1. Introduction
Lung cancer continues to be a significant global health challenge, claiming more lives than any other type of cancer. In 2013 alone, there were an estimated 1.8 million new cases and 1.6 million deaths attributed to this devastating disease, accounting for approximately 19% of all cancer-related deaths [
1,
2]. Despite advancements in treatment options, the overall survival rate for lung cancer remains dishearteningly low, with only 16.3% of patients surviving beyond five years following diagnosis [
3].
Efficient and personalized treatment strategies are crucial for maximizing the chances of a cure for early-stage lung cancer and avoiding unnecessary invasive procedures in advanced cases. Thus, accurate TNM staging plays a pivotal role in determining the most appropriate course of action for patients with Non-Small Cell Lung Cancer (NSCLC) [
4].
Among the NSCLC patient population, those diagnosed with stage IIIA NSCLC and harboring metastatic ipsilateral mediastinal nodes (N2) represent a unique and highly debated subgroup. Although initial surgical intervention is not recommended for this particular group, the possibility of radical surgery after neoadjuvant therapy and subsequent restaging offers a glimmer of hope [
5,
6,
7].
Various methods are employed for the staging of NSCLC, encompassing a range of imaging techniques, such as chest CT, PET-CT, and endoscopic/ultrasound approaches, such as endobronchial ultrasound/transbronchial needle aspiration (EBUS/TBNA) and endoscopic ultrasound/fine needle aspiration (EUS/FNA). Surgical techniques, including standard cervical mediastinoscopy (CM), video-assisted mediastinoscopy (VAM), extended mediastinoscopy, video-assisted mediastinoscopic lymphadenectomy (VAMLA), transcervical extended mediastinal lymphadenectomy (TEMLA), anterior mediastinotomy (Chamberlain procedure), and video thoracoscopy (VATS), are also employed [
8,
9,
10]. Presently, EBUS/TBNA and EUS/FNA are considered the next phase of mediastinal staging following CT and PET/CT, providing a comprehensive assessment of the mediastinal nodes [
11,
12,
13]. EBUS and EUS are complementary techniques that allow for visualization and biopsy of most of the mediastinal nodes [
14]. Notably, TEMLA, a relatively novel technique involving the complete removal of the mediastinal nodes and surrounding adipose tissue, aims to enhance the accuracy of NSCLC staging and restaging after neoadjuvant treatment [
10,
15,
16].
Restaging of mediastinal nodes holds significant importance in the context of multimodality treatment for stage IIIA NSCLC. Several studies have demonstrated that patients with residual metastatic nodes after neoadjuvant therapy exhibit inferior survival rates compared to those with nodal downstaging to ypN0-1 [
10,
17]. This disparity becomes more pronounced in patients with residual multilevel metastatic nodes [
18,
19]. Consequently, the decision to pursue surgery after neoadjuvant therapy should be based on the most reliable restaging method available. Currently, several approaches are available to restage mediastinal lymph nodes following neoadjuvant treatment, including imaging studies such as chest CT, PET/CT, EBUS, EUS, and combined EBUS/EUS, as well as invasive procedures such as repeated mediastinoscopy (re-mediastinoscopy), VATS, and TEMLA.
The objective of this study was to analyze the impact of restaging the mediastinal lymph nodes using endobronchial ultrasound (EBUS), endoesophageal ultrasound (EUS), and transcervical extended mediastinal lymphadenectomy (TEMLA) after neoadjuvant chemotherapy (CHT) or chemoradiotherapy (CRT) on the five-year overall survival of patients diagnosed with Non-Small Cell Lung Cancer (NSCLC) in clinical stage IIIA-IIIB with metastatic ipsilateral mediastinal nodes (N2) undergoing radical pulmonary resections.
2. Materials and Methods
This retrospective, single-center analysis included two groups of NSCLC patients diagnosed with stage IIIA disease based on CT scans that revealed enlargement of the ipsilateral mediastinal and/or subcarinal lymph nodes (N2) that responded to neoadjuvant chemotherapy (CTH) or chemoradiotherapy (CRT) and underwent surgery after restaging.
The first group (N = 56) was treated between 1999 and 2005 with restaging based exclusively on chest CT scans (clinical restaging group).
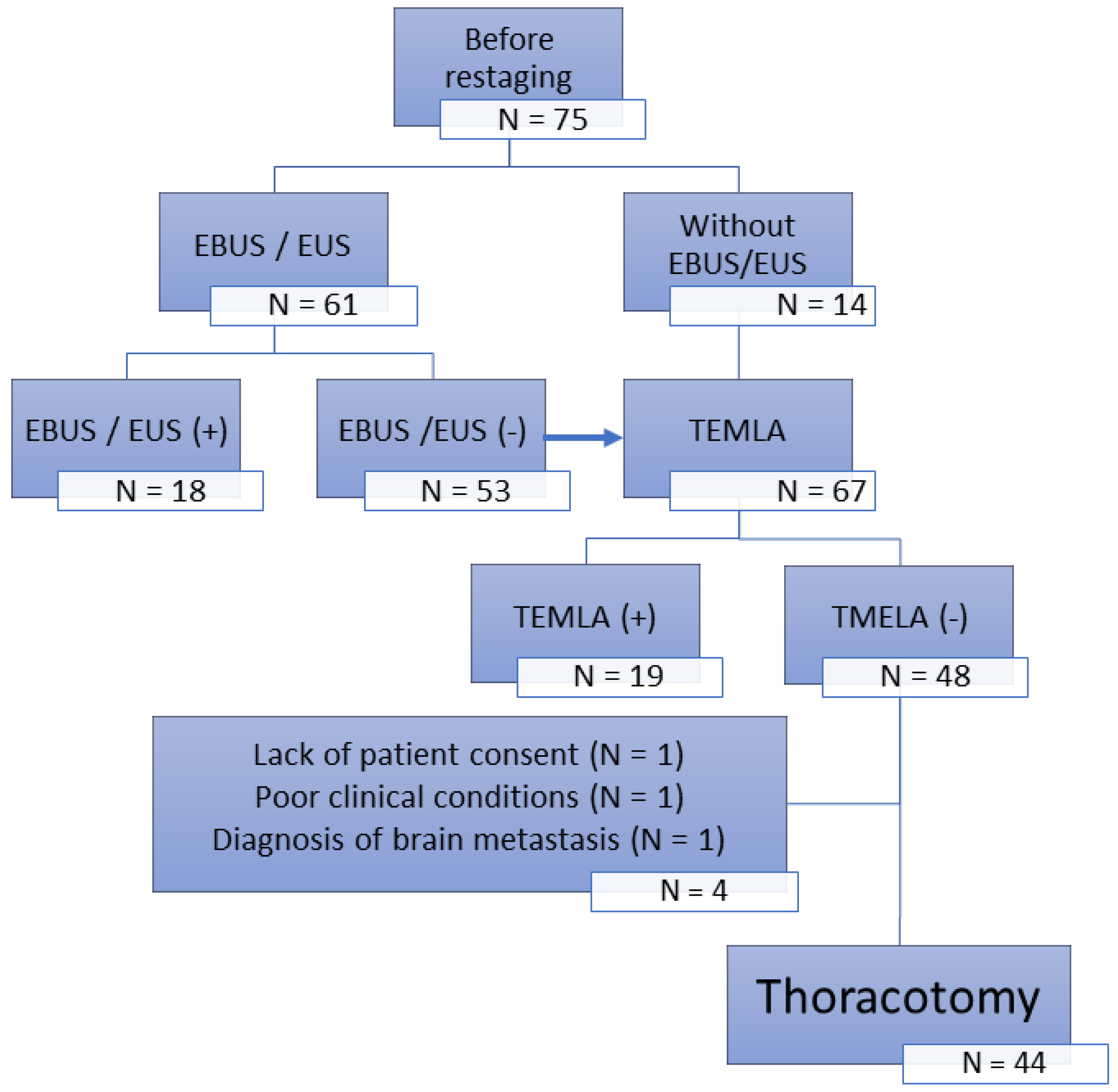
The second group (N = 75) was treated from 2004 to 2008 with restaging based on CT scans with verification of mediastinal lymph nodes by transcervical extended mediastinal lymphadenectomy (TEMLA) performed in 2006 by endobronchial ultrasound (EBUS)/endoesophageal ultrasound (EUS). The final analysis included 44 operated patients without lymph node involvement confirmed by TEMLA (TEMLA restaging group;
Figure 1).
2.1. Outcomes
The analysis of outcomes included percentages of exploratory thoracotomies, in-hospital postoperative mortality, overall survival (OS), and 5-year overall survival (5-year OS) in the clinical and TEMLA restaging groups.
2.2. Statistical Analysis
The statistical analysis was performed using STATISTICA 13.3 PL (13.3.0. TIBCO Software Inc., Palo Alto, CA, USA). The data are presented as mean values with standard deviations. The normality distribution was verified using the Shapiro–Wilk test. The Students’ t-test was used to compare differences in means. The statistical analysis also included the independence test c2, using which the two groups were compared in terms of sex, histologic type of cancer, type of neoadjuvant therapy, type of surgery performed, and clinical stage.
Differences in the Kaplan–Meier survival curves between the groups were calculated using the log-rank test. In addition, the median OS with a 95% confidence interval was calculated. For all analyses, a p-value below 0.05 was considered statistically significant.
3. Results
3.1. Analyzed Group Characteristics’
Squamous cell carcinoma was the predominant NSCLC type in both study groups (
Table 1). There were significant differences in adjuvant therapy. In the non-invasive restaging group, CRT was the most frequent method (80.4%), while in the TEMLA restaging group, it was CTH (86.4%). There was no difference between the study groups regarding age, sex, histologic type of tumor, type of surgical procedure, and resection margins.
3.2. Overall Survival
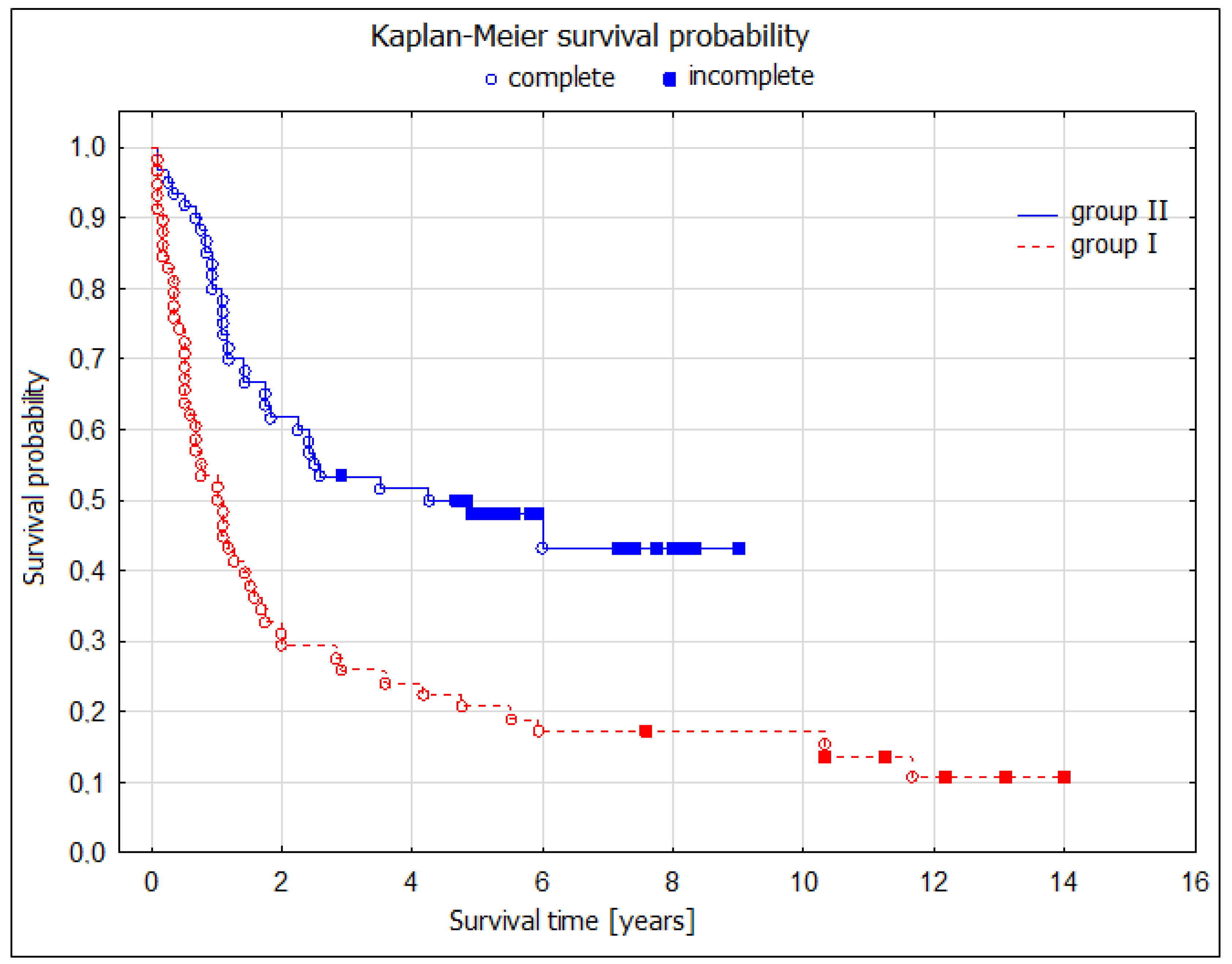
During the 5-year postoperative period, there were 42 deaths (75%) in the noninvasive restaging group and 20 deaths (45.5%) in the TEMLA restaging group. These numbers were reflected by significantly better 5-year overall survival (OS) after TEMLA restaging: 55.9 (95% CI: 40.6–71.1) vs. 25.0 (13.7–36.3)%,
p = 0.003; four times longer median OS (59 vs. 14 months); log-rank test,
p = 0.02 (
Figure 2).
We also obtained similar results and significant differences in 5-year OS when we analyzed separately men [58.1 (CI: 41.8–74.3) vs. 20.0 (CI: 8.3–31.7)%;
p < 0.001], patients in the age of less than 60 years old [42.9 (6.2–79.5) vs. 17.6 (0.1–35.8)%,
p = 0.01)], those with squamous cell carcinoma [56.1 (38.8–73.3) vs. 29.4 (14.1–44.7)%,
p = 0.009], after pneumonectomy [60.0 (38.5–81.5) vs. 28.0 (10.4–45.6)%,
p = 0.03], and R0 resections [55.9 (40.6–71.1) vs. 25.0 (12.8–37.3)%,
p < 0.001]. While there was no difference among women (
p = 0.21), patients aged 60 years or older (
p = 0.15), with adenocarcinoma (
p = 0.18), and non-other specified histology—NOS (
p = 0.09), after lobectomy (
p = 0.06), and R1-2 resections (
p = 0.78), these results were compiled and are presented in the table below (
Table 2).
There was also no difference in the 5-year OS between the subgroups of patients with different pathologic responses to neoadjuvant CTH/CRT (yp) (
Table 3).
4. Discussion
Current guidelines for the treatment of patients with stage IIIA (N2) Non-Small Cell Lung Cancer (NSCLC) are primarily based on the findings of two major prospective randomized trials conducted by the RTOG and the EORTC [
20,
21]. These trials concluded that concurrent chemoradiotherapy (CRT) is the preferred treatment method because it is less invasive than surgery. However, a more detailed analysis of the trial results revealed that the presence of persistent metastases in N2 lymph nodes had a significantly negative impact on survival outcomes. Interestingly, in the subgroup of patients who showed downstaging from N2 to N1/0 after neoadjuvant treatment, those treated with surgery demonstrated significantly better 5-year overall survival (OS) rates than those treated with CRT. This interpretation suggests that surgical intervention should be limited to patients who achieve remission of mediastinal node metastases (downstaging from N2 to N0/1) after neoadjuvant therapy. However, such selection can only be reliably determined through highly sensitive and specific restaging techniques, which were not utilized in the RTOG and EORTC studies that relied solely on chest CT for restaging purposes [
5,
6,
7,
19,
22,
23,
24,
25].
In our single-center retrospective analysis, we investigated two groups of patients with stage III NSCLC and ipsilateral mediastinal and/or subcarinal lymph node metastases (N2). Restaging was performed using different methods: chest CT alone in the group treated between 1999 and 2003 and the introduction of endobronchial ultrasound (EBUS), endoscopic ultrasound (EUS), and transcervical extended mediastinal lymphadenectomy (TEMLA) in the latter group (2004–2008). The transitional period from 2004 to 2005 involved some patients who qualified for treatment based on both scenarios. Notably, PET-CT was not utilized in either group because of its limited availability at the time.
The TEMLA restaging group demonstrated significantly better overall survival compared to the chest CT scan-only group (log-rank test,
p = 0.02). This was evident through a four-fold increase in median OS (59 vs. 14 months) and a higher 5-year OS rate of 55.9% (95% CI: 40.6–71.1) compared to 25.0% (95% CI: 13.7–36.3) in the chest CT scan-only group (
p = 0.003). The analysis revealed a significantly superior 5-year OS in patients who underwent TEMLA-based restaging and subsequently qualified for pulmonary resection (
Figure 2).
This difference was particularly pronounced in pneumonectomy cases, as lobectomy did not demonstrate statistical significance. These findings contradict those reported by Albain and Van Meerbeck [
20,
21]. In our opinion, poor results from pneumonectomy were probably due to technical problems or the lack of experience of the surgeons who performed these procedures. In the other publications on the role of pneumonectomy, 90-day mortality was 2–4%. There is no doubt that pneumonectomy is a demanding procedure for patients; however, this option should be considered if this is the only way to achieve a curative resection [
26,
27,
28].
Surprisingly, we observed a higher 5-year OS among patients who underwent more extensive surgery, namely pneumonectomy, than among those who underwent lobectomy. Although the difference was not statistically significant, it suggests the value of pneumonectomy in the treatment of patients with NSCLC following neoadjuvant therapy.
Further analysis revealed that the statistically significant superiority of TEMLA restaging was notable among younger patients (<60 years) with squamous cell carcinoma and R0 resection. However, no survival benefit was observed in women, patients over 60 years old, those who underwent non-radical operations (R1-2), and other histological types such as adenocarcinoma and not otherwise specified (NOS). It is important to note that these subgroups were small, rendering the analysis underpowered. Nevertheless, the lack of a survival benefit in non-radically operated patients (R1-2) underscores the importance of maximizing preoperative imaging with available methods to minimize the selection of patients for surgical treatment that would not derive significant benefits. In our opinion, TEMLA is one such method that can contribute to this goal. Based on our findings, we propose that surgical treatment for stage IIIA-IIIB (N2) NSCLC should only be offered to patients who demonstrate downstaging of ipsilateral metastatic mediastinal lymph nodes following neoadjuvant therapy, while it should be avoided in cases without nodular regression.
Another intriguing observation was the marked difference in 5-year OS between patients who underwent noninvasive restaging and TEMLA restaging with R0 radical resections. This difference (25.0% vs. 55.9%) suggests a potential therapeutic effect of bilateral extended lymphadenectomy (TEMLA). However, as chest CT was more frequently used in the TEMLA restaging group, this association could not be definitively proven. This observation underscores the need for further studies to explore potential therapeutic effects.
In conclusion, invasive mediastinal lymph node restaging improves the selection of patients with stage IIIA-IIIB (N2) NSCLC after neoadjuvant therapy who would benefit from a multidisciplinary treatment approach, including surgery. Our study highlights the importance of employing more advanced restaging techniques, such as TEMLA, to accurately assess the response to neoadjuvant therapy and guide appropriate treatment decisions. Further research is necessary to confirm these findings and to elucidate the potential therapeutic benefits of bilateral extended lymphadenectomy in this patient population.
5. Conclusions
Invasive mediastinal lymph node restaging, specifically through techniques such as TEMLA, improves the selection of patients with stage IIIA-IIIB (N2) NSCLC who would benefit from a multidisciplinary treatment approach, including surgery after neoadjuvant therapy.
Patients who demonstrate downstaging of ipsilateral metastatic mediastinal lymph nodes following neoadjuvant therapy are more likely to have better 5-year overall survival rates when undergoing surgical intervention.
Further research is needed to confirm these findings and explore the potential therapeutic benefits of bilateral extended lymphadenectomy (TEMLA) in this specific patient population.
Only patients who benefited from induction treatment, downstaging in the area of mediastinal lymph nodes, benefited from surgical treatment.
Author Contributions
Conceptualization, R.K. and M.Z.; methodology, R.K.; software, J.P.; validation, A.S.; formal analysis, J.G.; investigation, R.K.; resources, M.Z.; data curation, J.P.; writing—original draft preparation, R.K. and M.Z.; writing—review and editing, R.K., M.Z., A.S. and J.G.; visualization, M.Z.; supervision, R.K.; project administration, J.P.; funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Bioethics Committee of the Oncology Center in Gliwice (KB/493-29/11).
Informed Consent Statement
Patient consent was not required, as it was a retrospective analysis based on medical records.
Data Availability Statement
All data are contained within this article.
Acknowledgments
We are grateful to Michael Clark for reviewing this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, G.; Etxeberria, J.; Hao, Y. Global patterns and trends in lung cancer incidence: A population-based study. J. Thorac. Oncol. 2021, 16, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Petrella, F.; Rizzo, S.; Attili, I.; Passaro, A.; Zilli, T.; Martucci, F.; Bonomo, L.; Del Grande, F.; Casiraghi, M.; De Marinis, F.; et al. Stage III Non-Small-Cell Lung Cancer: An Overview of Treatment Options. Curr. Oncol. 2023, 30, 3160–3175. [Google Scholar] [CrossRef] [PubMed]
- Glatzer, M.; Leskow, P.; Caparrotti, F.; Elicin, O.; Furrer, M.; Gambazzi, F.; Dutly, A.; Gelpke, H.; Guckenberger, M.; Heuberger, J.; et al. Stage III N2 non-small cell lung cancer treatment: Decision-making among surgeons and radiation oncologists. Transl. Lung Cancer Res. 2021, 10, 1960–1968. [Google Scholar] [CrossRef]
- Watanabe, S.-I.; Nakagawa, K.; Suzuki, K.; Takamochi, K.; Ito, H.; Okami, J.; Aokage, K.; Saji, H.; Yoshioka, H.; Zenke, Y.; et al. Neoadjuvant and adjuvant therapy for Stage III non-small cell lung cancer. Ultrasound Med. Biol. 2017, 47, 1112–1118. [Google Scholar] [CrossRef]
- Heineman, D.J.; Beck, N.; Wouters, M.W.; van Brakel, T.J.; Daniels, J.M.; Schreurs, W.H.; Dickhoff, C. The dutch national clinical audit for lung cancer: A tool to improve clinical practice? An analysis of unforeseen ipsilateral mediastinal lymph node involvement in the Dutch Lung Surgery Audit (DLSA). Eur. J. Surg. Oncol. (EJSO) 2018, 44, 830–834. [Google Scholar] [CrossRef]
- De Leyn, P.; Dooms, C.; Kuzdzal, J.; Lardinois, D.; Passlick, B.; Rami-Porta, R.; Turna, A.; Schil, P.V.; Venuta, F.; Waller, D.; et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2014, 45, 787–798. [Google Scholar] [CrossRef]
- Gwóźdź, P.; Zieliński, M. Transcervical extended mediastinal lymphadenectomy for mediastinal restaging after induction therapy. Mediastinum 2019, 3, 400. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, F.; Zhu, R.; Wu, D.; Ding, Y.; Zhang, Z.; Gao, Y.; Wan, Y. Application of computed tomography, positron emission tomography-computed tomography, magnetic resonance imaging, endobronchial ultrasound, and mediastinoscopy in the diagnosis of mediastinal lymph node staging of non-small-cell lung cancer. Medicine 2020, 99, e19314. [Google Scholar] [CrossRef]
- von Bartheld, M.; van Breda, A.; Annema, J. Complication Rate of Endosonography (Endobronchial and Endoscopic Ultrasound): A Systematic Review. Respiration 2014, 87, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Bousema, J.E.; MEDIASTrial study group; Dijkgraaf, M.G.W.; Papen-Botterhuis, N.E.; Schreurs, H.W.; Maessen, J.G.; van der Heijden, E.H.; Steup, W.H.; Braun, J.; Noyez, V.J.J.M.; et al. MEDIASTinal staging of non-small cell lung cancer by endobronchial and endoscopic ultrasonography with or without additional surgical mediastinoscopy (MEDIASTrial): Study protocol of a multicenter randomised controlled trial. BMC Surg. 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Call, S.; Obiols, C.; Rami-Porta, R. Present indications of surgical exploration of the mediastinum. J. Thorac. Dis. 2018, 10, S2601–S2610. [Google Scholar] [CrossRef] [PubMed]
- Hartert, M.; Tripsky, J.; Huertgen, M. Video-assisted mediastinoscopic lymphadenectomy (VAMLA) for staging & treatment of non-small cell lung cancer (NSCLC). Mediastinum 2020, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, M.; Szlubowski, A.; Kołodziej, M.; Orzechowski, S.; Laczynska, E.; Pankowski, J.; Jakubiak, M.; Obrochta, A. Comparison of Endobronchial Ultrasound and/or Endoesophageal Ultrasound with Transcervical Extended Mediastinal Lymphadenectomy for Staging and Restaging of Non–Small-Cell Lung Cancer. J. Thorac. Oncol. 2013, 8, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Conte, S.C.; Spagnol, G.; Biolo, M.; Confalonieri, M. A retrospective study of endobronchial ultrasound transbronchial needle aspiration versus conventional transbronchial needle aspiration in diagnosis/staging of hilar/mediastinal lymph node in lung cancer: Which role in clinical practice? Monaldi Arch. Chest Dis. 2019, 89. [Google Scholar] [CrossRef]
- Nomori, H.; Watanabe, K.; Ohtsuka, T.; Naruke, T.; Suemasu, K.; Uno, K. The size of metastatic foci and lymph nodes yielding false-negative and false-positive lymph node staging with positron emission tomography in patients with lung cancer. J. Thorac. Cardiovasc. Surg. 2004, 127, 1087–1092. [Google Scholar] [CrossRef]
- Glover, J.; Velez-Cubian, F.O.; Toosi, K.; Ng, E.; Moodie, C.C.; Garrett, J.R.; Fontaine, J.P.; Toloza, E.M. Perioperative outcomes and lymph node assessment after induction therapy in patients with clinical N1 or N2 non-small cell lung cancer. J. Thorac. Dis. 2016, 8, 2165–2174. [Google Scholar] [CrossRef]
- Van Meerbeeck, J.P.; Kramer, G.W.; Van Schil, P.E.; Legrand, C.; Smit, E.F.; Schramel, F.; Tjan-Heijnen, V.C.; Biesma, B.; Debruyne, C.; Van Zandwijk, N.; et al. European Organisation for Research and Treatment of Cancer. Lung Cancer Group.Randomised controlled trail of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 nonsmall cell lung cancer. J. Natl. Cancer Inst. 2007, 99, 442–450. [Google Scholar] [CrossRef]
- Albain, K.S.; Swann, R.S.; Rusch, V.W.; Turrisi, A.T.; Shepherd, F.A.; Smith, C.; Chen, Y.; Livingston, R.B.; Feins, R.H.; Gandara, D.R.; et al. Radiotherapy plus Chemotherapy with or without Surgical Resection for Stage III Non-Small Cell Lung Cancer. Lancet 2009, 374, 379–386. [Google Scholar] [CrossRef]
- Yendamuri, S.; Battoo, A.; Dy, G.; Chen, H.; Gomez, J.; Singh, A.K.; Hennon, M.; Nwogu, C.E.; Dexter, E.U.; Huang, M.; et al. Transcervical Extended Mediastinal Lymphadenectomy: Experience From a North American Cancer Center. Ann. Thorac. Surg. 2017, 104, 1644–1649. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muriana, P.; Rossetti, F. The role of EBUS-TBNA in lung cancer restaging and mutation analysis. Mediastinum 2020, 4, 23. [Google Scholar] [CrossRef]
- Castello, A.; Rossi, S.; Lopci, E. 18F-FDG PET/CT in Restaging and Evaluation of Response to Therapy in Lung Cancer: State of the Art. Curr. Radiopharm. 2020, 13, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Szlubowski, A.; Ski, M.Z.; Soja, J.; Filarecka, A.; Orzechowski, S.; Pankowski, J.; Obrochta, A.; Jakubiak, M.; Grzyn, J.W.; Miel, A. Accurate and safe mediastinal restaging by combined endobronchial and endoscopic ultrasound-guided needle aspiration performed by single ultrasound bronchoscope. Eur. J. Cardio-Thorac. Surg. 2014, 46, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, M.; Guarize, J.; Sandri, A.; Maisonneuve, P.; Brambilla, D.; Romano, R.; Galetta, D.; Petrella, F.; Gasparri, R.; Gridelli, C.; et al. Pneumonectomy in Stage IIIA-N2 NSCLC: Should It Be Considered After Neoadjuvant Chemotherapy? Clin. Lung Cancer 2019, 20, 97–106. [Google Scholar] [CrossRef]
- Weder, W.; Collaud, S.; Eberhardt, W.E.; Hillinger, S.; Welter, S.; Stahel, R.; Stamatis, G. Pneumonectomy is a valuable treatment option after neoadjuvant therapy for stage III non–small-cell lung cancer. J. Thorac. Cardiovasc. Surg. 2010, 139, 1424–1430. [Google Scholar] [CrossRef]
- Skrzypczak, P.J.; Roszak, M.; Kasprzyk, M.; Kopczyńska, A.; Gabryel, P.; Dyszkiewicz, W. Pneumonectomy–permanent injury or still effective method of treatment? Early and long-term results and quality of life after pneumonectomy due to non- small cell lung cancer. Pol. J. Cardio-Thorac. Surg. 2019, 16, 7–12. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).