Quantification of hs-Troponin Levels and Global Longitudinal Strain among Critical COVID-19 Patients with Myocardial Involvement
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Study Population and Design
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Subjects
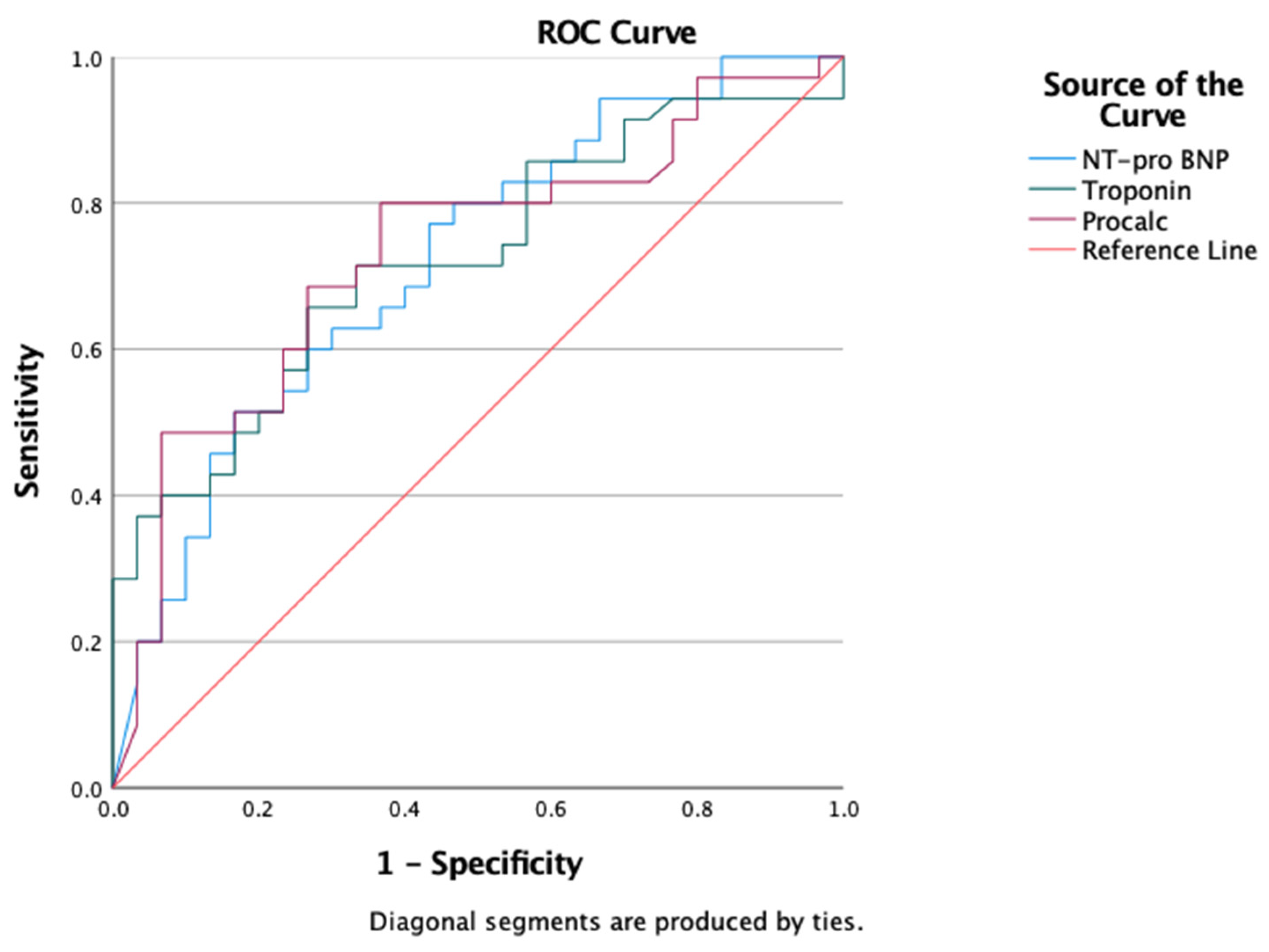
3.2. Subanalysis of Myocardial Injury and Severe COVID-19 Outcome
3.3. Subanalysis of Echocardiography Parameters and Severe COVID-19 Outcome
3.4. Subanalysis of Clinical Characteristics and Myocarditis in Severe COVID-19
3.5. Subanalyses of Myocarditis Clinical Characteristics and Mortality
4. Discussion
4.1. Clinical Characteristics of the Research Subjects
4.2. Subanalysis of Myocardial Injury and the Outcomes of the Patients
4.3. Subanalysis of the Echocardiographic Profile of Severe COVID-19 Patient Outcomes
4.4. Analysis of Relationship of Clinical Characteristics to Incidence of Myocarditis
4.5. Study Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kemenkes, R.I. Situasi Terkini COVID19. 2020. Internet. Available online: https://covid19.kemkes.go.id/situasi-infeksi-emerging/situasi-terkini-perkembangan-coronavirus-disease-covid-19-5-mei-2020 (accessed on 1 June 2020).
- Hageman, J.R. The Coronavirus Disease 2019 (COVID-19). Pediatr. Ann. 2020, 49, e99–e100. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yan, J.T.; Zhou, N.; Zhao, J.P.; Wang, D.W. Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19. Zhonghua Xin Xue Guan Bing Za Zhi 2020, 48, e008. [Google Scholar]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.R.; et al. COVID-19 and Cardiovascular Disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Sagarad, S.V. NT-proBNP in Myocarditis after a Scorpion Sting Envenomation. J. Clin. Diagn. Res. 2013, 7, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Ma, F.; Wei, X.; Fang, Y. Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin. Eur. Heart J. 2020, 42, 206. [Google Scholar] [CrossRef] [PubMed]
- Hékimian, G.; Combes, A. Myocardites. Rev. Méd. Interne 2017, 38, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.H.; Hays, A.G.; A Gilotra, N. The Evolving Role of Echocardiography During the Coronavirus Disease 2019 Pandemic. Heart Int. 2022, 16, 28–36. [Google Scholar] [CrossRef]
- Han, H.; Xie, L.; Liu, R.; Yang, J.; Liu, F.; Wu, K.; Chen, L.; Hou, W.; Feng, Y.; Zhu, C. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J. Med. Virol. 2020, 92, 819–823. [Google Scholar] [CrossRef]
- Hu, L.; Chen, S.; Fu, Y.; Gao, Z.; Long, H.; Ren, H.-W.; Zuo, Y.; Wang, J.; Li, H.; Xu, Q.-B.; et al. Risk Factors Associated With Clinical Outcomes in 323 Coronavirus Disease 2019 (COVID-19) Hospitalized Patients in Wuhan, China. Clin. Infect. Dis. 2020, 71, 2089–2098. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Shao, C.; Huang, J.; Gan, J.; Huang, X.; Bucci, E.; Piacentini, M.; Ippolito, G.; Melino, G. COVID-19 infection: The perspectives on immune responses. Cell Death Differ. 2020, 27, 1451–1454. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury with Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Bavishi, C.; Bonow, R.O.; Trivedi, V.; Abbott, J.D.; Messerli, F.H.; Bhatt, D.L. Special Article—Acute myocardial injury in patients hospitalized with COVID-19 infection: A review. Prog. Cardiovasc. Dis. 2020, 63, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Zhang, Y.; Jee, S.H.; Jung, K.J.; Li, B.; Xiu, Q. Obesity survival paradox in pneumonia: A meta-analysis. BMC Med. 2014, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- de Almeida-Pititto, B.; Dualib, P.M.; Zajdenverg, L.; Dantas, J.R.; de Souza, F.D.; Rodacki, M.; Bertoluci, M.C.; Brazilian Diabetes Society Study Group (SBD). Severity and mortality of COVID 19 in patients with diabetes, hypertension and cardiovascular disease: A meta-analysis. Diabetol. Metab. Syndr. 2020, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.R.; Adhikari, S.; Pulgarin, C.; Troxel, A.B.; Iturrate, E.; Johnson, S.B.; Hausvater, A.; Newman, J.D.; Berger, J.S.; Bangalore, S.; et al. Renin–Angiotensin–Aldosterone System Inhibitors and Risk of Covid-19. N. Engl. J. Med. 2020, 382, 2441–2448. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA. 2020, 17, 1061–1069. [Google Scholar] [CrossRef]
- Jaffe, A.S.; Cleland, J.G.F.; Katus, H.A. Myocardial injury in severe COVID-19 infection. Eur. Heart J. 2020, 41, 2080–2082. [Google Scholar] [CrossRef]
- Maino, A.; Di Stasio, E.; Grimaldi, M.C.; Cappannoli, L.; Rocco, E.; Vergallo, R.; Biscetti, F.; Baroni, S.; Urbani, A.; Landolfi, R.; et al. Prevalence and characteristics of myocardial injury during COVID-19 pandemic: A new role for high-sensitive troponin. Int. J. Cardiol. 2021, 338, 278–285. [Google Scholar] [CrossRef]
- Ostermann, M.; Ayis, S.; Tuddenham, E.; Lo, J.; Lei, K.; Smith, J.; Sanderson, B.; Moran, C.; Collinson, P.; Peacock, J.; et al. Cardiac Troponin Release is Associated with Biomarkers of Inflammation and Ventricular Dilatation During Critical Illness. Shock 2017, 47, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.J.; Khan, I.A.; Gupta, V.; Jani, K.; Gowda, R.M.; Smith, P.R. Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int. J. Cardiol. 2004, 95, 13–17. [Google Scholar] [CrossRef]
- Wibowo, A.; Pranata, R.; Astuti, A.; Tiksnadi, B.B.; Martanto, E.; Martha, J.W.; Purnomowati, A.; Akbar, M.R. Left and right ventricular longitudinal strains are associated with poor outcome in COVID-19: A systematic review and meta-analysis. J. Intensiv. Care 2021, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Jiang, D.; Wen, X.-S.; Cheng, X.-C.; Sun, M.; He, B.; You, L.-N.; Lei, P.; Tan, X.-W.; Qin, S.; et al. Prognostic value of NT-proBNP in patients with severe COVID-19. Respir. Res. 2020, 21, 83. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, C.C.; Ahmed, A.; Burger, A.L.; Muthspiel, M.; Jäger, B.; Wojta, J.; Huber, K. Biomarkers Associated with Cardiovascular Disease in COVID-19. Cells 2022, 11, 922. [Google Scholar] [CrossRef] [PubMed]
- Mudatsir, M.; Fajar, J.K.; Wulandari, L.; Soegiarto, G.; Ilmawan, M.; Purnamasari, Y.; Mahdi, B.A.; Jayanto, G.D.; Suhendra, S.; Setianingsih, Y.A.; et al. Predictors of COVID-19 severity: A systematic review and meta-analysis. F1000Research 2020, 9, 1107. [Google Scholar] [CrossRef]
- Firani, N.K.; Prisilla, J. Procalcitonin and Troponin-I as Predictor of Mortality in Acute Myocardial Infarction Patients. Indones. J. Clin. Pathol. Med. Lab. 2022, 28, 170–174. [Google Scholar] [CrossRef]
- Lippi, G.; Plebani, M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chim. Acta 2020, 505, 190–191. [Google Scholar] [CrossRef]
- Verdonschot, J.A.; Henkens, M.T.; Wang, P.; Schummers, G.; Raafs, A.G.; Krapels, I.P.; van Empel, V.; Heymans, S.R.; Rocca, H.B.; Knackstedt, C. A global longitudinal strain cut-off value to predict adverse outcomes in individuals with a normal ejection fraction. ESC Heart Fail. 2021, 8, 4343–4345. [Google Scholar] [CrossRef]
- Huang, S.; Vignon, P.; Mekontso-Dessap, A.; Tran, S.; Prat, G.; Chew, M.; Balik, M.; Sanfilippo, F.; Banauch, G.; Clau-Terre, F.; et al. Echocardiography findings in COVID-19 patients admitted to intensive care units: A multi-national observational study (the ECHO-COVID study). Intensiv. Care Med. 2022, 48, 667–678. [Google Scholar] [CrossRef]
- Wieczorkiewicz, P.; Supel, K.; Przybylak, K.; Kacprzak, M.; Zielinska, M. Acute coronary syndrome versus acute myocarditis in young adults–value of speckle tracking echocardiography. PLoS ONE 2022, 17, e0271483. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, E.D.; Mercado, P.; Pairumani, R.; Medel, J.N.; Petruska, E.; Ugalde, D.; Morales, F.; Eisen, D.; Araya, C.; Montoya, J.; et al. Cardiac function in critically ill patients with severe COVID: A prospective cross-sectional study in mechanically ventilated patients. J. Crit. Care 2022, 72, 154166. [Google Scholar] [CrossRef] [PubMed]
- Lairez, O.; Blanchard, V.; Houard, V.; Vardon-Bounes, F.; Lemasle, M.; Cariou, E.; Lavie-Badie, Y.; Ruiz, S.; Cazalbou, S.; Delmas, C.; et al. Cardiac imaging phenotype in patients with coronavirus disease 2019 (COVID-19): Results of the cocarde study. Int. J. Cardiovasc. Imaging 2021, 37, 449–457. [Google Scholar] [CrossRef]
- Bevilacqua, M.; De Togni, P.; Cattazzo, F.; Dell’Atti, D.; Dalbeni, A.; Mazzaferri, F.; Tacconelli, E.; Farzaneh-Far, A.; Fava, C.; Minuz, P.; et al. Global Longitudinal Strain to Predict Respiratory Failure and Death in Patients Admitted for COVID-19–Related Disease. Am. J. Cardiol. 2022, 165, 109–115. [Google Scholar] [CrossRef]
- Huang, S.; Vieillard-Baron, A.; Evrard, B.; Prat, G.; Chew, M.S.; Balik, M.; Clau-Terré, F.; De Backer, D.; Mekontso Dessap, A.; Orde, S.; et al. Echocardiography phenotypes of right ventricular involvement in COVID-19 ARDS patients and ICU mortality: Post-hoc (exploratory) analysis of repeated data from the ECHO-COVID study. Intensive Care Med. 2023, 49, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Urban, S.; Fułek, M.; Błaziak, M.; Iwanek, G.; Jura, M.; Fułek, K.; Guzik, M.; Garus, M.; Gajewski, P.; Lewandowski, Ł.; et al. COVID-19 Related Myocarditis in Adults: A Systematic Review of Case Reports. J. Clin. Med. 2022, 21, 5519. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, A.; Pranata, R.; Akbar, M.R.; Purnomowati, A.; Martha, J.W. Prognostic performance of troponin in COVID-19: A diagnostic meta-analysis and meta-regression. Int. J. Infect. Dis. 2021, 105, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Janardhanan, R. Myocarditis with very high troponins: Risk stratification by cardiac magnetic resonance. J. Thorac Dis. 2016, 8, E1333–E1336. [Google Scholar] [CrossRef]
- Kim, C.W.; Aronow, W.S. COVID-19, cardiovascular diseases and cardiac troponins. Futur. Cardiol. 2022, 18, 135–142. [Google Scholar] [CrossRef]
- Lovell, J.P.; Čiháková, D.; A Gilotra, N. COVID-19 and Myocarditis: Review of Clinical Presentations, Pathogenesis and Management. Heart Int. 2022, 16, 20–27. [Google Scholar] [CrossRef]
- AlJaroudi, W.; Alraies, M.C.; Halley, C.; Rodriguez, L.; Grimm, R.A.; Thomas, J.D.; Jaber, W.A. Impact of progression of diastolic dysfunction on mortality in patients with normal ejection fraction. Circulation 2012, 125, 782–788. [Google Scholar] [CrossRef] [PubMed]

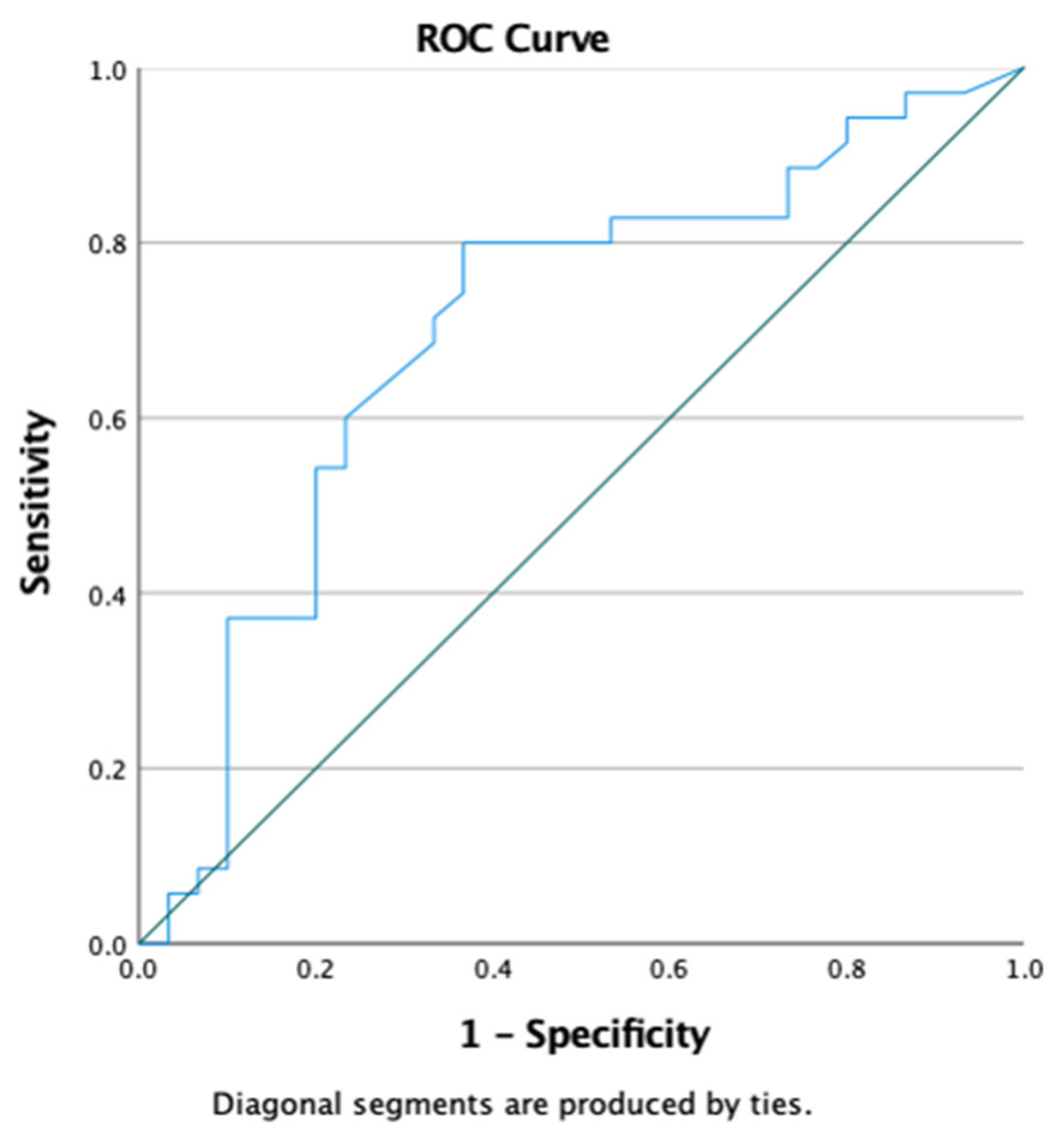
| Variable | Total (n = 65) | Survived (n = 32) | Mortality (n = 33) | p-Value |
|---|---|---|---|---|
| Age | 51 (20, 75) | 46 (20, 75) | 56 (25, 75) | 0.085 |
| Gender Male Female | 41 (63.1%) 24 (36.9%) | 20 (48.8%) 10 (41.7%) | 21 (51.2%) 14 (58.3%) | 0.579 |
| Body Mass Index (kg/m2) | 27 (17, 45) | 28 (22, 37) | 26 (17, 45) | 0.044 |
| Past Medical History | ||||
| Hypertension | 45 (69.2%) | 18 (40%) | 27 (60%) | 0.135 |
| Diabetes Mellitus | 39 (60%) | 15 (38.5%) | 24 (61.5%) | 0.128 |
| Pregnancy | 8 (12.3%) | 5 (62.5%) | 3 (37.5%) | 0.270 |
| Acute Kidney Injury | 23 (35.4%) | 3 (13%) | 20 (87%) | <0.001 |
| Laboratory Parameters | ||||
| Serum Creatinine (mg/dL) | 1.10 (0.10, 10.00) | 0.80 (0.40, 3.70) | 1.70 (0.10, 10.00) | <0.001 |
| Increased NT-proBNP | 54 (83.1%) | 23 (42.6%) | 31 (57.4%) | 0.173 |
| NT-proBNP (pg/mL) | 581.90 (31, 10,000) | 238.10 (32, 10,000) | 1380.50 (82, 10,000) | 0.002 |
| Patients with Elevated Hs-Troponin-I | 37 (56.9%) | 12 (32.4%) | 25 (67.6%) | 0.011 |
| Mean Level of Hs-Troponin-I (ng/L) | 17.56 (1, 1407) | 5.76 (2, 162) | 34.57 (1, 1407) | 0.002 |
| Procalcitonin (ng/mL) | 1.41 (0.01, 100) | 0.65 (0.01, 100) | 2.32 (0.05, 100) | 0.001 |
| CRP (mg/dL) | 14.90 (0.36, 90.80) | 14.6 (0.36, 28.90) | 15.6 (0.50, 90.80) | 0.571 |
| NLR | 10.85 (2.10, 44.80) | 9.86 (3.87, 28.76) | 11.78 (2.10, 44.80) | 0.519 |
| D-dimer | 5030.00 (700, 25,230) | 4620 (940, 22,300) | 5240 (700, 25,230) | 0.146 |
| Echocardiography Parameters | ||||
| Biplane EF | 66.00 (51, 85) | 67.50 (53, 79) | 65.00 (51, 85) | 0.391 |
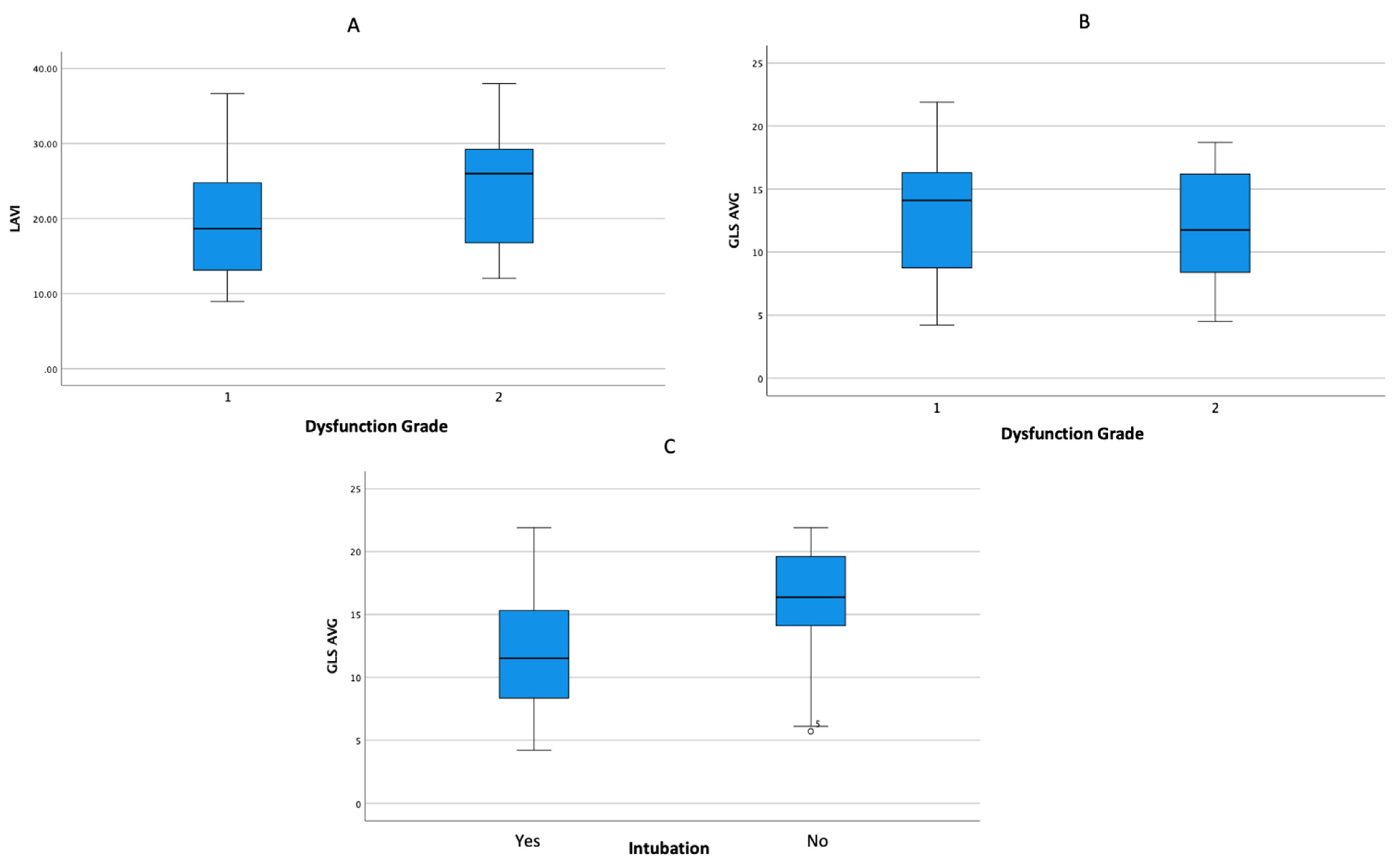
| Average GLS | 12.92 ± 4.99 | 14.74 ± 4.82 | 11.36 ± 4.64 | 0.005 |
| LAVI | 20.72 ± 7.31 | 20.05 ± 6.11 | 21.28 ± 8.24 | 0.503 |
| Velocity MV E | 0.59 (0.36, 0.92) | 0.59 (0.41, 0.85) | 0.58 (0.36, 0.92) | 0.818 |
| Average E/e’ | 6.99 (3.68, 14.00) | 6.39 (4.62, 10.65) | 6.47 (3.68, 14.00) | 0.927 |
| TR MaxPG | 00.00 (0.00, 83.18) | 0.00 (0.00, 56.66) | 0.00 (00.00, 83.18) | 0.018 |
| TR Vmax | 00.00 (00.00, 4.56) | 0.00 (0.00, 3.76) | 0.00 (0.00, 4.56) | 0.019 |
| Degree of Diastolic Dysfunction Grade 1 Grade 2 | 47 (72.3%) 18 (27.7%) | 27 (57.4%) 3 (16.7%) | 20 (42.6%) 15 (83.3%) | 0.003 |
| Other Clinical Parameters | ||||
| Length of Stay | 19 ± 9 | 21 ± 9 | 18 ± 10 | 0.115 |
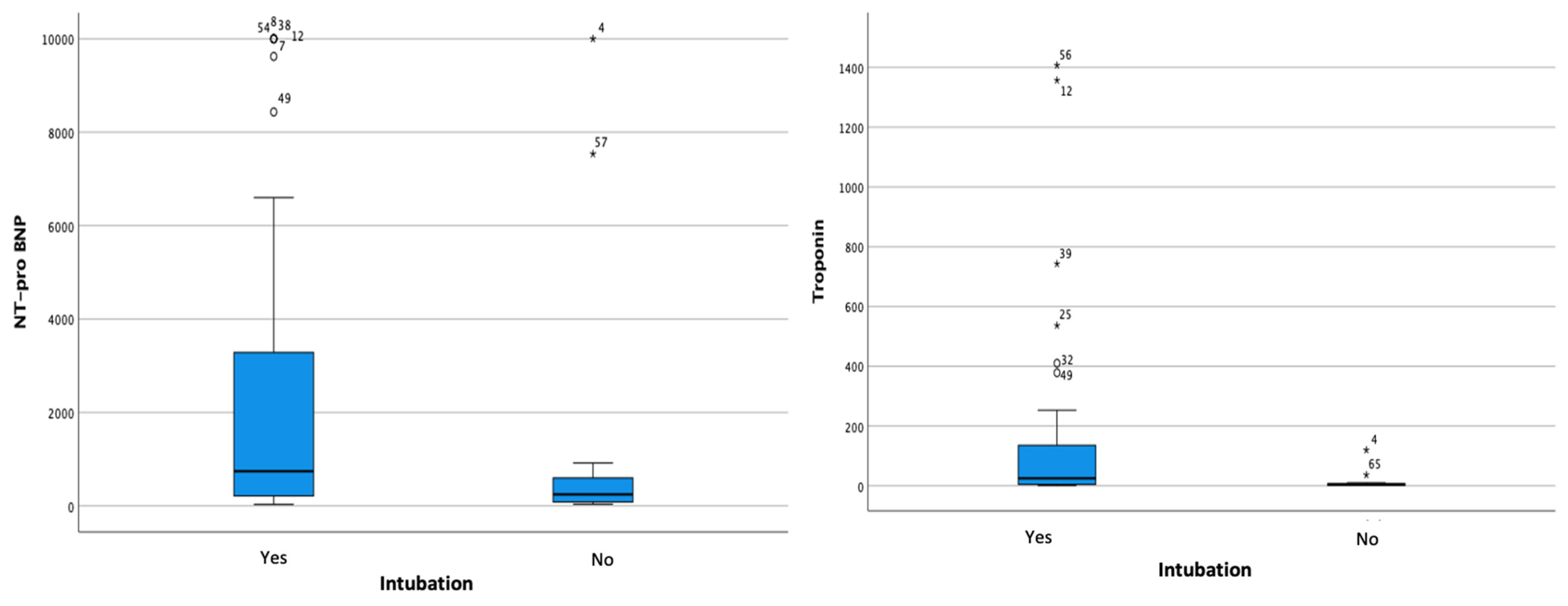
| Intubation | 51 (78.5%) | 17 (33.3%) | 34 (66.7%) | <0.001 |
| ECMO | 13 (20%) | 7 (53.8%) | 6 (46.2%) | 0.534 |
| Myocarditis | 33 (50.8%) | 9 (27.3%) | 24 (72.7%) | 0.002 |
| Variable | Event | Non-Event | p-Value |
|---|---|---|---|
| Intubation | Intubation | Non-Intubation | |
| NT-proBNP Hs-Troponin Procalcitonin Serum Creatinine | 742 (32–10,000) 25.33 (1–1407) 1.85 (0.05–100) 1.40 (0.10–10) | 246.30 (37–10,000) 3.56 (2–120) 0.59 (0.05–100) 0.75 (0.50–1.10) | 0.068 0.002 0.044 0.001 |
| ECMO | ECMO | Non-ECMO | |
| NT-proBNP Hs-Troponin Procalcitonin Serum Creatinine | 742 (32–10,000) 32.79 (2–1357) 2.01 (0.38–100) 0.80 (0.10–3.30) | 419.20 (37–10,000) 16.44 (1–1407) 1.38 (0.01–100) 1.10 (0.40–10.00) | 0.640 0.210 0.623 0.370 |
| Diastolic Dysfunction | Grade 1 | Grade 2 | |
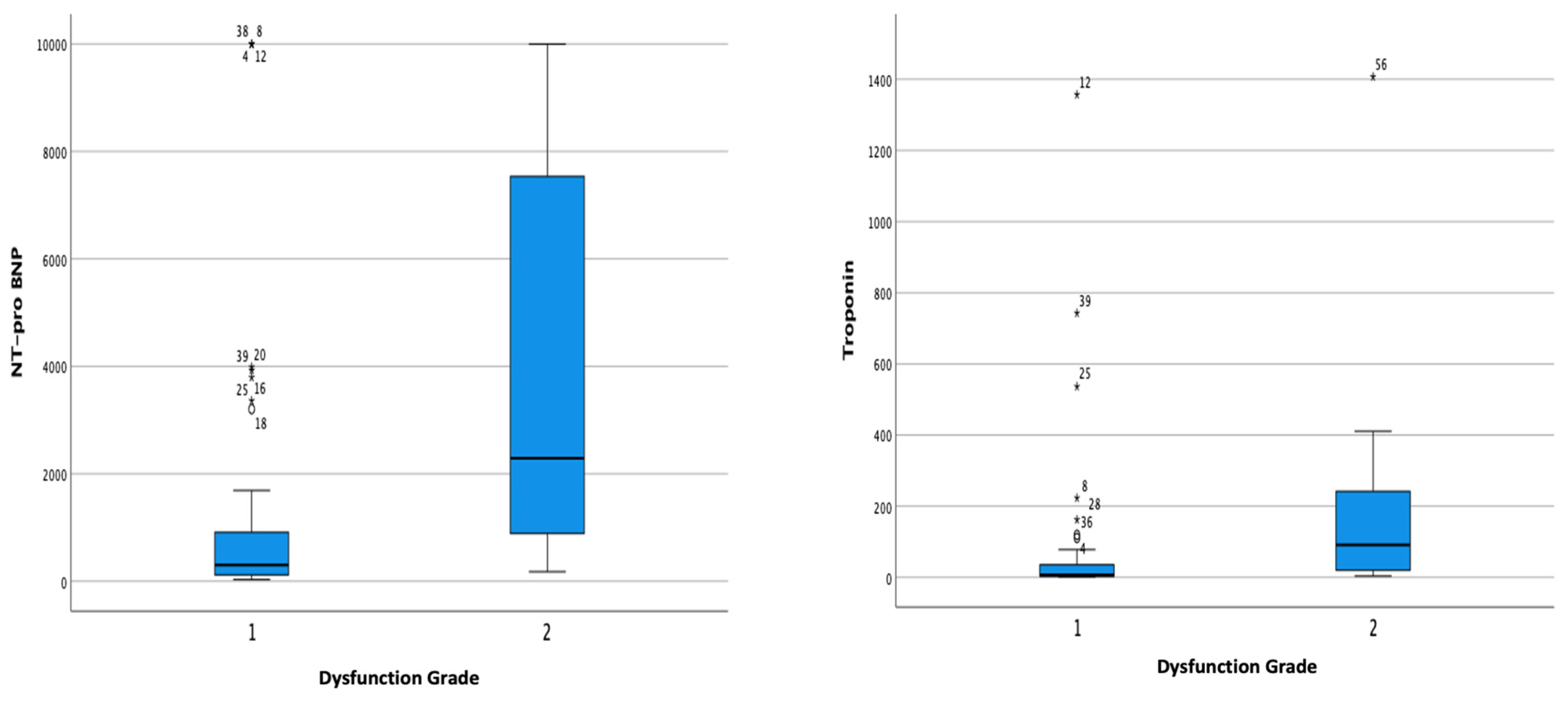
| NT-proBNP Hs-Troponin Procalcitonin Serum Creatinine | 302.30 (32–10,000) 7.19 (1–1356) 1.20 (0.09–100) 1.00 (0.40–10.00) | 2290.05 (178–10,000) 91.47 (4–1407) 1.97 (0.01–100) 1.30 (0.10–6.80) | <0.001 0.001 0.655 0.454 |
| Variable | Event | Non-Event | p-Value |
|---|---|---|---|
| Intubation | Intubated | Not intubated | |
| Length of Stay | 19.71 ± 9.93 | 18.07 ± 7.05 | 0.567 |
| GLS Average | 12.17 ± 4.79 | 15.65 ± 4.90 | 0.02 |
| LAVI | 20.36 ± 7.74 | 22.02 ± 5.51 | 0.455 |
| ECMO | ECMO | Non- ECMO | |
| Length of Stay | 23.15 ± 9.88 | 18.40 ± 9.08 | 0.102 |
| GLS Average | 11.94 ± 4.71 | 13.17 ± 5.07 | 0.432 |
| LAVI | 22.21 ± 6.54 | 20.34 ± 7.50 | 0.415 |
| Diastolic Dysfunction | Grade 1 | Grade 2 | |
| Length of Stay | 20.49 ± 9.67 | 16.39 ± 7.99 | 0.115 |
| GLS Average | 13.35 ± 5.14 | 11.79 ± 4.49 | 0.261 |
| LAVI | 19.26 ± 6.77 | 24.52 ± 7.47 | 0.008 |
| Variable | Non-Myocarditis (n = 32) | Myocarditis (n = 33) | p-Value |
|---|---|---|---|
| Age | 49 (20, 75) | 55 (25, 73) | 0.753 |
| Gender Male Female | 21 (51.2%) 11 (45.8%) | 20 (48.8%) 13 (54.2%) | 0.675 |
| Body Mass Index | 27 (22.37) | 27 (17, 45) | 0.693 |
| Past Medical History | |||
| Hypertension | 20 (44.4%) | 25 (55.6%) | 0.247 |
| Diabetes Mellitus | 21 (53.8%) | 18 (46.2%) | 0.362 |
| Pregnancy | 4 (50%) | 4 (50%) | 0.628 |
| Acute Kidney Injury | 8 (34.8%) | 15 (65.2%) | 0.085 |
| Laboratory Parameters | |||
| Serum Creatinine | 0.80 (0.40, 10.00) | 1.60 (0.10, 8.30) | <0.001 |
| NT-proBNP | 238.10 (32, 7536) | 2524.40 (72, 10,000) | <0.001 |
| Increased NT-proBNP | 24 (44.4%) | 30 (55.6%) | 0.087 |
| Hs-Troponin | 3.56 (1, 36) | 78.32 (2, 1407) | <0.001 |
| Increased Hs-Troponin | 6 (16.2%) | 31 (83.8%) | <0.001 |
| Procalcitonin | 1.29 (0.01, 97.65) | 1.92 (0.05, 100) | 0.158 |
| CRP | 11.75 (0.36, 30.10) | 15.70 (0.50, 90.80) | 0.462 |
| NLR | 9.47 (3.21, 25.21) | 13.22 (2.10, 44.80) | 0.181 |
| D-dimer | 4440.00 (960, 25,230) | 5720 (700, 22,300) | 0.300 |
| Echocardiography Parameters | |||
| Biplane EF | 67 (60, 79) | 66 (51, 85) | 0.669 |
| MV Evel | 0.58 (0.36, 0.81) | 0.62 (0.42, 0.92) | 0.047 |
| Average E/E’ | 6.38 (3.77, 10.65) | 6.76 (3.68, 14.00) | 0.679 |
| TR maxPG | 0.00 (0.00, 56.66) | 7.01 (0.00, 83.18) | 0.026 |
| TR Vmax | 0.00 (0.00, 3.76) | 1.32 (0.00, 4.56) | 0.029 |
| LAVI | 19.99 ± 5.68 | 21.42 ± 8.63 | 0.436 |
| Average GLS | 14.70 ± 5.20 | 11.19 ± 4.16 | 0.004 |
| Degree of Diastolic Dysfunction Grade 1 Grade 2 | 27 (57.4%) 5 (27.8%) | 20 (42.6%) 13 (72.2%) | 0.032 |
| Other Clinical Parameters | |||
| Intubation | 19 (37.3%) | 32 (62.7%) | <0.001 |
| ECMO | 6 (46.2%) | 7 (53.8%) | 0.804 |
| Length of Stay | 21 ± 9 | 18 ± 10 | 0.309 |
| Mortality | 11 (31.4%) | 24 (68.6%) | 0.002 |
| Myocarditis Variables | Survived (%) | Mortality (%) | p-Value |
|---|---|---|---|
| Total | 9 (27.3%) | 24 (72.7%) | - |
| Length of Stay | 23 ± 11 | 16 ± 9 | 0.095 |
| Intubation | 8 (25%) | 24 (75%) | 0.273 |
| ECMO | 4 (57.1%) | 3 (42.9%) | 0.068 |
| Demographic Parameters | |||
| Age | 44.0 (29, 63) | 56.6 (25, 73) | 0.392 |
| Gender Male Female | 7 (35%) 2 (15.4%) | 13 (65%) 11 (84.6%) | 0.216 |
| Anthropometry | |||
| Body Mass Index | 28 (22, 36) | 26.5 (17, 45) | 0.766 |
| Past Medical History | |||
| Hypertension | 5 (20%) | 20 (80%) | 0.117 |
| Diabetes Mellitus | 4 (22.2%) | 14 (77.8%) | 0.475 |
| Pregnancy | 1 (25%) | 3 (75%) | 0.705 |
| Acute Kidney Injury | 2 (13.3%) | 13 (86.7%) | 0.101 |
| Laboratory Parameters | |||
| Increased NT-proBNP | 8 (26.7%) | 22 (73.3%) | 0.629 |
| Increased HS-Troponin | 8 (25.8%) | 23 (74.2%) | 0.477 |
| Serum Creatinine | 1.00 (0.70, 3.70) | 0.10 (0.10, 8.30) | 0.036 |
| NT-proBNP | 895.70 (72, 10,000) | 3180.10 (120, 10,000) | 0.179 |
| Hs-Troponin | 46.55 (2, 162) | 135.99 (5, 1407) | 0.079 |
| Procalcitonin | 0.70 (0.33, 100) | 2.80 (0.05, 100) | 0.437 |
| CRP | 14.30 (4.60, 23.30) | 17.85 (0.50, 90.80) | 0.538 |
| NLR | 13.22 (4.64, 28.76) | 13.24 (2.10, 44.80) | 0.890 |
| D-dimer | 5720 (940, 22,300) | 5580 (700, 12,870) | 0.984 |
| Echocardiography Parameters | |||
| Biplane EF | 57 (53, 77) | 65 (51, 85) | 0.592 |
| Average GLS | 10.90 (4, 16) | 10.40 (5, 22) | 0.981 |
| LAVI | 19.36 ± 7.00 | 22.19 ± 9.18 | 0.409 |
| MV E Vel | 0.62 (0.42, 0.85) | 0.63 (0.43, 0.92) | 0.238 |
| Average E/E’ | 5.87 (4.74, 10.53) | 7.09 (3.68, 14.00) | 0.619 |
| TR Vmax | 0.00 (0.00, 2.84) | 3.10 (0.00, 4.56) | 0.018 |
| TR MaxPG | 0.00 (00.00, 32.00) | 38.37 (00.00, 83.18) | 0.018 |
| Diastolic Dysfunction Grade 1 Grade 2 | 9 (45%) 0 (0%) | 11 (55%) 13 (100%) | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsagaff, M.Y.; Wardhani, L.F.K.; Nugraha, R.A.; Putra, T.S.; Khrisna, B.P.D.; Al-Farabi, M.J.; Gunadi, R.I.; Azmi, Y.; Budianto, C.P.; Fagi, R.A.; et al. Quantification of hs-Troponin Levels and Global Longitudinal Strain among Critical COVID-19 Patients with Myocardial Involvement. J. Clin. Med. 2024, 13, 352. https://doi.org/10.3390/jcm13020352
Alsagaff MY, Wardhani LFK, Nugraha RA, Putra TS, Khrisna BPD, Al-Farabi MJ, Gunadi RI, Azmi Y, Budianto CP, Fagi RA, et al. Quantification of hs-Troponin Levels and Global Longitudinal Strain among Critical COVID-19 Patients with Myocardial Involvement. Journal of Clinical Medicine. 2024; 13(2):352. https://doi.org/10.3390/jcm13020352
Chicago/Turabian StyleAlsagaff, Mochamad Yusuf, Louisa Fadjri Kusuma Wardhani, Ricardo Adrian Nugraha, Tony Santoso Putra, Bagus Putra Dharma Khrisna, Makhyan Jibril Al-Farabi, Ruth Irena Gunadi, Yusuf Azmi, Christian Pramudita Budianto, Rosi Amrilla Fagi, and et al. 2024. "Quantification of hs-Troponin Levels and Global Longitudinal Strain among Critical COVID-19 Patients with Myocardial Involvement" Journal of Clinical Medicine 13, no. 2: 352. https://doi.org/10.3390/jcm13020352
APA StyleAlsagaff, M. Y., Wardhani, L. F. K., Nugraha, R. A., Putra, T. S., Khrisna, B. P. D., Al-Farabi, M. J., Gunadi, R. I., Azmi, Y., Budianto, C. P., Fagi, R. A., Luthfah, N., Subagjo, A., Oktaviono, Y. H., Lefi, A., Dharmadjati, B. B., Alkaff, F. F., & Pikir, B. S. (2024). Quantification of hs-Troponin Levels and Global Longitudinal Strain among Critical COVID-19 Patients with Myocardial Involvement. Journal of Clinical Medicine, 13(2), 352. https://doi.org/10.3390/jcm13020352





