height from DL, cm (IQR) | 6.5 (4.3–7.0) | 6.0 (4.5–8.5) | 5.0 (3.0–6.0) | 0.190 |
| Median anastomosis height from DL, cm (IQR) | 3.0 (1.0–4.8) | 3.5 (2.3–5.3) | 1.0 (0.0–3.0) | 0.081 |
| Type of anastomosis, n (%) | 0.969 | |||
| 7 (58.3%) | 4 (66.7%) | 19 (59.4%) | |
| 5 (41.7%) | 2 (33.3%) | 13 (40.6%) | |
| Concomitant procedures, n (%) | 0.268 | |||
| 2 (16.6%) | 0 (0.0) | 0 (0.0) | |
| 0 (0.0) | 0 (0.0) | 1 (3.1%) | |
| 0 (0.0) | 0 (0.0) | 1 (3.1%) | |
| Intraoperative complications, n (%) | ||||
| 1 (8.3%) | 0 (0.0) | 2 (6.3%) | 0.778 |
| 1 (8.3%) | 0 (0.0) | 1 (3.1%) | 0.637 |
| Operative time, hours, median (IQR) | 3.1 (2.8–3.6) | 3.6 (2.9–4.25) | 31 (2.8–4.1) | 0.560 |
| Postoperative complications, n (%) | ||||
| 1 (8.3%) | 1 (16.6%) | 1 (3.1%) | 0.408 |
| 7 (58.3%) | 1 (16.6%) | 15 (46.9%) | 0.244 |
| Adjuvant chemotherapy, n (%) | 9 (75.0%) | 5 (83.3%) | 17 (53.1%) | 0.253 |
| Time to stoma reversal, months, median (IQR) | 6.5 (5.3–8.8) | 5.0 (2.8–9.5) | 5.0 (3.0–7.0) | 0.147 |
| Follow-up time after stoma reversal, months, median (IQR) | 8.0 (5.0–9.0) | 13.0 (6.0–21.0) | 6.5 (3.8–11.0) | 0.322 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parnasa, S.Y.; Mizrahi, I.; Helou, B.; Cohen, A.; Abu Gazala, M.; Pikarsky, A.J.; Shussman, N. Incidence and Risk Factors for Low Anterior Resection Syndrome following Trans-Anal Total Mesorectal Excision. J. Clin. Med. 2024, 13, 437. https://doi.org/10.3390/jcm13020437
Parnasa SY, Mizrahi I, Helou B, Cohen A, Abu Gazala M, Pikarsky AJ, Shussman N. Incidence and Risk Factors for Low Anterior Resection Syndrome following Trans-Anal Total Mesorectal Excision. Journal of Clinical Medicine. 2024; 13(2):437. https://doi.org/10.3390/jcm13020437
Chicago/Turabian StyleParnasa, Shani Y., Ido Mizrahi, Brigitte Helou, Adiel Cohen, Mahmoud Abu Gazala, Alon J. Pikarsky, and Noam Shussman. 2024. "Incidence and Risk Factors for Low Anterior Resection Syndrome following Trans-Anal Total Mesorectal Excision" Journal of Clinical Medicine 13, no. 2: 437. https://doi.org/10.3390/jcm13020437
APA StyleParnasa, S. Y., Mizrahi, I., Helou, B., Cohen, A., Abu Gazala, M., Pikarsky, A. J., & Shussman, N. (2024). Incidence and Risk Factors for Low Anterior Resection Syndrome following Trans-Anal Total Mesorectal Excision. Journal of Clinical Medicine, 13(2), 437. https://doi.org/10.3390/jcm13020437






