Different Analgesia Techniques for Postoperative Pain in Children Undergoing Abdominal Surgery for Intractable Constipation: A Retrospective Cohort Study in a Single Tertiary Children’s Hospital
Abstract
1. Introduction
2. Materials and Methods
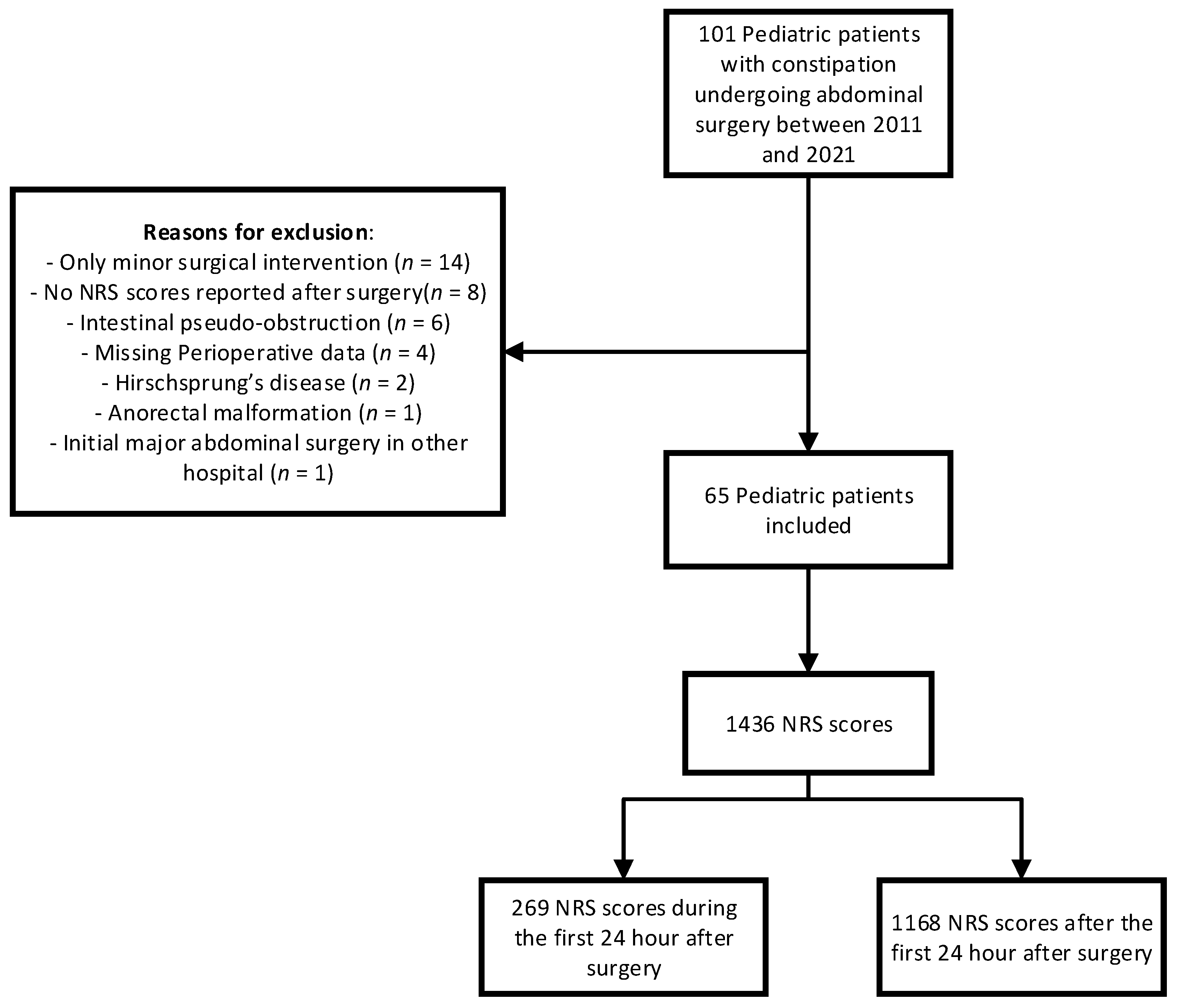
2.1. Study Design and Participants
2.2. Procedures
2.3. Data Collection and Measurements
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Anesthetic and Surgical Characteristics
3.3. Primary Outcome
3.4. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Vriesman, M.H.; Koppen, I.J.N.; Camilleri, M.; Di Lorenzo, C.; Benninga, M.A. Management of functional constipation in children and adults. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Koppen, I.J.N.; Vriesman, M.H.; Saps, M.; Rajindrajith, S.; Shi, X.; van Etten-Jamaludin, F.S.; Di Lorenzo, C.; Benninga, M.A.; Tabbers, M.M. Prevalence of Functional Defecation Disorders in Children: A Systematic Review and Meta-Analysis. J. Pediatr. 2018, 198, 121–130.e6. [Google Scholar] [CrossRef] [PubMed]
- Benninga, M.A.; Faure, C.; Hyman, P.E.; St James Roberts, I.; Schechter, N.L.; Nurko, S. Childhood Functional Gastrointestinal Disorders: Neonate/Toddler. Gastroenterology 2016, 150, 1443–1455. [Google Scholar] [CrossRef] [PubMed]
- Bokova, E.; Svetanoff, W.J.; Rosen, J.M.; Levitt, M.A.; Rentea, R.M. State of the Art Bowel Management for Pediatric Colorectal Problems: Functional Constipation. Children 2023, 10, 1078. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Shanahan, S.; Livingston, M.H.; Walton, J.M. Malone appendicostomy versus cecostomy tube insertion for children with intractable constipation: A systematic review and meta-analysis. J. Pediatr. Surg. 2018, 53, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Kuizenga-Wessel, S.; Koppen, I.J.N.; Zwager, L.W.; Di Lorenzo, C.; de Jong, J.R.; Benninga, M.A. Surgical management of children with intractable functional constipation; experience of a single tertiary children’s hospital. Neurogastroenterol. Motil. 2017, 29, e13005. [Google Scholar] [CrossRef]
- Freedman, S.B.; Thull-Freedman, J.; Rumantir, M.; Eltorki, M.; Schuh, S. Pediatric Constipation in the Emergency Department: Evaluation, Treatment, and Outcomes. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 327–333. [Google Scholar] [CrossRef]
- Glare, P.; Aubrey, K.R.; Myles, P.S. Transition from acute to chronic pain after surgery. Lancet 2019, 393, 1537–1546. [Google Scholar] [CrossRef]
- Pas, R.; Ickmans, K.; Van Oosterwijck, S.; Van der Cruyssen, K.; Foubert, A.; Leysen, L.; Nijs, J.; Meeus, M. Hyperexcitability of the Central Nervous System in Children with Chronic Pain: A Systematic Review. Pain Med. 2018, 19, 2504–2514. [Google Scholar] [CrossRef]
- Farmer, A.D.; Holt, C.B.; Downes, T.J.; Ruggeri, E.; Del Vecchio, S.; De Giorgio, R. Pathophysiology, diagnosis, and management of opioid-induced constipation. Lancet Gastroenterol. Hepatol. 2018, 3, 203–212. [Google Scholar] [CrossRef]
- Walker, B.J.; Long, J.B.; Sathyamoorthy, M.; Birstler, J.; Wolf, C.; Bosenberg, A.T.; Flack, S.H.; Krane, E.J.; Sethna, N.F.; Suresh, S.; et al. Complications in Pediatric Regional Anesthesia: An Analysis of More than 100,000 Blocks from the Pediatric Regional Anesthesia Network. Anesthesiology 2018, 129, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Van den Heuvel, M.; Rickard, M.; El-Bardisi, Y.; Mistry, N.; Koyle, M.; Farhat, W.; Santos, J.D. Neurodevelopmental and psychiatric disorders in pediatric bladder and bowel dysfunction. J. Pediatr. Urol. 2021, 17, 450.e1–450.e6. [Google Scholar] [CrossRef] [PubMed]
- Westwell-Roper, C.; Best, J.R.; Naqqash, Z.; Afshar, K.; MacNeily, A.E.; Stewart, S.E. Bowel and Bladder Dysfunction Is Associated with Psychiatric Comorbidities and Functional Impairment in Pediatric Obsessive-Compulsive Disorder. J. Child Adolesc. Psychopharmacol. 2022, 32, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Taenzer, P.; Melzack, R.; Jeans, M.E. Influence of psychological factors on postoperative pain, mood and analgesic requirements. Pain 1986, 24, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Rasquin, A.; Di Lorenzo, C.; Forbes, D.; Guiraldes, E.; Hyams, J.S.; Staiano, A.; Walker, L.S. Childhood functional gastrointestinal disorders: Child/adolescent. Gastroenterology 2006, 130, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Tabbers, M.M.; DiLorenzo, C.; Berger, M.Y.; Faure, C.; Langendam, M.W.; Nurko, S.; Staiano, A.; Vandenplas, Y.; Benninga, M.A. Evaluation and treatment of functional constipation in infants and children: Evidence-based recommendations from ESPGHAN and NASPGHAN. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 258–274. [Google Scholar] [CrossRef]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13, S31–S34. [Google Scholar] [CrossRef]
- Aldrete, J.A. The post-anesthesia recovery score revisited. J. Clin. Anesth. 1995, 7, 89–91. [Google Scholar] [CrossRef]
- Geary, T.; Negus, A.; Anderson, B.J.; Zernikow, B. Perioperative management of the child on long-term opioids. Paediatr. Anaesth. 2012, 22, 189–202. [Google Scholar] [CrossRef]
- Voepel-Lewis, T.; Burke, C.N.; Jeffreys, N.; Malviya, S.; Tait, A.R. Do 0–10 numeric rating scores translate into clinically meaningful pain measures for children? Anesth. Analg. 2011, 112, 415–421. [Google Scholar] [CrossRef]
- Gerbershagen, H.J.; Aduckathil, S.; van Wijck, A.J.; Peelen, L.M.; Kalkman, C.J.; Meissner, W. Pain intensity on the first day after surgery: A prospective cohort study comparing 179 surgical procedures. Anesthesiology 2013, 118, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Ecoffey, C.; Bosenberg, A.; Lonnqvist, P.A.; Suresh, S.; Delbos, A.; Ivani, G. Practice advisory on the prevention and management of complications of pediatric regional anesthesia. J. Clin. Anesth. 2022, 79, 110725. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Ecoffey, C.; Bosenberg, A.; Lonnqvist, P.-A.; de Oliveira, J.G.S.; Casasola, O.d.L.; Andrés, J.d.; Ivani, G. The European Society of Regional Anaesthesia and Pain Therapy/American Society of Regional Anesthesia and Pain Medicine Recommendations on Local Anesthetics and Adjuvants Dosage in Pediatric Regional Anesthesia. Reg. Anesth. Pain Med. 2018, 43, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Baeriswyl, M.; Zeiter, F.; Piubellini, D.; Kirkham, K.R.; Albrecht, E. The analgesic efficacy of transverse abdominis plane block versus epidural analgesia: A systematic review with meta-analysis. Medicine 2018, 97, e11261. [Google Scholar] [CrossRef] [PubMed]
- İpek, C.B.; Kara, D.; Yılmaz, S.; Yeşiltaş, S.; Esen, A.; Dooply, S.; Karaaslan, K.; Türköz, A. Comparison of ultrasound-guided transversus abdominis plane block, quadratus lumborum block, and caudal epidural block for perioperative analgesia in pediatric lower abdominal surgery. Turk. J. Med. Sci. 2019, 49, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Sethi, N.; Pant, D.; Dutta, A.; Koul, A.; Sood, J.; Chugh, P.T. Comparison of caudal epidural block and ultrasonography-guided transversus abdominis plane block for pain relief in children undergoing lower abdominal surgery. J. Clin. Anesth. 2016, 33, 322–329. [Google Scholar] [CrossRef]
- Mansfield, S.A.; Woodroof, J.; Murphy, A.J.; Davidoff, A.M.; Morgan, K.J. Does epidural analgesia really enhance recovery in pediatric surgery patients? Pediatr. Surg. Int. 2021, 37, 1201–1206. [Google Scholar] [CrossRef]
- Krell, R.W.; Girotti, M.E.; Dimick, J.B. Extended length of stay after surgery: Complications, inefficient practice, or sick patients? JAMA Surg. 2014, 149, 815–820. [Google Scholar] [CrossRef]
- Pogatzki-Zahn, E.; Schnabel, K.; Kaiser, U. Patient-reported outcome measures for acute and chronic pain: Current knowledge and future directions. Curr. Opin. Anaesthesiol. 2019, 32, 616–622. [Google Scholar] [CrossRef]
- Weldring, T.; Smith, S.M.S. Article Commentary: Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs). Health Serv. Insights 2013, 6, HSI.S11093. [Google Scholar] [CrossRef]
- Admiraal, M.; Hermanides, J.; Meinsma, S.L.; Wartenberg, H.C.H.; Rutten, M.V.H.; Heine, Y.; Kallewaard, J.W.; Hollmann, M.W.; Hermanns, H. The effectiveness of a transitional pain service in patients undergoing surgery with an increased risk of developing chronic postsurgical pain (TRUSt study). A randomized clinical trial. J. Clin. Anesth. 2023, 91, 111262. [Google Scholar] [CrossRef] [PubMed]
| N = 65 | |
|---|---|
| Female sex | 46 (70.8%) |
| Age at first surgery, years † | 13.5 [8.8, 16.1] |
| BMI at first surgery, kg/m2 † | 18.3 [15.8, 21.3] |
| Age of onset constipation, years † | 2.0 [0, 7.0] |
| Age of first contact with tertiary hospital, years † | 9.1 [5.9, 14.0] |
| sychological history | |
| PTSD | 4 (6.2%) |
| Anxiety disorder | 8 (12.3%) |
| Depression | 2 (3.1%) |
| Behavioral problems | |
| Autism/PDD-NOS | 10 (15.4%) |
| ADHD/ADD | 5 (7.7%) |
| Eating disorder | 2 (3.1%) |
| Developmental delay | 8 (12.3%) |
| Previous surgeries | |
| Cecostomy button | 22 (30.6%) |
| PEG tube | 21 (32.8%) |
| Appendicostomy | 3 (4.6%) |
| Systemic Analgesia (n = 43) | Neuraxial Analgesia (n = 17) | Truncal Block (n = 5) | |
|---|---|---|---|
| Age at first surgery, years † | 13.5 [8.9, 16.2] | 11.3 [4.8, 14.0] | 15.9 [15.8, 17.6] |
| Type of procedure ‡ | |||
| Ileostomy | 29 (67.4%) | 7 (41.2%) | 4 (80.0%) |
| Colostomy | 9 (20.9%) | 3 (17.6%) | - |
| Sigmoidectomy | 6 (14.0%) | 8 (47.1%) | 1 (20.0%) |
| Subtotal colectomy | 1 (2.3%) | 3 (17.6%) | 1 (20.0%) |
| Technique | |||
| Laparoscopic | 36 (83.7%) | 6 (35.3%) | 3 (60.0%) |
| Open | 4 (9.3%) | 4 (23.5%) | - |
| Hand-assisted | 3 (7.0%) | 7 (41.2%) | 2 (40.0%) |
| Duration surgery, minutes | 122.0 [77.5, 145.5] | 150.0 [135.0, 198.0] | 202.0 [109.0, 220.0] |
| Analgesia intraoperative | |||
| Paracetamol | 15 (34.9%) | 9 (52.9%) | 1 (20.0%) |
| Metamizole | 22 (51.2%) | 9 (52.9%) | 4 (80.0%) |
| Total opioids in IV MME † | 29.0 [18.8, 50.0] | 45.0 [12.5, 70.0] | 40.0 [40.0, 47.0] |
| Total opioids in IV MME/kg † | 0.4 [0.3, 0.6] | 0.3 [0.3, 0.5] | 0.2 [0.2, 0.4] |
| Clonidine or dexmedetomidine | 7 (16.3%) | 5 (29.4%) | 3 (60.0%) |
| Esketamine continuous | 5 (11.6%) | - | 2 (40.0%) |
| Lidocaine continuous | 3 (7.0%) | - | 1 (20.0%) |
| Analgesia postoperative | |||
| Paracetamol, orally | 42 (97.7%) | 15 (88.2%) | 5 (100.0%) |
| NSAID, orally | 18 (41.9%) | 7 (41.2%) | 2 (40.0%) |
| Metamizole, IV | 25 (58.1%) | 12 (63.2%) | 4 (80.0%) |
| Tramadol, orally | 8 (18.6%) | 2 (11.8%) | - |
| Oxycodone, orally | 9 (20.9%) | 4 (23.5%) | 3 (60.0%) |
| PCA opioids | 13 (27.1%) | 6 (31.6%) | 1 (20.0%) |
| Duration PCA opioids, days † | 3.0 [2.0, 4.0] | 5.0 [4.0, 6.0] | 6.0 [3.0, 6.0] |
| Duration strong opioids, days † | 2.0 [1.0, 4.0] | 0.0 [0.0, 3.0] | 6.0 [5.0, 6.0] |
| Clonidine, orally | 10 (23.3%) | 6 (35.3%) | 3 (60.0%) |
| Esketamine, IV | 4 (9.3%) | 2 (11.8%) | 2 (40.0%) |
| Duration esketamine, day † | 2.0 [2.0, 3.2] | 5.0 [3.5, 6.5] | 2.0 [2.0, 2.0] |
| Postoperative nausea and vomiting | 2 (4.7%) | 3 (17.6%) | 3 (60.0%) |
| Systemic Analgesia (n = 43) | Neuraxial Analgesia (n = 17) | Truncal Block (n = 5) | p-Value | |
|---|---|---|---|---|
| NRS 0–24 h (269 NRS scores) †,‡ | 5.0 [2.0, 7.0] | 2.0 [0, 4.0] | 5.0 [3.0, 6.5] | <0.001 *** |
| NRS 24–48 h (187 NRS scores) †,‡ | 4.0 [1.0, 6.0] | 2.0 [0, 4.0] | 4.0 [3.0, 6.5] | 0.003 ** |
| NRS 48–72 h (190 NRS scores) †,‡ | 4.5 [2.0, 6.0] | 2.0 [0, 5.0] | 5.0 [4.0, 6.8] | 0.009 ** |
| NRS 72–96 h (136 NRS scores) | 3.5 [0, 5.0] | 3.0 [0, 5.0] | 5.0 [3.0, 6.0] | 0.117 |
| NRS 96–120 h (120 NRS scores) | 4.0 [1.0, 6.0] | 2.0 [0, 5.0] | 4.0 [2.0, 8.0] | 0.250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Admiraal, M.; van der Burg, F.A.E.; Hermanns, H.; Hermanides, J.; Hollmann, M.W.; Benninga, M.A.; de Jong, J.; Gorter, R.R.; Stevens, M.F. Different Analgesia Techniques for Postoperative Pain in Children Undergoing Abdominal Surgery for Intractable Constipation: A Retrospective Cohort Study in a Single Tertiary Children’s Hospital. J. Clin. Med. 2024, 13, 349. https://doi.org/10.3390/jcm13020349
Admiraal M, van der Burg FAE, Hermanns H, Hermanides J, Hollmann MW, Benninga MA, de Jong J, Gorter RR, Stevens MF. Different Analgesia Techniques for Postoperative Pain in Children Undergoing Abdominal Surgery for Intractable Constipation: A Retrospective Cohort Study in a Single Tertiary Children’s Hospital. Journal of Clinical Medicine. 2024; 13(2):349. https://doi.org/10.3390/jcm13020349
Chicago/Turabian StyleAdmiraal, Manouk, Fleur A. E. van der Burg, Henning Hermanns, Jeroen Hermanides, Markus W. Hollmann, Marc A. Benninga, Justin de Jong, Ramon R. Gorter, and Markus F. Stevens. 2024. "Different Analgesia Techniques for Postoperative Pain in Children Undergoing Abdominal Surgery for Intractable Constipation: A Retrospective Cohort Study in a Single Tertiary Children’s Hospital" Journal of Clinical Medicine 13, no. 2: 349. https://doi.org/10.3390/jcm13020349
APA StyleAdmiraal, M., van der Burg, F. A. E., Hermanns, H., Hermanides, J., Hollmann, M. W., Benninga, M. A., de Jong, J., Gorter, R. R., & Stevens, M. F. (2024). Different Analgesia Techniques for Postoperative Pain in Children Undergoing Abdominal Surgery for Intractable Constipation: A Retrospective Cohort Study in a Single Tertiary Children’s Hospital. Journal of Clinical Medicine, 13(2), 349. https://doi.org/10.3390/jcm13020349





