Influence of Platelet-Rich Plasma on Recurrent Vesicovaginal Fistula—A Histological and Immunohistochemical Study
Abstract
1. Introduction
2. Materials and Methods
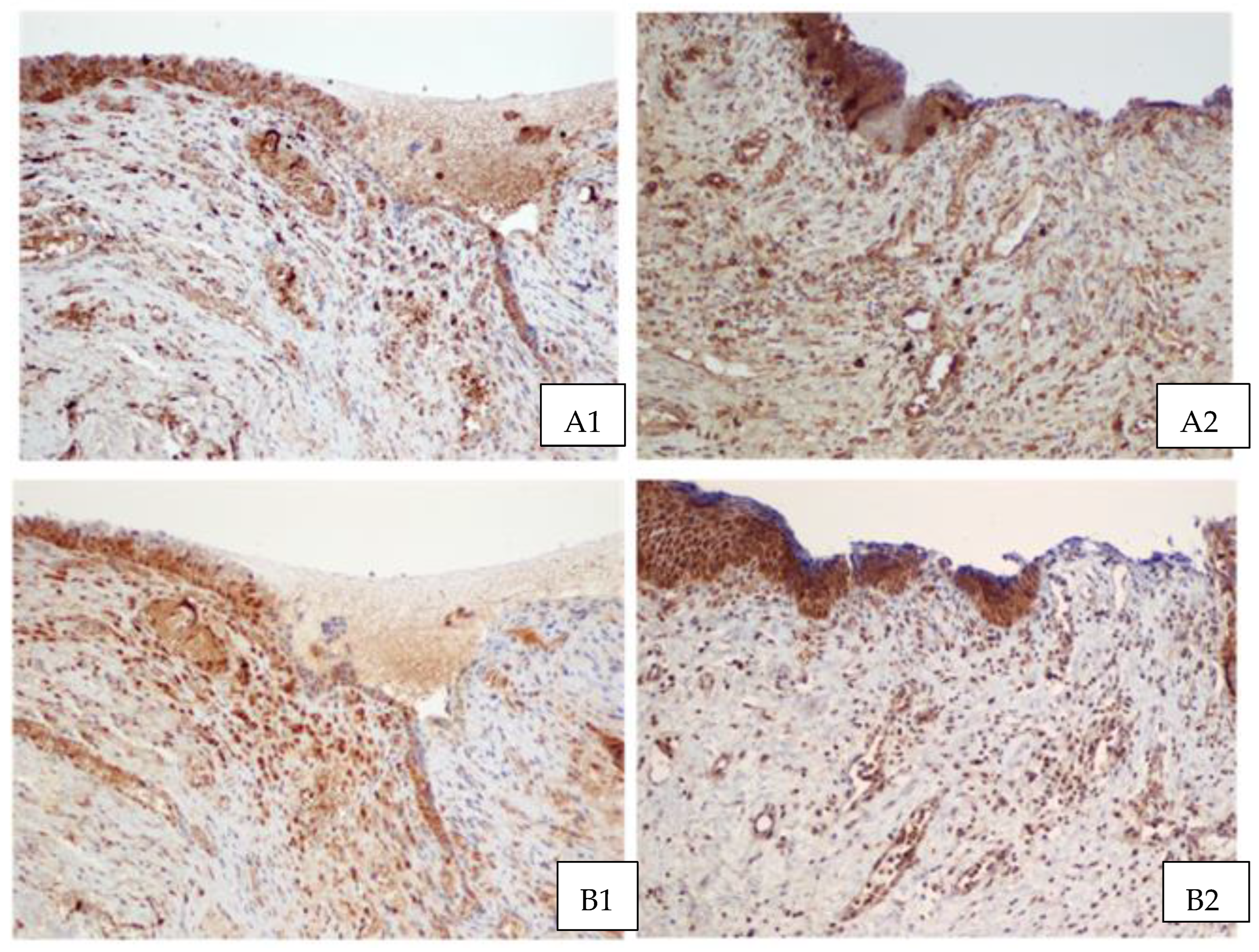
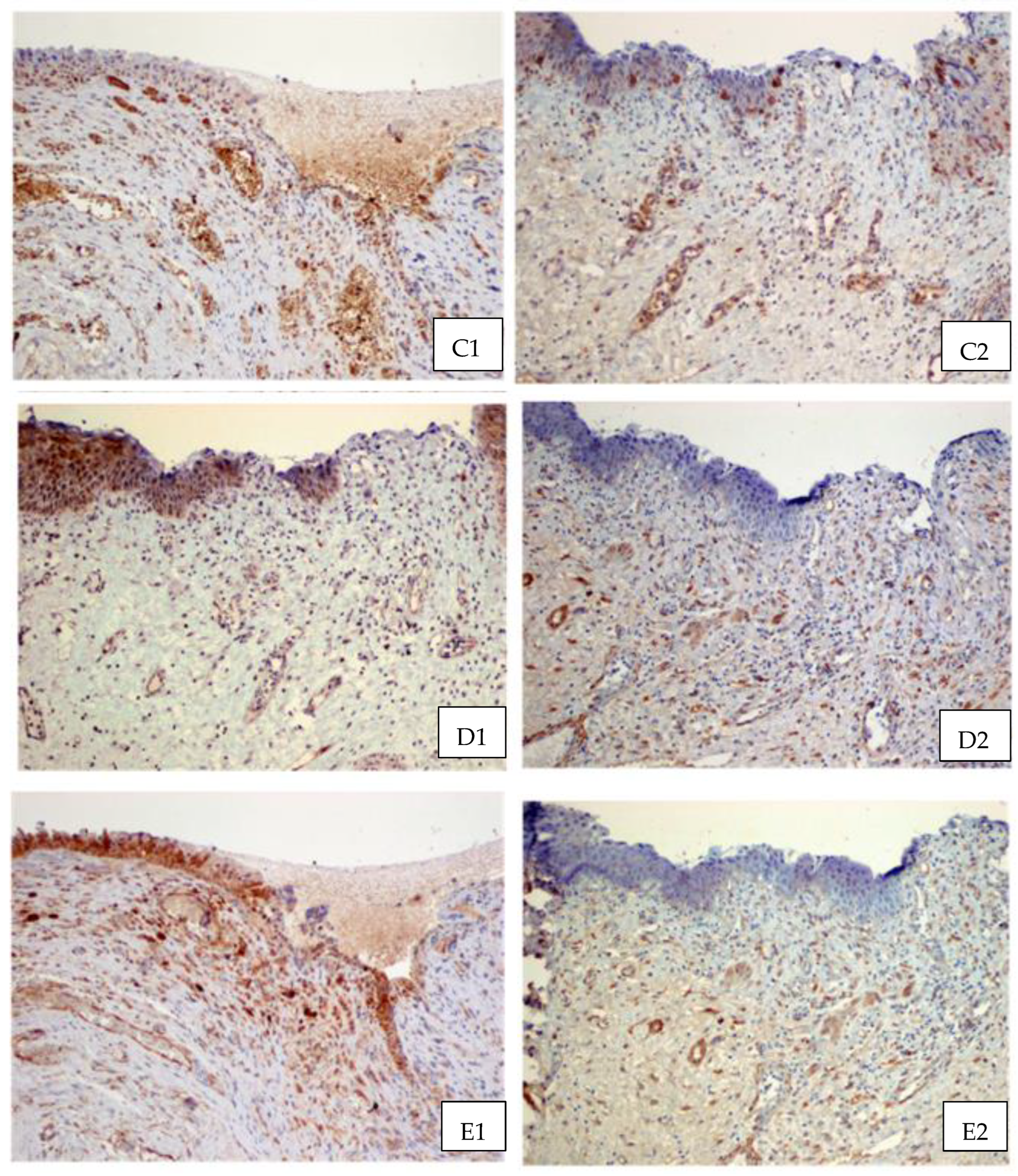
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghoniem, G.M.; Langford, C.F. Current concepts and treatment strategies for genitourinary fistulas. In Continence. Current Concepts and Treatment Strategies; Badlani, G.H., Davilla, G.W., Michel, M.C., de la Rosette, J.M.C.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 453–459. [Google Scholar]
- De Bernis, L. Obstetric fistula: Guiding principles for clinical management and programme development, a new WHO guideline. Int. Gynaecol. Obstet. 2007, 99, S117. [Google Scholar] [CrossRef] [PubMed]
- Amable, P.R.; Carias, R.B.V.; Teixeira, M.V.T.; da Cruz Pacheco, Í.; Corrêa do Amaral, R.J.F.; Granjeiro, J.M.; Borojevic, R. Platelet-Rich Plasma Preparation for regenerative medicine: Optimization and quantification of cytokines and growth factors. Stem Cell Res. Ther. 2013, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Streit-Ciećkiewicz, D.; Kołodyńska, A.; Futyma-Gąbka, K.; Grzybowska, M.E.; Gołacki, J.; Futyma, K. Platelet-Rich Plasma in Gynecology—Discovering Undiscovered. Int. J. Environ. Res. Public Health 2022, 19, 5284. [Google Scholar] [CrossRef] [PubMed]
- Gołos, A.; Treliński, J. Kliniczne zastosowanie osocza bogatopłytkowego. Hematologia 2014, 5, 252–259. [Google Scholar]
- Lubkowska, A.; Dołęgowska, B.; Banfi, G. Growth factor content in PRP and their applicability in medicine. J. Biol. Regul. Homeost. Agents 2012, 26, 3–22. [Google Scholar]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair. Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Dhillon, R.S.; Schwarz, E.M.; Maloney, M.D. Platelet-rich plasma therapy—Future or trend? Arthritis Res. Ther. 2012, 14, 219. [Google Scholar] [CrossRef]
- Berlanga-Acosta, J.; Gavilondo-Cowley, J.; López-Saura, P.; González-López, T.; Castro-Santana, M.D.; López-Mola, E.; Guillén-Nieto, G.; Herrera-Martinez, L. Epidermal growth factor in clinical practice—A review of its biological actions, clinical indications and safety implications. Int. Wound J. 2009, 6, 331–346. [Google Scholar] [CrossRef]
- Jameson, C. Autologous Platelet Concentrate for the production of Platelet Gel. Lab. Med. 2007, 38, 39–42. [Google Scholar] [CrossRef]
- Gieseck, R.L., 3rd; Wilson, M.S.; Wynn, T.A. Type 2 immunity in tissue repair and fibrosis. Nat. Rev. Immunol. 2018, 18, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Berek, C. Eosinophils can more than kill. J. Exp. Med. 2018, 215, 1967–1969. [Google Scholar] [CrossRef] [PubMed]
- Flood-Page, P.; Menzies-Gow, A.; Phipps, S.; Ying, S.; Wangoo, A.; Ludwig, M.S.; Barnes, N.; Robinson, D.; Kay, A.B. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J. Clin. Investig. 2003, 112, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.; Mathur, S.K.; Espenshade, B.M.; Mori, Y.; Varga, J.; Ackerman, S.J. Eosinophil-fibroblast interactions induce fibroblast IL-6 secretion and extracellular matrix gene expression: Implications in fibrogenesis. J. Allergy Clin. Immunol. 2005, 116, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Pegorier, S.; Wagner, L.A.; Gleich, G.J.; Pretolani, M. Eosinophil-derived cationic proteins activate the synthesis of remodeling factors by airway epithelial cells. J. Immunol. 2006, 177, 4861–4869. [Google Scholar] [CrossRef]
- Zagai, U.; Dadfar, E.; Lundahl, J.; Venge, P.; Skold, C.M. Eosinophil cationic protein stimulates TGF-beta1 release by human lung fibroblasts in vitro. Inflammation 2007, 30, 153–160. [Google Scholar] [CrossRef]
- Minns, D.; Smith, K.J.; Alessandrini, V. The neutrophil antimicrobial peptide cathelicidin promotes Th17 differentiation. Nat. Commun. 2021, 12, 1285. [Google Scholar] [CrossRef]
- Brockmann, L.; Giannou, A.D.; Gagliani, N.; Huber, S. Regulation of T(H)17 Cells and Associated Cytokines in Wound Healing, Tissue Regeneration, and Carcinogenesis. Int. J. Mol. Sci. 2017, 18, 1033. [Google Scholar] [CrossRef]
- Medvedev, V.L.; Opolskiy, A.M.; Gorban, N.A.; Kogan, M.I. Morphological analysis of the structure of parafistulous tissue in patients with vesicovaginal fistulas by the local interstitial application of platelet-rich-plasma. Urologiia 2021, 1, 21–26. [Google Scholar] [CrossRef]
- Xu, P.; Wu, Y.; Zhou, L.; Yang, Z.; Zhang, X.; Hu, X.; Yang, J.; Wang, M.; Wang, B.; Luo, G.; et al. Platelet-rich plasma accelerates skin wound healing by promoting re-epithelialization. Burn. Trauma 2020, 8, tkaa028. [Google Scholar] [CrossRef]
- Nolan, G.S.; Smith, O.J.; Heavey, S.; Jell, G.; Mosahebi, A. Histological analysis of fat grafting with platelet-rich plasma for diabetic foot ulcers—A randomised controlled trial. Int. Wound J. 2022, 19, 389–398. [Google Scholar] [CrossRef] [PubMed]

| Name of GF | Abbreviation | Function |
|---|---|---|
| Platelet Derived Growth Factor | PDGF | Enhances collagen synthesis, fibroblast chemotaxis and macrophage activation |
| Transforming Growth Factor β | TGF β | Enhances synthesis collagen type I, promotes angiogenesis |
| Vascular Endothelial Growth Factor | VEGF | Stimulates angiogenesis, migration and mitosis of endothelial cells, as well as chemotaxis of macrophages, increases permeability of the vessels |
| Fibroblast Growth Factor | FGF | Promotes proliferation of mesenchymal cells |
| Epidermal Growth Factor | EGF | Stimulates cellular proliferation and differentiation of epithelial cells |
| Number of the Sample | Age | Parity | BMI | Surgery Before VVF Occurrence |
|---|---|---|---|---|
| 0.01 | 49 | 1 | 24.2 | Op. m. Meigs |
| 0.0.1.1 | 49 | 1 | 24.2 | Op. m. Meigs |
| 0.02 | 54 | 1 | 30 | TAH/BSO |
| 0.03 | 42 | 0 | 19.53 | TAH |
| 0.04 | 33 | 1 | 20.2 | Op. m. Meigs |
| 0.05 | 74 | 1 | 19.71 | TAH |
| 0.06 | 60 | 4 | 26.7 | TAH/BSO |
| 0.07 | 44 | 0 | 23.5 | TAH/BSO |
| Type of Reaction | |||
|---|---|---|---|
| Growth Factors | Cytoplasmic | Nuclear | Membrane–Cytoplasmic |
| EGF | + | ||
| TGFβ1 | + | ||
| TGFβ2 | + | + | |
| PDGFAA | + | ||
| PDGFB | + | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Streit-Ciećkiewicz, D.E.; Szumiło, J.; Grzybowska, M.E.; Futyma, K. Influence of Platelet-Rich Plasma on Recurrent Vesicovaginal Fistula—A Histological and Immunohistochemical Study. J. Clin. Med. 2024, 13, 370. https://doi.org/10.3390/jcm13020370
Streit-Ciećkiewicz DE, Szumiło J, Grzybowska ME, Futyma K. Influence of Platelet-Rich Plasma on Recurrent Vesicovaginal Fistula—A Histological and Immunohistochemical Study. Journal of Clinical Medicine. 2024; 13(2):370. https://doi.org/10.3390/jcm13020370
Chicago/Turabian StyleStreit-Ciećkiewicz, Dominika Ewelina, Justyna Szumiło, Magdalena Emilia Grzybowska, and Konrad Futyma. 2024. "Influence of Platelet-Rich Plasma on Recurrent Vesicovaginal Fistula—A Histological and Immunohistochemical Study" Journal of Clinical Medicine 13, no. 2: 370. https://doi.org/10.3390/jcm13020370
APA StyleStreit-Ciećkiewicz, D. E., Szumiło, J., Grzybowska, M. E., & Futyma, K. (2024). Influence of Platelet-Rich Plasma on Recurrent Vesicovaginal Fistula—A Histological and Immunohistochemical Study. Journal of Clinical Medicine, 13(2), 370. https://doi.org/10.3390/jcm13020370






