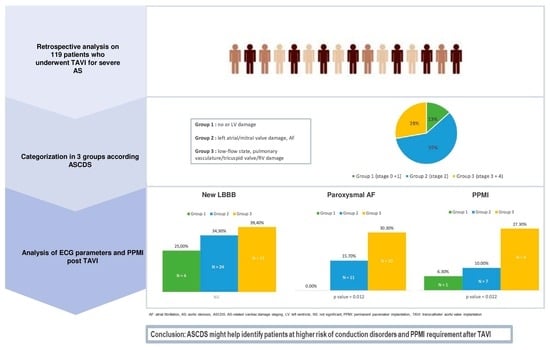
Cardiac Damage and Conduction Disorders after Transcatheter Aortic Valve Implantation
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Echocardiography and Cardiac Staging
2.3. TAVI Procedure and ECG Assessment
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Staging and ECG Outcomes after TAVI
3.3. Predictors of PPMI and In-Hospital Mortality
4. Discussion
4.1. TAVI-Related ECG Changes
4.2. Cardiac Damage and Post-TAVI Conduction Disorders
4.3. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosseel, L.; Mylotte, D.; Cosyns, B.; Vanhaverbeke, M.; Zweiker, D.; Teles, R.C.; Angerås, O.; Neylon, A.; Rudolph, T.K.; Wykrzykowska, J.J.; et al. Contemporary European Practice in Transcatheter Aortic Valve Implantation: Results from the 2022 European TAVI Pathway Registry. Front. Cardiovasc. Med. 2023, 10, 1227217. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Karyofillis, P.; Kostopoulou, A.; Thomopoulou, S.; Habibi, M.; Livanis, E.; Karavolias, G.; Voudris, V. Conduction Abnormalities after Transcatheter Aortic Valve Implantation. J. Geriatr. Cardiol. JGC 2018, 15, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Auffret, V.; Puri, R.; Urena, M.; Chamandi, C.; Rodriguez-Gabella, T.; Philippon, F.; Rodés-Cabau, J. Conduction Disturbances after Transcatheter Aortic Valve Replacement. Circulation 2017, 136, 1049–1069. [Google Scholar] [CrossRef] [PubMed]
- Mangieri, A.; Montalto, C.; Pagnesi, M.; Lanzillo, G.; Demir, O.; Testa, L.; Colombo, A.; Latib, A. TAVI and Post Procedural Cardiac Conduction Abnormalities. Front. Cardiovasc. Med. 2018, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Scarsini, R.; De Maria, G.L.; Joseph, J.; Fan, L.; Cahill, T.J.; Kotronias, R.A.; Burzotta, F.; Newton, J.D.; Kharbanda, R.; Prendergast, B.; et al. Impact of Complications during Transfemoral Transcatheter Aortic Valve Replacement: How Can They Be Avoided and Managed? J. Am. Heart Assoc. 2019, 8, e013801. [Google Scholar] [CrossRef] [PubMed]
- Sammour, Y.; Krishnaswamy, A.; Kumar, A.; Puri, R.; Tarakji, K.G.; Bazarbashi, N.; Harb, S.; Griffin, B.; Svensson, L.; Wazni, O.; et al. Incidence, Predictors, and Implications of Permanent Pacemaker Requirement after Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2021, 14, 115–134. [Google Scholar] [CrossRef]
- Auffret, V.; Webb, J.G.; Eltchaninoff, H.; Muñoz-García, A.J.; Himbert, D.; Tamburino, C.; Nombela-Franco, L.; Nietlispach, F.; Morís, C.; Ruel, M.; et al. Clinical Impact of Baseline Right Bundle Branch Block in Patients Undergoing Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2017, 10, 1564–1574. [Google Scholar] [CrossRef]
- Van Rosendael, P.J.; Delgado, V.; Bax, J.J. Pacemaker Implantation Rate after Transcatheter Aortic Valve Implantation with Early and New-Generation Devices: A Systematic Review. Eur. Heart J. 2018, 39, 2003–2013. [Google Scholar] [CrossRef]
- Lenders, G.D.; Collas, V.; Hernandez, J.M.; Legrand, V.; Danenberg, H.D.; den Heijer, P.; Rodrigus, I.E.; Paelinck, B.P.; Vrints, C.J.; Bosmans, J.M. Depth of Valve Implantation, Conduction Disturbances and Pacemaker Implantation with CoreValve and CoreValve Accutrak System for Transcatheter Aortic Valve Implantation, a Multi-Center Study. Int. J. Cardiol. 2014, 176, 771–775. [Google Scholar] [CrossRef]
- Prihadi, E.A.; Leung, M.; Vollema, E.M.; Ng, A.C.T.; Ajmone Marsan, N.; Bax, J.J.; Delgado, V. Prevalence and Prognostic Relevance of Ventricular Conduction Disturbances in Patients with Aortic Stenosis. Am. J. Cardiol. 2017, 120, 2226–2232. [Google Scholar] [CrossRef] [PubMed]
- Sebag, F.A.; Lellouche, N.; Chaachoui, N.; Dubois-Rande, J.-L.; Monin, J.-L. Prevalence and Clinical Impact of QRS Duration in Patients with Low-flow/Low-gradient Aortic Stenosis Due to Left Ventricular Systolic Dysfunction. Eur. J. Heart Fail. 2014, 16, 639–647. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1002/ejhf.63 (accessed on 19 November 2023). [CrossRef] [PubMed]
- Dhingra, R.C.; Amat-y-Leon, F.; Pietras, R.J.; Wyndham, C.; Deedwania, P.C.; Wu, D.; Denes, P.; Rosen, K.M. Sites of Conduction Disease in Aortic Stenosis: Significance of Valve Gradient and Calcification. Ann. Intern. Med. 1977, 87, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Pibarot, P.; Redfors, B.; Mack, M.J.; Makkar, R.R.; Jaber, W.A.; Svensson, L.G.; Kapadia, S.; Tuzcu, E.M.; Thourani, V.H.; et al. Staging Classification of Aortic Stenosis Based on the Extent of Cardiac Damage. Eur. Heart J. 2017, 38, 3351–3358. [Google Scholar] [CrossRef] [PubMed]
- Tastet, L.; Tribouilloy, C.; Maréchaux, S.; Vollema, E.M.; Delgado, V.; Salaun, E.; Shen, M.; Capoulade, R.; Clavel, M.-A.; Arsenault, M.; et al. Staging Cardiac Damage in Patients with Asymptomatic Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2019, 74, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Viva, T.; Postolache, A.; Nguyen Trung, M.L.; Danthine, P.; Petitjean, H.; Bruno, V.D.; Martinez, C.; Lempereur, M.; Guazzi, M.; Aghezzaf, S.; et al. A New Integrative Approach Combining Right Heart Catheterization and Echocardiography to Stage Aortic Stenosis-Related Cardiac Damage. Front. Cardiovasc. Med. 2023, 10, 1184308. [Google Scholar] [CrossRef]
- Faroux, L.; Chen, S.; Muntané-Carol, G.; Regueiro, A.; Philippon, F.; Sondergaard, L.; Jørgensen, T.H.; Lopez-Aguilera, J.; Kodali, S.; Leon, M.; et al. Clinical Impact of Conduction Disturbances in Transcatheter Aortic Valve Replacement Recipients: A Systematic Review and Meta-Analysis. Eur. Heart J. 2020, 41, 2771–2781. [Google Scholar] [CrossRef]
- Kim, K.; Ko, Y.-G.; Shim, C.Y.; Ryu, J.; Lee, Y.-J.; Seo, J.; Lee, S.-J.; Cho, I.; Hong, S.-J.; Ahn, C.-M.; et al. Impact of New-Onset Persistent Left Bundle Branch Block on Reverse Cardiac Remodeling and Clinical Outcomes after Transcatheter Aortic Valve Replacement. Front. Cardiovasc. Med. 2022, 9, 893878. [Google Scholar] [CrossRef]
- Jørgensen, T.H.; De Backer, O.; Gerds, T.A.; Bieliauskas, G.; Svendsen, J.H.; Søndergaard, L. Mortality and Heart Failure Hospitalization in Patients with Conduction Abnormalities after Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 52–61. [Google Scholar] [CrossRef]
- Wang, B.; Mei, Z.; Ge, X.; Li, Y.; Zhou, Q.; Meng, X.; An, G. Comparison of Outcomes of Self-Expanding versus Balloon-Expandable Valves for Transcatheter Aortic Valve Replacement: A Meta-Analysis of Randomized and Propensity-Matched Studies. BMC Cardiovasc. Disord. 2023, 23, 382. [Google Scholar] [CrossRef]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Aladham, A.; Gada, H.; Wang, Y.; Mumtaz, M.; Sultan, I.; Mulukutla, S.; Vora, A.N. Incidence of Permanent Pacemaker Implantation Using the Cusp Overlap Technique. JACC Cardiovasc. Interv. 2022, 15, 1006–1008. [Google Scholar] [CrossRef] [PubMed]
- Szotek, M.; Drużbicki, Ł.; Sabatowski, K.; Amoroso, G.R.; Schouwer, K.D.; Matusik, P.T. Transcatheter Aortic Valve Implantation and Cardiac Conduction Abnormalities: Prevalence, Risk Factors and Management. J. Clin. Med. 2023, 12, 6056. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, A.; Abdul-Jawad Altisent, O.; Del Trigo, M.; Campelo-Parada, F.; Puri, R.; Urena, M.; Philippon, F.; Rodés-Cabau, J. Impact of New-Onset Left Bundle Branch Block and Periprocedural Permanent Pacemaker Implantation on Clinical Outcomes in Patients Undergoing Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Interv. 2016, 9, e003635. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, S.; Han, X.; Chen, Y.; Chen, H.; Wan, Z.; Song, B. Prognostic Outcome of New-Onset Left Bundle Branch Block after Transcatheter Aortic Valve Replacement in Patients with Aortic Stenosis: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 842929. [Google Scholar] [CrossRef]
- Chamandi, C.; Barbanti, M.; Munoz-Garcia, A.; Latib, A.; Nombela-Franco, L.; Gutiérrez-Ibanez, E.; Veiga-Fernandez, G.; Cheema, A.N.; Cruz-Gonzalez, I.; Serra, V.; et al. Long-Term Outcomes in Patients with New-Onset Persistent Left Bundle Branch Block Following TAVR. JACC Cardiovasc. Interv. 2019, 12, 1175–1184. [Google Scholar] [CrossRef]
- Avvedimento, M.; Franzone, A.; Leone, A.; Piccolo, R.; Castiello, D.S.; Ilardi, F.; Mariani, A.; Esposito, R.; Iapicca, C.; Angellotti, D.; et al. Extent of Cardiac Damage and Mortality in Patients Undergoing Transcatheter Aortic Valve Implantation. J. Clin. Med. 2021, 10, 4563. [Google Scholar] [CrossRef]
- Pellegrini, C.; Duesmann, C.; Rheude, T.; Berg, A.; Alvarez-Covarrubias, H.A.; Trenkwalder, T.; Mayr, N.P.; Schürmann, F.; Nicol, P.; Xhepa, E.; et al. The Impact of Extra-Valvular Cardiac Damage on Mid-Term Clinical Outcome Following Transcatheter Aortic Valve Replacement in Patients with Severe Aortic Stenosis. Front. Cardiovasc. Med. 2022, 9, 1039208. [Google Scholar] [CrossRef]
- Mills, R.W.; Wright, A.T.; Narayan, S.M.; McCulloch, A.D. The Effects of Wall Stretch on Ventricular Conduction and Refractoriness in the Whole Heart. In Cardiac Mechano-Electric Coupling and Arrhythmias; Kohl, P., Sachs, F., Franz, M.R., Eds.; Oxford University Press: Oxford, UK, 2011; ISBN 978-0-19-957016-4. [Google Scholar]

| Total | Group 1 | Group 2 | Group 3 | p-Value | |
|---|---|---|---|---|---|
| Clinical characteristics | |||||
| Number of patients (%) | 119 (100%) | 16 (13.5%) | 70 (58.8%) | 33 (27.7%) | |
| Age (years) | 84 (79–86) | 78 (73.5–84.5) | 83.5 (79–87) | 85 (81–87) | 0.017 |
| Male, (%) | 64 (53.78) | 9 (56.3) | 37 (52.9) | 18 (54.6) | 0.97 |
| Body mass index, kg/m2 | 25.51 (23.2–29.6) | 27.1 (23.3–29.5) | 26.4 (23.1–30.1) | 24.9 (23.5–26.9) | 0.58 |
| Current smoker (%) | 45 (37.8) | 8 (50) | 26 (37.1) | 11 (33.3) | 0.30 |
| Hypertension n (%) | 106 (89.1) | 14 (87.5) | 62 (88.6) | 30 (90.9) | 0.82 |
| Diabetes (%) | 35 (29.4) | 6 (37.5) | 20 (28.6) | 9 (27.3) | 0.52 |
| Dyslipidemia (%) | 96 (80.7) | 14 (87.5) | 55 (78.6) | 27 (81.8) | 0.86 |
| Atrial fibrillation (%) | 48 (40.3) | 3 (18.8) | 25 (35.7) | 20 (60.6) | 0.003 |
| Stroke (%) | 22 (18.5) | 0 (0) | 15 (21.4) | 7 (21.2) | 0.19 |
| Prior peripheral vascular disease (%) | 59 (49.6) | 7 (43.8) | 37 (52.9) | 15 (45.5) | 0.90 |
| Coronary artery disease (%) | 53 (44.5) | 8 (50) | 29 (41.4) | 16 (48.5) | 0.89 |
| Chronic kidney diseases (eGFR < 45 mL/min) | 50 (42.0) | 5 (31.3) | 29 (41.4) | 16 (48.5) | 0.25 |
| Medical therapy | |||||
| Beta-blockers (%) | 84 (70.6) | 9 (56.3) | 49 (70) | 26 (78.8) | 0.11 |
| Calcium antagonists (%) | 28 (23.5) | 4 (25) | 16 (22.9) | 8 (24.2) | 1.00 |
| Amiodarone (%) | 21 (17.7) | 2 (12.5) | 12 (17.1) | 7 (21.2) | 0.57 |
| Procedure | |||||
| Euroscore II (%) | 3.98 (2.51–6.02) | 3.92 (2.8–4.9) | 3.64 (2.35–6.06) | 4.8 (2.8–11.27) | 0.24 |
| Calcium score | 2471.45 (1652–3768) | 1582 (869–3197) | 2990 (1853–4111) | 2211 (1855–3117) | 0.10 |
| Pre-dilatation (%) | 38 (31.9) | 3 (18.8) | 23 (32.9) | 12 (36.4) | 0.28 |
| Post-dilatation (%) | 15 (12.6) | 1 (6.3) | 12 (17.1) | 2 (6.1) | 0.66 |
| Prosthesis size | 0.55 | ||||
| 23 mm (%) | 8 (6.72) | 0 (0) | 4 (5.7) | 4 (12.1) | |
| 26 mm (%) | 33 (27.7) | 5 (31.3) | 19 (27.1) | 9 (27.3) | |
| 29 mm (%) | 45 (37.8) | 7 (43.8) | 27 (38.6) | 11 (33.3) | |
| 34 mm (%) | 33 (27.7) | 4 (25) | 20 (28.6) | 9 (27.3) | |
| Approach (%) | 0.32 | ||||
| Femoral | 103 (86.6) | 14 (87.5) | 60 (85.7) | 29 (87.9) | |
| Trans-Axillary | 15 (12.6) | 2 (12.5) | 10 (14.3) | 3 (9.1) | |
| Carotid | 1 (0.84) | 0 (0) | 0 (0) | 1 (3) | |
| General anesthesia (%) | 0.42 | ||||
| General | 80 (67.2) | 7 (43.8) | 52 (74.3) | 21 (63.6) | |
| Local anesthesia + sedation | 39 (32.8) | 9 (56.3) | 18 (25.7) | 12 (36.4) | |
| Procedure time(min) | 122 (101–140) | 123.5 (102.5–132.5) | 123 (102–140) | 115 (99–147) | 0.84 |
| Electrocardiogram | |||||
| Heart rate (bpm) | 71 (62–81) | 73.5 (65–85) | 69 (60–80) | 72 (62–81) | 0.59 |
| Rhythm | <0.0001 | ||||
| Sinus (%) | 94 (78.99) | 16 (100) | 61 (87.1) | 17 (51.5) | |
| Atrial fibrillation (%) | 25 (21.01) | 0 (0) | 9 (12.9) | 16 (48.5) | |
| PR interval (ms) | 180 (164–204) | 165 (161–177) | 180 (164–204) | 202 (180–214) | 0.020 |
| <200 ms (%) | 63 (67.0) | 15 (93.8) | 40 (57.1) | 8 (24.2) | |
| 200–239 ms (%) | 20 (21.3) | 1 (6.3) | 13 (18.6) | 6 (18.2) | |
| ≥240 ms (%) | 11 (11.70) | 0 (0) | 8 (11.4) | 3 (9.1) | |
| QRS (ms) | 96 (84–112) | 89 (78–98) | 96 (86–119) | 94 (84–110) | 0.13 |
| <120 ms (%) | 96 (80.7) | 14 (87.5) | 54 (77.1) | 28 (84.9) | |
| 120–149 ms (%) | 17 (14.3) | 2 (12.5) | 10 (14.3) | 3 (15.1) | |
| ≥150 ms (%) | 6 (5.04) | 0 (0) | 6 (8.6) | 0 (0) | |
| Prior bundle branch block | 0.47 | ||||
| Left (%) | 10 (8.4) | 1 (6.3) | 7 (10) | 2 (6.1) | |
| Right (%) | 6 (5.04) | 1 (6.3) | 5 (7.1) | 0 (0) |
| Total | Group 1 n = 16 (13.5%) | Group 2 n = 70 (58.8%) | Group 3 n = 33 (27.7%) | p-Value | |
|---|---|---|---|---|---|
| Electrocardiogram | |||||
| Heart rate (bpm) | 73 (65–86) | 72 (61–95) | 72 (65–81) | 79 (67.5–88.5) | 0.41 |
| Rhythm | |||||
| Atrial fibrillation (%) | 25 (21.7) | 0 (0) | 9 (12.9) | 16 (48.5) | <0.0001 |
| PR interval (ms) | 190 (166–214) | 170 (162–194) | 192 (172–215) | 214 (168–226) | 0.052 |
| <200 ms (%) | 55 (63.2) | 13 (81.3) | 36 (51.4) | 6 (18.2) | |
| 200–239 ms (%) | 19 (21.8) | 2 (12.5) | 11 (15.7) | 3 (18.2) | |
| ≥240 ms (%) | 13 (14.9) | 1 (6.3) | 9 (12.9) | 3 (9.1) | |
| Delta PR | 8 (−6–20) | 4 (−3–23) * | 8 (−6–18) * | 2 (−22–26) | 0.71 |
| QRS (ms) | 122 (96–140) | 101 (88–129) | 122 (94–140) | 124.5 (103–142) | 0.13 |
| <120 ms (%) | 54 (47.4) | 10 (62.5) | 30 (42.9) | 14 (42.4) | |
| 120–149 ms (%) | 42 (36.8) | 6 (37.5) | 23 (32.9) | 13 (39.4) | |
| ≥150 ms (%) | 18 (15.8) | 0 (0) | 13 (18.6) | 5 (15.2) | |
| Delta QRS | 8 (2–36) | 11 (6–24) * | 5 (0–33) * | 19 (4–54) * | 0.082 |
| New bundle branch block | 0.17 | ||||
| Left (%) | 41 (34.5) | 4 (25) | 24 (34.3) | 13 (39.4) | |
| Right (%) | 3 (2.52) | 0 (0) | 1 (1.4) | 2 (6.1) | |
| Complications | |||||
| Paroxysmal atrial fibrillation (%) | 21 (17.7) | 0 (0) | 11 (15.7) | 10 (30.3) | 0.012 |
| High degree AV block (%) | 12 (10) | 0 (0) | 7 (10) | 5 (15) | 0.38 |
| Permanent pacemaker implantation (%) | 17 (14.3) | 1 (6.3) | 7 (10) | 9 (27.3) | 0.022 |
| Stroke (%) | 8 (6.7) | 1 (6.3) | 4 (5.7) | 3 (9.1) | 0.32 |
| Death (%) | 6 (5) | 0 (0) | 4 (5.7) | 2 (6.1) | 0.52 |
| Variables | OR (95% CI) | p-Value | Units |
|---|---|---|---|
| Local vs. general anesthesia | 2.77 (1.11–6.89) | 0.028 | |
| Heart rate ECG pre-TAVI | 0.96 (0.93–1) | 0.047 | 1 beat |
| Duration QRS ECG pre-TAVI | 1.16 (1–1.34) | 0.051 | 10 ms |
| PR pre-TAVI 200–239 ms vs. <200 ms | 3.35 (0.97–11.59) | 0.056 | |
| Per-procedure: AV block 2 type 2 vs. No AV block | 142.6 (8.71–2333.5) | 0.0005 | |
| Per-procedure: AV block 3 vs. No AV block | 6.2 (2.19–17.54) | 0.0006 | |
| Prosthesis size 34 vs. 29 | 5.27 (1.45–19.17) | 0.012 | |
| Atrial fibrillation | 2.95 (1.16–7.51) | 0.023 | |
| Heart rate ECG post-TAVI | 0.97 (0.94–1) | 0.082 | 1 beat |
| Interval PR ECG post-TAVI | 1.20 (1.06–1.36) | 0.004 | 10 ms |
| Delta PR | 1.02 (1–1.04) | 0.11 | 1 ms |
| Duration QRS ECG post-TAVI | 1.55 (1.26–1.91) | <0.0001 | 10 ms |
| Delta QRS | 1.03 (1.01–1.05) | 0.001 | 1 ms |
| ECG post-TAVI: left bundle-branch block vs. No bundle-branch block | 5.76 (1.62–20.44) | 0.007 | |
| ECG post-TAVI: right bundle-branch block vs. No bundle-branch block | 7.15 (1.44–35.49) | 0.016 | |
| PR post-TAVI < 200 ms vs. ≥240 ms | 0.13 (0.02–0.79) | 0.027 | |
| PR post-TAVI 200–239 ms vs. <200 ms | 6.59 (1.2–35.99) | 0.030 | |
| QRS post-TAVI ≥ 150 ms vs. <120 ms | 16.97 (4.55–63.29) | <0.0001 | |
| QRS post-TAVI 120–149 ms vs. ≥150 ms | 0.17 (0.06–0.48) | 0.0009 | |
| Post-TAVI: AV block 3 vs. No AV block | 9.95 (3.67–26.92) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damas, F.; Nguyen Trung, M.-L.; Postolache, A.; Petitjean, H.; Lempereur, M.; Viva, T.; Oury, C.; Dulgheru, R.; Lancellotti, P. Cardiac Damage and Conduction Disorders after Transcatheter Aortic Valve Implantation. J. Clin. Med. 2024, 13, 409. https://doi.org/10.3390/jcm13020409
Damas F, Nguyen Trung M-L, Postolache A, Petitjean H, Lempereur M, Viva T, Oury C, Dulgheru R, Lancellotti P. Cardiac Damage and Conduction Disorders after Transcatheter Aortic Valve Implantation. Journal of Clinical Medicine. 2024; 13(2):409. https://doi.org/10.3390/jcm13020409
Chicago/Turabian StyleDamas, François, Mai-Linh Nguyen Trung, Adriana Postolache, Hélène Petitjean, Mathieu Lempereur, Tommaso Viva, Cécile Oury, Raluca Dulgheru, and Patrizio Lancellotti. 2024. "Cardiac Damage and Conduction Disorders after Transcatheter Aortic Valve Implantation" Journal of Clinical Medicine 13, no. 2: 409. https://doi.org/10.3390/jcm13020409
APA StyleDamas, F., Nguyen Trung, M.-L., Postolache, A., Petitjean, H., Lempereur, M., Viva, T., Oury, C., Dulgheru, R., & Lancellotti, P. (2024). Cardiac Damage and Conduction Disorders after Transcatheter Aortic Valve Implantation. Journal of Clinical Medicine, 13(2), 409. https://doi.org/10.3390/jcm13020409