Innovative Approaches to Monitor Central Line Associated Bloodstream Infections (CLABSIs) Bundle Efficacy in Intensive Care Unit (ICU): Role of Device Standardized Infection Rate (dSIR) and Standardized Utilization Ratio (SUR)—An Italian Experience
Abstract
1. Introduction
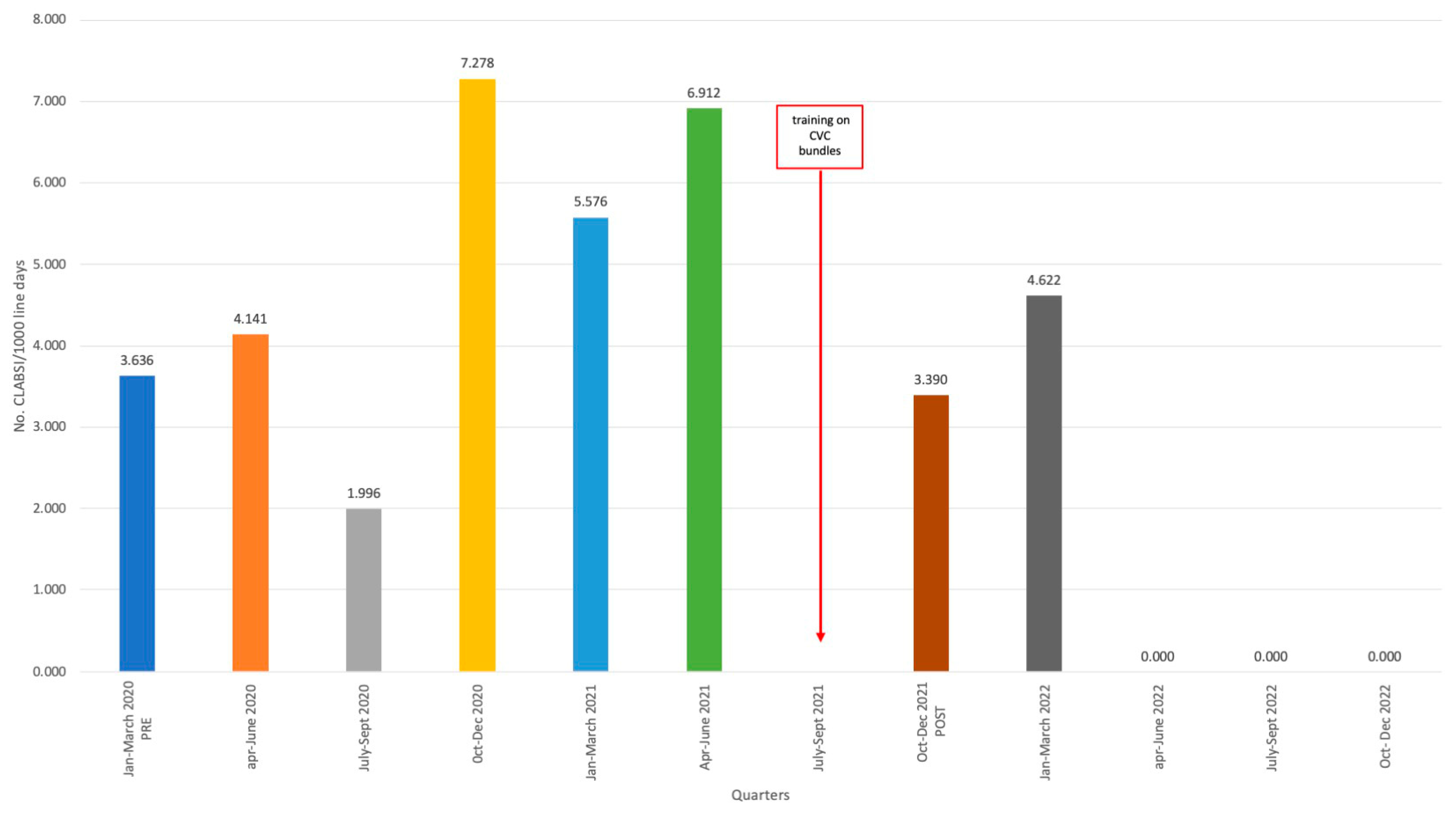
2. Results
Intervention
3. Discussion
3.1. Limitations
3.2. Future Implications
4. Materials and Methods
4.1. Population
4.2. Description of Training on CVC Bundles
- device insertion,
- guided ultrasound procedure,
- surgical hand washing aseptic technique,
- skin antisepsis with 2% chlorhexidine in 70% isopropyl alcohol,
- use of sutureless fixation device,
- management,
- hand washing with alcohol solution before and after using the catheter,
- hub scrub with chlorhexidine,
- keep the dressing intact and replace it every 7 days,
- remove as soon as possible.
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HAIs | Healthcare-associated infections |
| ICUs | intensive care units |
| MDROs | multidrug-resistant organisms |
| CLABSIs | central lines associated bloodstream infections |
| COVID-19 | coronavirus disease |
| SIR | Standardized Infection Rate |
| SUR | Standardized Utilization Rate |
| dSIR | Device Standardized Infection Rate |
| CVCs | central venous catheters |
| DUR | Device Utilization Rate |
| pSIR | population Standardized Infection Rate. |
References
- Burchardi, H.; Schneider, H. Economic aspects of severe sepsis: A review of intensive care unit costs, cost of illness and cost effectiveness of therapy. PharmacoEconomics 2004, 22, 793–813. [Google Scholar] [CrossRef]
- Tiru, B.; DiNino, E.K.; Orenstein, A.; Mailloux, P.T.; Pesaturo, A.; Gupta, A.; McGee, W.T. The Economic and Humanistic Burden of Severe Sepsis. PharmacoEconomics 2015, 33, 925–937. [Google Scholar] [CrossRef]
- Mathur, P.; Malpiedi, P.; Walia, K.; Srikantiah, P.; Gupta, S.; Lohiya, A.; Chakrabarti, A.; Ray, P.; Biswal, M.; Taneja, N.; et al. EIndian Healthcare Associated Infection Surveillance Network collaborators. Health-care-associated bloodstream and urinary tract infections in a network of hospitals in India: A multicentre, hospital-based, prospective surveillance study. Lancet Glob. Health 2022, 10, e1317–e1325. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, C.; Polk, R.E. Antimicrobial stewardship programs in health care systems. Clin. Microbiol. Rev. 2005, 18, 638–656. [Google Scholar] [CrossRef] [PubMed]
- Bertollo, L.G.; Lutkemeyer, D.S.; Levin, A.S. Are antimicrobial stewardship programs effective strategies for preventing antibiotic resistance? A systematic review. Am. J. Infect. Control. 2018, 46, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Sticchi, C.; Alberti, M.; Artioli, S.; Assensi, M.; Baldelli, I.; Battistini, A.; Boni, S.; Cassola, G.; Castagnola, E.; Cattaneo, M.; et al. Collaborative Group for the Point Prevalence Survey of healthcare-associated infections in Liguria. Regional point prevalence study of healthcare-associated infections and antimicrobial use in acute care hospitals in Liguria, Italy. J. Hosp. Infect. 2018, 99, 8–16. [Google Scholar] [CrossRef]
- Gupta, P.; Thomas, M.; Patel, A.; George, R.; Mathews, L.; Alex, S.; John, S.; Simbulan, C.; Garcia, M.L.; Al-Balushi, S.; et al. Bundle approach used to achieve zero central line-associated bloodstream infections in an adult coronary intensive care unit. BMJ Open Qual. 2021, 10, e001200. [Google Scholar] [CrossRef]
- Pronovost, P.; Needham, D.; Berenholtz, S.; Sinopoli, D.; Chu, H.; Cosgrove, S.; Sexton, B.; Hyzy, R.; Welsh, R.; Roth, G.; et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N. Engl. J. Med. 2006, 355, 2725–2732. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Aceituno, A.; Vega-Costa, V.; Ruiz-Álvarez, M.; Figuerola-Tejerina, A.; Méndez-Hernández, R.; Ramasco-Rueda, F. Effectiveness of a bundle of measures for reducing central line-associated bloodstream infections. Rev. Esp. Anestesiol. Reanim. 2020, 67, 227–236. [Google Scholar] [CrossRef]
- Goldman, J.; Rotteau, L.; Shojania, K.G.; Baker, G.R.; Rowland, P.; Christianson, M.K.; Vogus, T.J.; Cameron, C.; Coffey, M. Implementation of a central-line bundle: A qualitative study of three clinical units. Implement. Sci. Commun. 2021, 2, 105. [Google Scholar] [CrossRef]
- Salama, M.F.; Jamal, W.; Al Mousa, H.; Rotimi, V. Implementation of central venous catheter bundle in an intensive care unit in Kuwait: Effect on central line-associated bloodstream infections. J. Infect. Public Health 2016, 9, 34–41. [Google Scholar] [CrossRef]
- Ray-Barruel, G.; Xu, H.; Marsh, N.; Cooke, M.; Rickard, C.M. Effectiveness of insertion and maintenance bundles in preventing peripheral intravenous catheter-related complications and bloodstream infection in hospital patients: A systematic review. Infect. Dis. Health 2019, 24, 152–168. [Google Scholar] [CrossRef]
- Barker, A.K.; Ngam, C.; Musuuza, J.S.; Vaughn, V.M.; Safdar, N. Reducing Clostridium difficile in the Inpatient Setting: A Systematic Review of the Adherence to and Effectiveness of C. difficile Prevention Bundles. Infect. Control. Hosp. Epidemiol. 2017, 38, 639–650. [Google Scholar] [CrossRef]
- Pettit, N.N.; Han, Z.; Nguyen, C.T.; Choksi, A.; Charnot-Katsikas, A.; Beavis, K.G.; Tesic, V.; Pisano, J. Antimicrobial Stewardship Review of Automated Candidemia Alerts Using the Epic Stewardship Module Improves Bundle-of-Care Adherence. Open Forum Infect. Dis. 2019, 6, ofz412. [Google Scholar] [CrossRef]
- Tripathi, S.; Pallotto, E.; Hord, J.; Staubach, K.; Sisso, P.; Mack, E.; Coffey, M. 1048: Association of bundle compliance with CLABSI rates: A solutions for patient safety (sps) report. Crit. Care Med. 2022, 51, 518. [Google Scholar] [CrossRef]
- Simoneaux, C.; Guerra, P. Implementation of Evidence-based Maintenance Bundles to Reduce Central Line-Associated Bloodstream Infection (CLABSI) and Catheter-Associated Urinary Tract Infection (CAUTI) Rates. Am. J. Infect. Control. 2022, 50, S34. [Google Scholar] [CrossRef]
- Kampmeier, S.; Tönnies, H.; Correa-Martinez, C.L.; Mellmann, A.; Schwierzeck, V. A nosocomial cluster of vancomycin resistant enterococci among COVID-19 patients in an intensive care unit. Antimicrob. Resist. Infect. Control 2020, 9, 154. [Google Scholar] [CrossRef]
- Brooks, S.K.; Greenberg, N.; Wessely, S.; Rubin, G.J. Factors affecting healthcare workers’ compliance with social and behavioural infection control measures during emerging infectious disease outbreaks: Rapid evidence review. BMJ Open 2021, 11, e049857. [Google Scholar] [CrossRef]
- Abed Alah, M.; Abdeen, S.; Selim, N.; Hamdani, D.; Radwan, E.; Sharaf, N.; Al-Katheeri, H.; Bougmiza, I. Compliance and barriers to the use of infection prevention and control measures among health care workers during COVID-19 pandemic in Qatar: A national survey. J. Nurs. Manag. 2021, 29, 2401–2411. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, A.; Kajihara, T.; Yahara, K.; Shibayama, K.; Sugai, M. Impact of the COVID-19 pandemic on the surveillance of antimicrobial resistance. J. Hosp. Infect. 2021, 117, 147–156. [Google Scholar] [CrossRef]
- de Carvalho Hessel Dias, V.M.; Tuon, F.; de Jesus Capelo, P.; Telles, J.P.; Fortaleza, C.M.C.B.; Pellegrino Baena, C. Trend analysis of carbapenem-resistant Gram-negative bacteria and antimicrobial consumption in the post-COVID-19 era: An extra challenge for healthcare institutions. J. Hosp. Infect. 2022, 120, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Weldetinsae, A.; Alemu, Z.A.; Tefaye, K.; Gizaw, M.; Alemahyehu, E.; Tayachew, A.; Derso, S.; Abate, M.; Getachew, M.; Abera, D.; et al. Adherence to infection prevention and control measures and risk of exposure among health-care workers: A cross-sectional study from the early period of COVID-19 pandemic in Addis Ababa, Ethiopia. Health Sci. Rep. 2023, 6, e1365. [Google Scholar] [CrossRef]
- Oweidat, K.A.; Toubasi, A.A.; Khraisat, F.A.; Aldahabi, M.N.; Alghrabli, A.; Khater, Y.; Saleh, N.; Al-Sayegh, T.N.; Albtoosh, A.S. The Impact of COVID-19 on Antibiotic Resistance and Clinical Outcomes among Critically Ill Patients. Am. J. Infect. Control. 2023. [Google Scholar] [CrossRef]
- Parisini, A.; Boni, S.; Vacca, E.B.; Bobbio, N.; Puente, F.D.; Feasi, M.; Prinapori, R.; Lattuada, M.; Sartini, M.; Cristina, M.L.; et al. Impact of the COVID-19 Pandemic on Epidemiology of Antibiotic Resistance in an Intensive Care Unit (ICU): The Experience of a North-West Italian Center. Antibiotics 2023, 12, 1278. [Google Scholar] [CrossRef]
- Haque, M.; McKimm, J.; Sartelli, M.; Dhingra, S.; Labricciosa, F.M.; Islam, S.; Jahan, D.; Nusrat, T.; Chowdhury, T.S.; Coccolini, F.; et al. Strategies to Prevent Healthcare-Associated Infections: A Narrative Overview. Risk Manag. Healthc. Policy 2020, 13, 1765–1780. [Google Scholar] [CrossRef] [PubMed]
- Sartini, M.; Del Puente, F.; Oliva, M.; Carbone, A.; Blasi Vacca, E.; Parisini, A.; Boni, S.; Bobbio, N.; Feasi, M.; Battistella, A.; et al. Riding the COVID Waves: Clinical Trends, Outcomes, and Remaining Pitfalls of the SARS-CoV-2 Pandemic: An Analysis of Two High-Incidence Periods at a Hospital in Northern Italy. J. Clin. Med. 2021, 10, 5239. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M.G.; Bufalino, A.; Sturm, L.; Huang, R.H.; Ottenbacher, A.; Saake, K.; Winegar, A.; Fogel, R.; Cacchione, J. Coronavirus disease 2019 (COVID-19) pandemic, central-line-associated bloodstream infection (CLABSI), and catheter-associated urinary tract infection (CAUTI): The urgent need to refocus on hardwiring prevention efforts. Infect. Control. Hosp. Epidemiol. 2022, 43, 26–31. [Google Scholar] [CrossRef]
- Bruyneel, A.; Gallani, M.C.; Tack, J.; d’Hondt, A.; Canipel, S.; Franck, S.; Reper, P.; Pirson, M. Impact of COVID-19 on nursing time in intensive care units in Belgium. Intensive Crit. Care Nurs. 2021, 62, 102967. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, D.; Fu, S.; Zhang, J.; Yang, X.; Xu, L.; Xu, J.; Wu, Y.; Huang, C.; Ouyang, Y.; et al. Patients with COVID-19 in 19 ICUs in Wuhan, China: A cross-sectional study. Crit. Care 2020, 24, 219. [Google Scholar] [CrossRef]
- Weiner-Lastinger, L.M.; Pattabiraman, V.; Konnor, R.Y.; Patel, P.R.; Wong, E.; Xu, S.Y.; Smith, B.; Edwards, J.R.; Dudeck, M.A. The impact of coronavirus disease 2019 (COVID-19) on healthcare-associated infections in 2020: A summary of data reported to the National Healthcare Safety Network. Infect. Control. Hosp. Epidemiol. 2022, 43, 12–25. [Google Scholar] [CrossRef]
- Tiri, B.; Sensi, E.; Marsiliani, V.; Cantarini, M.; Priante, G.; Vernelli, C.; Martella, L.A.; Costantini, M.; Mariottini, A.; Andreani, P.; et al. Antimicrobial Stewardship Program, COVID-19, and Infection Control: Spread of Carbapenem-Resistant Klebsiella Pneumoniae Colonization in ICU COVID-19 Patients. What Did Not Work? J. Clin. Med. 2020, 9, 2744. [Google Scholar] [CrossRef] [PubMed]
- Pontali, E.; Volpi, S.; Signori, A.; Antonucci, G.; Castellaneta, M.; Buzzi, D.; Montale, A.; Bustaffa, M.; Angelelli, A.; Caorsi, R.; et al. Efficacy of early anti-inflammatory treatment with high doses of intravenous anakinra with or without glucocorticoids in patients with severe COVID-19 pneumonia. J. Allergy Clin. Immunol. 2021, 147, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Kyriazopoulou, E.; Huet, T.; Cavalli, G.; Gori, A.; Kyprianou, M.; Pickkers, P.; Eugen-Olsen, J.; Clerici, M.; Veas, F.; Chatellier, G.; et al. International Collaborative Group for Anakinra in COVID-19. Effect of anakinra on mortality in patients with COVID-19: A systematic review and patient-level meta-analysis. Lancet Rheumatol. 2021, 3, e690–e697. [Google Scholar] [CrossRef] [PubMed]
- McMullen, K.M.; Smith, B.A.; Rebmann, T. Impact of SARS-CoV-2 on hospital acquired infection rates in the United States: Predictions and early results. Am. J. Infect. Control. 2020, 48, 1409–1411. [Google Scholar] [CrossRef] [PubMed]
- Tetaj, N.; Capone, A.; Stazi, G.V.; Marini, M.C.; Garotto, G.; Busso, D.; Scarcia, S.; Caravella, I.; Macchione, M.; De Angelis, G.; et al. ICU COVID-19 Study Group. Epidemiology of ventilator-associated pneumonia in ICU COVID-19 patients: An alarming high rate of multidrug-resistant bacteria. J. Anesth. Analg. Crit. Care 2022, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Lastinger, L.M.; Alvarez, C.R.; Kofman, A.; Konnor, R.Y.; Kuhar, D.T.; Nkwata, A.; Patel, P.R.; Pattabiraman, V.; Xu, S.Y.; Dudeck, M.A. Continued increases in the incidence of healthcare-associated infection (HAI) during the second year of the coronavirus disease 2019 (COVID-19) pandemic. Infect. Control. Hosp. Epidemiol. 2023, 44, 997–1001. [Google Scholar] [CrossRef]
- Alsaffar, M.J.; Alsheddi, F.M.; Humayun, T.; Aldalbehi, F.Z.; Alshammari, W.H.S.; Aldecoa, Y.S.; Burhan, N.M.; El-Saed, A.; Tawfeeq, S.; Alanazi, K.H. Impact of COVID-19 pandemic on the rates of central line-associated bloodstream infection and catheter-associated urinary tract infection in an intensive care setting. A National experience. Am. J. Infect. Control. 2023, 51, 1108–1113. [Google Scholar] [CrossRef]
- Sahrmann, J.M.; Nickel, K.B.; Stwalley, D.; Dubberke, E.R.; Lyons, P.G.; Michelson, A.P.; McMullen, K.M.; Gandra, S.; Olsen, M.A.; Kwon, J.H.; et al. Healthcare-associated infections (HAIs) during the coronavirus disease 2019 (COVID-19) pandemic: A time-series analysis. Antimicrob. Steward. Healthc. Epidemiol. 2023, 3, e14. [Google Scholar] [CrossRef]
- Snyder, G.M.; Wagester, S.; Harris, P.L.; Valek, A.L.; Hodges, J.C.; Bilderback, A.L.; Kader, F.; Tanner, C.A.; Metzger, A.P.; DiNucci, S.E.; et al. Healthcare-associated infections during the coronavirus disease 2019 (COVID-19) pandemic and the modulating effect of centralized surveillance. Antimicrob. Steward. Healthc. Epidemiol. 2023, 3, e72. [Google Scholar] [CrossRef]
- Sands, K.E.; Blanchard, E.J.; Fraker, S.; Korwek, K.; Cuffe, M. Health Care-Associated Infections Among Hospitalized Patients With COVID-19, March 2020–March 2022. JAMA Netw. Open 2023, 6, e238059. [Google Scholar] [CrossRef]
- Scaramuzzo, G.; Gamberini, L.; Tonetti, T.; Zani, G.; Ottaviani, I.; Mazzoli, C.A.; Capozzi, C.; Giampalma, E.; Bacchi Reggiani, M.L.; Bertellini, E.; et al. ICU-RER COVID-19 Collaboration Sustained oxygenation improvement after first prone positioning is associated with liberation from mechanical ventilation and mortality in critically ill COVID-19 patients: A cohort study. Ann. Intensive Care 2021, 11, 63. [Google Scholar] [CrossRef]
- Okin, D.; Huang, C.Y.; Alba, G.A.; Jesudasen, S.J.; Dandawate, N.A.; Gavralidis, A.; Chang, L.L.; Moin, E.E.; Ahmad, I.; Witkin, A.S.; et al. Prolonged Prone Position Ventilation Is Associated With Reduced Mortality in Intubated COVID-19 Patients. Chest 2023, 163, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Gabiatti, D.; Vieira, L.G.; Margatho, A.S.; Dos Santos, B.N.; Clark, A.M.; Vasques, C.I.; Silveira, R.C.C.P. Prevalence of adverse events in pronated intubated adult COVID-19 patients: A systematic review with meta-analysis. J. Clin. Nurs. 2023, 33, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Frattari, A.; Polilli, E.; Rapacchiale, G.; Coladonato, S.; Ianniruberto, S.; Mazzotta, E.; Patarchi, A.; Battilana, M.; Ciulli, R.; Moretta, A.; et al. Predictors of bacteremia and death, including immune status, in a large single-center cohort of unvaccinated ICU patients with COVID-19 pneumonia. Eur. J. Med. Res. 2023, 28, 219. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.R.; Weiner-Lastinger, L.M.; Dudeck, M.A.; Fike, L.V.; Kuhar, D.T.; Edwards, J.R.; Pollock, D.; Benin, A. Impact of COVID-19 pandemic on central-line-associated bloodstream infections during the early months of 2020, National Healthcare Safety Network. Infect. Control. Hosp. Epidemiol. 2022, 43, 790–793. [Google Scholar] [CrossRef]
- Menegueti, M.G.; Ardison, K.M.; Bellissimo-Rodrigues, F.; Gaspar, G.G.; Martins-Filho, O.A.; Puga, M.L.; Laus, A.M.; Basile-Filho, A.; Auxiliadora-Martins, M. The Impact of Implementation of Bundle to Reduce Catheter-Related Bloodstream Infection Rates. J. Clin. Med. Res. 2015, 7, 857–861. [Google Scholar] [CrossRef]
- Dumyati, G.; Concannon, C.; van Wijngaarden, E.; Love, T.M.; Graman, P.; Pettis, A.M.; Greene, L.; El-Daher, N.; Farnsworth, D.; Quinlan, G.; et al. Sustained reduction of central line-associated bloodstream infections outside the intensive care unit with a multimodal intervention focusing on central line maintenance. Am. J. Infect. Control. 2014, 42, 723–730. [Google Scholar] [CrossRef]
- CDC. Bloodstream Infection Event (Central Line-Associated Bloodstream Infection and Non-Central Line Associated Bloodstream Infection). 2023. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf (accessed on 16 August 2023).
- CDC. The NHSN Standardized Infection Ratio (SIR) A Guide to the SIR (Based on 2015 National Baseline) Updated April 2022. Available online: https://www.cdc.gov/nhsn/pdfs/ps-analysis-resources/nhsn-sir-guide.pdf (accessed on 16 August 2023).
- CDC. THE NHSN Standardized Utilization Ratio (SUR) A Guide to the SUR (Based on 2015 National Baseline) Updated April 2022. Available online: https://www.cdc.gov/nhsn/pdfs/ps-analysis-resources/nhsn-sur-guide-508.pdf (accessed on 16 August 2023).
- Fakih, M.G.; Huang, R.H.; Bufalino, A.; Erlinger, T.; Sturm, L.; Hendrich, A.; Haydar, Z. The case for a population standardized infection ratio (SIR): A metric that marries the device SIR to the standardized utilization ratio (SUR). Infect. Control. Hosp. Epidemiol. 2019, 40, 979–982. [Google Scholar] [CrossRef]





| Year | Number of Patients | Patients Stay in ICU (Total Days) | Central Line Days |
|---|---|---|---|
| 2018 | 375 | 2284 | 1716 |
| 2019 | 371 | 2238 | 1867 |
| 2020 | 337 | 2643 | 2221 |
| 2021 | 309 | 2613 | 1915 |
| 2022 | 287 | 2252 | 1932 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boni, S.; Sartini, M.; Del Puente, F.; Adriano, G.; Blasi Vacca, E.; Bobbio, N.; Carbone, A.; Feasi, M.; Grasso, V.; Lattuada, M.; et al. Innovative Approaches to Monitor Central Line Associated Bloodstream Infections (CLABSIs) Bundle Efficacy in Intensive Care Unit (ICU): Role of Device Standardized Infection Rate (dSIR) and Standardized Utilization Ratio (SUR)—An Italian Experience. J. Clin. Med. 2024, 13, 396. https://doi.org/10.3390/jcm13020396
Boni S, Sartini M, Del Puente F, Adriano G, Blasi Vacca E, Bobbio N, Carbone A, Feasi M, Grasso V, Lattuada M, et al. Innovative Approaches to Monitor Central Line Associated Bloodstream Infections (CLABSIs) Bundle Efficacy in Intensive Care Unit (ICU): Role of Device Standardized Infection Rate (dSIR) and Standardized Utilization Ratio (SUR)—An Italian Experience. Journal of Clinical Medicine. 2024; 13(2):396. https://doi.org/10.3390/jcm13020396
Chicago/Turabian StyleBoni, Silvia, Marina Sartini, Filippo Del Puente, Giulia Adriano, Elisabetta Blasi Vacca, Nicoletta Bobbio, Alessio Carbone, Marcello Feasi, Viviana Grasso, Marco Lattuada, and et al. 2024. "Innovative Approaches to Monitor Central Line Associated Bloodstream Infections (CLABSIs) Bundle Efficacy in Intensive Care Unit (ICU): Role of Device Standardized Infection Rate (dSIR) and Standardized Utilization Ratio (SUR)—An Italian Experience" Journal of Clinical Medicine 13, no. 2: 396. https://doi.org/10.3390/jcm13020396
APA StyleBoni, S., Sartini, M., Del Puente, F., Adriano, G., Blasi Vacca, E., Bobbio, N., Carbone, A., Feasi, M., Grasso, V., Lattuada, M., Nelli, M., Oliva, M., Parisini, A., Prinapori, R., Santarsiero, M. C., Tigano, S., Cristina, M. L., & Pontali, E. (2024). Innovative Approaches to Monitor Central Line Associated Bloodstream Infections (CLABSIs) Bundle Efficacy in Intensive Care Unit (ICU): Role of Device Standardized Infection Rate (dSIR) and Standardized Utilization Ratio (SUR)—An Italian Experience. Journal of Clinical Medicine, 13(2), 396. https://doi.org/10.3390/jcm13020396








