Abstract
Atrial fibrillation (AF) is the most common arrhythmia in adults. Prevention of the ischaemic risk with oral anticoagulants (OACs) is widely recommended, and current clinical guidelines recommend direct oral anticoagulants (DOACs) as preference therapy for stroke prevention. However, there are currently no clinical practice guidelines or recommendation documents on the optimal management of OACs in patients with AF that specifically address and adapt to the Central American and Caribbean context. The aim of this Delphi-like study is to respond to doubts that may arise in the management of OACs in patients with non-valvular AF in this geographical area. A consensus project was performed on the basis of a systematic review of the literature, a recommended ADOLOPMENT-like approach, and the application of a two-round Delphi survey. In the first round, 31 recommendations were evaluated and 30 reached consensus, of which, 10 unanimously agreed. The study assessed expert opinions in a wide variety of contextualized recommendations for the optimal management of DOACs in patients with non-valvular atrial fibrillation (NVAF). There is a broad consensus on the clinical practice guideline (CPG) statements used related to anticoagulation indication, patient follow-up, anticoagulation therapy complications, COVID-19 management and prevention, and cardiac interventions.
1. Introduction
TAF is the most common cardiac arrhythmia in adults [1,2] and can be either asymptomatic or very disabling [3]. During the last few decades, its prevalence has increased to between 2% and 4% of the adult population, which represents about 46.3-million people worldwide, and it is expected to increase by 2.3 million in the coming decades largely owing to the extended longevity of the general population. The reasons for the observed increase in AF prevalence are not completely understood but may include enhanced detection, rising incidence, improved survival in patients with cardiovascular (CV) conditions predisposing to atrial fibrillation, and greater survival following atrial fibrillation onset [4].
Atrial fibrillation (AF) is associated with an increased risk of cerebral and peripheral arterial thromboembolic incidents [5], thus representing a major public health problem with high comorbidity, an increased relapse and mortality risk, and soaring health care costs [4]. Appropriate preventive treatment for people with ischemic risks is, therefore, essential [1,6]. The simple Atrial Fibrillation Better Care (ABC) holistic pathway (“A” Anticoagulation/Avoid stroke; “B” Better symptom management; “C” Cardiovascular and Comorbidity optimization) is the framework for the general care of AF patients. It has been significantly associated with a lower risk of all causes of death, adverse cardiovascular events, hospitalizations, and lower health-related costs [7,8,9].
The prevention of stroke and systemic thromboembolism with OAC is the cornerstone for the management of atrial fibrillation. Prior to 2009, Vitamin K antagonists (VKA) such as warfarin were the drugs of choice with a high efficacy and known safety profile [10,11]. Since 2009, four DOACs, including one direct thrombin inhibitor (dabigatran etexilate) and three factor Xa inhibitors (apixaban, edoxaban, and rivaroxaban) [12], have been compared with VKA therapy for stroke prevention in non-valvular AF (NVAF) [2]. They have been shown to be at least as effective as VKA in antithrombotic prevention and treatment with a better safety profile. Indeed, they present two major advantages: (1) They are less likely to lead to hemorrhagic events, especially the most severe ones; and (2) They do not require INR monitoring as their anticoagulant effect is very stable and more independent of factors such as patients’ diets or concomitant treatment [12]. Current clinical guidelines recommend DOACs as the preferable therapy for stroke prevention in patients with NVAF [13,14,15].
Multiple risk assessment models have been developed to estimate an individual patient’s risk of stroke or systemic thromboembolism. The first model was the CHADS2 Score, which was developed in 2001 by expert consensus [2]. In 2010, Lip and colleagues published the CHA2DS2-VASc score as an update to the CHADS2 [16]. It was designed to reduce the number of patients with an intermediate risk and to better identify those who were at a low risk of thromboembolic complications [2]. A second model, called the ABC stroke risk score, has also been validated [1]. These two models have been identified as possessing the best evidence for predicting thromboembolic risk. However, in various analyses, the CHA2DS2-VASc score has been shown to have similar or modestly better predictive ability than its predecessor (the CHADS2 score) [1], which led to it being incorporated into most major guidelines as the recommended stroke risk stratification tool [2]. The decision to start a patient on an OAC should not only be based on the benefits but also on the risks (e.g., bleeding) for the individual patient. Several bleeding risk-assessment tools based on patient risk factors have been developed: HAS-BLED is a balanced tool in terms of sensitivity and specificity, whereas the European score, ABC, and mOBRI are high-sensitivity tools, and ORBIT, ATRIA, Shireman, and GARFIELD-AF are high-specificity tools [17]. The HAS-BLED tool has been validated in several clinical trials and is currently the most frequently used tool to screen for the risk of bleeding [18]. This tool incorporates the following risk factors: hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly patients (>65 years), and drugs/alcohol concomitantly.
Prevention of the ischaemic risk with OACs is widely recommended by different global clinical practice guidelines, and recommendations have been made on the management of patients on OACs in different, complex clinical situations [1]. However, there are currently no clinical practice guidelines or recommendation documents on the optimal management of OACs in patients with NVAF that specifically address and adapt to the Central American and Caribbean context.
In order to meet this need, a consensus based on scientific evidence, and the opinion of experts, has been proposed to respond to those doubts that may arise in the management of OACs as a preventive treatment of thrombotic events related to NVAF in this geographical area.
2. Materials and Methods
This study is based on a review of clinical practice guidelines (CPG) and a two-round Delphi-type consensus survey (See Figure 1).
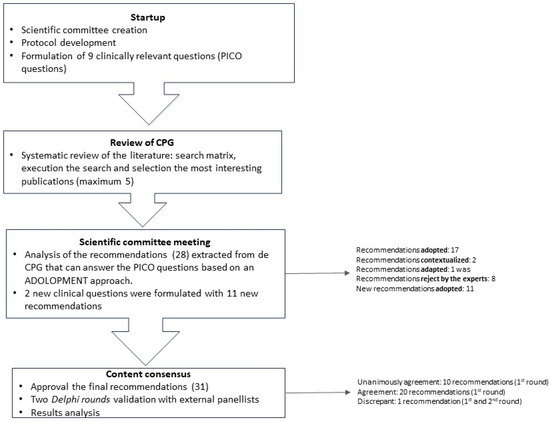
Figure 1.
Methodology flowchart.
A scientific committee was formed, consisting of 19 cardiologists with significant experience in the management of patients with NVAF from El Salvador, Guatemala, Honduras, Panamá, Nicaragua, Costa Rica, Dominican Republic, and Spain. This committee was responsible for the decision-making, including establishing the topics to answer in the study, approving the methodology, identifying the bibliography, proposing the panelists, developing the Delphi statements, and analyzing the results.
A research protocol was developed that described the objectives and methodology of the project, as well as the criteria and requirements for the selection of survey respondents. The scientific committee validated the protocol and developed nine clinical questions following the PICO method (Patient, Intervention, Comparison, Outcomes) [19].
In April 2022, a review of CPGs on NVAF management was conducted, and five guidelines were selected to meet the consensus needs using the following criteria: title, national or international guideline, country, year of publication, and relevance for the project (Table 1). Twenty-eight recommendations that responded to the clinical questions established were extracted from the CPG.

Table 1.
CPG selection.
The recommendations were analyzed in a meeting with the scientific committee in May 2022, applying a structured process, in order to categorize them in terms of accordance, utility, relevance, validity, and feasibility for Central America and the Caribbean, based on an ADOLOPMENT approach [26,27]. This approach combines the advantages of adoption, adaption, and de novo development of guidelines and facilitates structured interaction and deliberation with experts during meetings saving important resources [27] (See Figure 2).
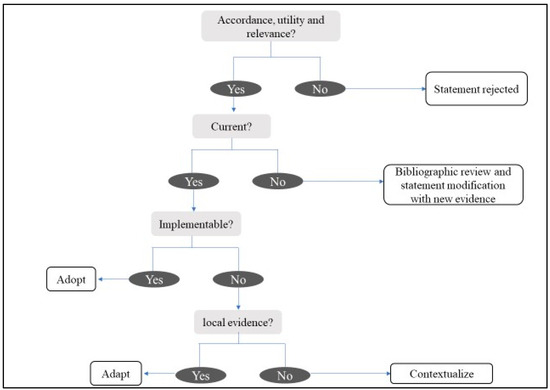
Figure 2.
Analysis and evaluation of recommendations.
During the meeting, the experts discussed the relevance to adopt, adapt, or contextualize the international recommendations, specifically in the Central America and Caribbean region. They also proposed new topics not covered in the initial draft of the project. Moreover, two extra clinical questions were also considered. To answer these new questions, a review of CPGs or consensus documents was conducted, two new CPGs were selected and analyzed, and 11 new recommendations were extracted. The scientific committee ratified the statements to be included in the Delphi-type questionnaire through an online survey (Table 2, Table 3, Table 4, Table 5, Table 6 and Table 7).

Table 2.
First-section consensus statements and level of agreement achieved during the two Delphi rounds.

Table 3.
Second-section consensus statements and level of agreement achieved during the two Delphi rounds.

Table 4.
Third-section consensus statements and level of agreement achieved during the two Delphi rounds.

Table 5.
Fourth-section consensus statements and level of agreement achieved during the two Delphi rounds.

Table 6.
Fifth-section consensus statements and level of agreement achieved during the two Delphi rounds (part 1).

Table 7.
Fifth-section consensus statements and level of agreement achieved during the two Delphi rounds (Part 2).
A panel of 30 participants was selected from a representative series of hospitals and geographic areas from Central America and the Caribbean. Panelists had to meet the following criteria: (1) Experienced in the management of patients with NVAF; (2) Leadership within the medical community; (3) Attending a reasonable number of patients with this disease. The panelists participated in the consensus process to validate the statements through a two-round Delphi-like methodology [28]. The statement questionnaire was designed to be completed online, with a voting system to indicate the level of agreement and fields for the panelists’ comments. It was uploaded on an online platform that also offered access to the CPGs that were used for the questionnaire development. The level of agreement was assessed on a four-point Likert scale (1: Strongly disagree; 2: Disagree; 3: Agree; 4: Strongly agree). Consensus was pre-defined as ≥80% of all respondents rating their agreement as 3 or 4, and unanimous consensus as 100% agreement (all participants voting 4). After the first round, the results and comments of the panelists were analyzed, and statements that reached consensus and those that did not require modification were not submitted to the second round.
In addition, in order to understand the need to adapt and contextualize the international recommendations and the requirements for their implementation in the Central American and Caribbean area, it was decided to ask four open questions during the second round.
3. Results
Related to the ADOLOPMENT-like process, from the initial 28 recommendations, 17 of them were adopted (statement accepted), two were contextualized (statement’s evidence is not modified but some local information is added), one was adapted (statement is modified following local evidence), and eight were rejected by the experts. The 11 recommendations formulated to answer new questions were all adopted. Finally, the statements questionnaire included 31 recommendations (Table 2, Table 3, Table 4, Table 5, Table 6 and Table 7).
For the Delphi survey, all 30 invited panelists participated in the two rounds. Among them, six were from Guatemala, three from El Salvador, three from Honduras, three from Nicaragua, four from Costa Rica, two from Panama, seven from Dominican Republic, and two from Puerto Rico. Twenty-seven out of the 30 were cardiologists, two were neurologists, and one was a geriatric specialist. Half of the experts reported visiting between 25 and 50 patients a month, 40% less than 25 patients a month, and only 10% reported visiting more than 50 patients a month.
In the first round, 31 recommendations were evaluated and 30 reached consensus, of which 10 were unanimously agreed upon. The only recommendation presenting discrepancy [“For patients at “low stroke risk” (CHA2DS2-VASc score = 0 in men, or 1 in women) antithrombotic therapy should not be offered”] was re-submitted unchanged in the second Delphi round as the Scientific Group did not consider it necessary to rephrase it. The results of both rounds can be seen in Table 2, Table 3, Table 4, Table 5, Table 6 and Table 7.
Regarding the additional questions, the first one referred to the considerations that, according to the panelists, should be considered for the implementation and the follow-up of the Delphi statements as recommendations in their country. The primary considerations were the necessity to implement medical training on AF and anticoagulation and the transmission of the latest scientific evidence to all the professionals implicated in the healthcare process. Then, panelists also considered the importance of a better access to practical information about DOACs (e.g., situations of use, early treatment data or impact on morbidity, mortality, and cost) in order to optimize therapeutic decision-making, and highlighted the importance of the economic aspects of the area. (Figure S1).
With the second question, the panelists were asked about the actions to implement in order to facilitate the use and access to DOACs in this area. More than half emphasized the need to act on the economics and prices of DOACs, arguing that their cost is one of the main barriers to their access and use. The other proposed actions were mainly related to information and training on the use of DOACs, and more particularly to the need to inform patients about the risks and benefits of DOACs throughout informative campaigns (Figure S2).
The third question was about the availability of the panelists for monitoring the implementation grade of the consensus recommendations in their country in a particular time frame (about 1 year), and whether they would be interested in collaborating. Most of the experts answered that they would be available, even if some of them pointed out the lack of time and some obligations as reasons for not being able to commit.
Finally, the experts were asked whether they thought it was necessary to implement a monographic consultation dedicated to anticoagulation for the follow-up of patients. A great majority responded positively. They were also asked about the optimal frequency of this intervention. The responses were disparate, with various ranges of frequency (from every month up to 2 years) and depending on whether or not it was the first year of treatment.
4. Discussion
Our study focused on defining the optimal management of OACs in patients with NVAF in Central America and the Caribbean area through the adaptation or contextualization of recommendations from international and recognized CPGs. The recommendations were validated through a two-round Delphi-type consensus with experienced cardiologists, neurologists, and geriatricians from the eight countries of the area [1,20,21,22,23,24,25].
The selection of an anticoagulant agent should be based on shared decision-making that considers risk factors, cost, tolerability, patient preference, potential for drug interactions, and other clinical characteristics, including time in the INR therapeutic range if the patient has been on VKA [22]. As it is shown in the results, DOACs, if they are available, are the recommended drugs for the management of patients with NVAF. The results of our study not only showed agreement in the statements related to anticoagulation indication but also in most of the statements regarding patient follow-up, anticoagulation therapy complications, COVID-19 management and prevention, and cardiac interventions.
The scientific committee included information to contextualize about cost and access to DOACs or to prothrombin complex concentrates, and it considered VKA in absence of DOACs as a valid first-line therapeutic option. The ADOLOPMENT approach, which consists of adapting and contextualizing recommendations, may have had a positive effect among the panelists on achieving consensus in some of the statements. This approach is considered a rigorous, valid, and reproducible alternative methodology for obtaining clinical recommendations at a local level in a lesser time and using fewer resources. Indeed, the use of guidelines developed in other settings may be inappropriate because of different contextual factors such as acceptability or feasibility. There are some limitations to their adaptation, as recommendations are modified to reflect these contextual factors. However, it has been hypothesized that adapting the guidelines to the local setting is expected to improve their uptake and implementation [26,27,29].
Only one of the 31 recommendations did not reach consensus and showed discrepancies between panelists. The discrepant recommendation was related to the management of patients with low stroke risk (CHA2DS2-VASC = 0 in men or 1 in women) and the need of antithrombotic therapy. In the first Delphi round, only 60% of the experts agreed on not offering antithrombotic therapy in this group of patients. Although the percentage of agreement increased to 70% in the second round, it did not meet the consensus level (80%), and the statement remained discrepant.
The scientific committee considered that atrial fibrillation, by itself, is not a reason to initiate anticoagulation therapy, and it must be considered that in low-risk stroke patients anticoagulation risks (mainly bleeding) outweigh the benefits. However, some of the panelists considered that the indication of anticoagulant therapy must be broader. Discrepancy could be explained because there is less agreement on whether to recommend anticoagulation or antiagregation in low-risk patients among reference guideline recommendations. For men with a CHA2DS2-VASC score of 0 and 1 in women, ACCP and ESC guidelines [14,15] recommend omitting antithrombotic therapy, but AHA/ACC/HRS [13] makes a weaker recommendation, stating that it is reasonable to omit anticoagulant therapy. Among panelists’ comments, it stands out that some of them consider patients with atrial fibrillation candidates to anticoagulant therapy if there is no absolute contradiction, regardless of the CHA2DS2-VASC score. Other experts noted that this decision should be individualized, arguing that the CHA2DS2-VASC score does not include pro-coagulant factors such as AF burden or left atrial volume index.
Results of the open-ended questions formulated to panelists are aligned with the process followed by the scientific committee to adapt and contextualize recommendations. In regard to the implementation of recommendations, panelists have pointed out the need to dispose of more practical information about DOAC, to develop local medical training projects, and to implement initiatives for lowering the DOAC’s costs and improving their local access. Experts consider that elevated costs are one of the main limitations to promote the use and access to DOACs in the Central America and Caribbean area and actions must be considered in order to reduce them. VKAs have a lower cost but enable poor anticoagulation control, which has a strong impact on health loss and on increased health system expenses [30]. Reference-pricing policies and the use of generic drugs could lead to decreases in drug prices and to increases in utilization of targeted medications, while also reducing payer and patient expenditures [31,32]. Generic drugs are considered to have the same qualitative and quantitative composition in active substance and the same pharmaceutical form, and whose bioequivalence with the reference drug has been demonstrated by appropriate bioavailability studies [33].
This consensus document can serve as an adapted local guide for the management of OACs in patients with NVAF in the Central American and Caribbean area. In the near future, it will be necessary to readapt it due to the use artificial intelligence (AI) and machine learning in cardiology [34]. AI applications have shown effectiveness in managing AF, aiding in risk assessment beyond CHA2DS2-VASc and HAS-BLED, diagnosis, treatment selection, and remote monitoring. Despite these challenges, such as the need for extensive, high-quality data and ethical considerations, for example, preparing for the AI era is essential for physicians to enhance patient outcomes in chronic diseases.
The design of this study has some limitations inherent to the chosen methodology. Although it was conducted using a robust, well-known, rigorous methodology based on the Delphi technique, it only provides us with qualitative information on the degree of agreement among the panelists based on the available evidence, as well as their clinical practice and experience.
5. Conclusions
The present Delphi-like study assessed expert opinions in a wide variety of contextualized recommendations for the optimal management of DOACs in patients with NVAF. There is a broad consensus on the CPG statements used related to anticoagulation indication, patient follow-up, anticoagulation therapy complications, COVID-19 management and prevention, and cardiac interventions. Considering this, this consensus document can serve as an adapted local guide for the management of these patients.
The results manifest that DOACS are recommended over VKAs. It is important to individualize treatment according to patient’s thrombotic and bleeding risk and to select the best therapeutic strategy conditioned by the level of access to medicines and the clinical context of the patient. As cost and access are important limitation factors, efforts must be made to allow for a better access to DOACs as first line-treatment options in patients with NVAF from Central America and the Caribbean.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13020314/s1, Figure S1: Considerations that, according to the panelists, should be considered for the implementation and follow-up of the Delphi statements as recommended in their country. Figure S2. Actions to implement to facilitate the use and access to DOACs in the Central America and Caribbean area. Table S1: Sources of information. Table S2: Search strategy, specific filters, key keywords.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; provided final approval of the version to be published; and agreed to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Mefasa Farma (Grupo Profarmaco). Mefasa Farma did not contribute to the design of the consensus study, the preparation of statements, or the analysis of the results.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Patient consent was waived because this study is a consensus document among healthcare professionals and the approval by an ethics committee is not mandatory.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors are thankful to the 30 panelists that participated in the Delphi to express their degree of agreement with the statements: Juliana Salas Segura; Oswaldo Gutiérrez Sotelo; Isabel Barrientos Calvo; Miguel Barboza Elizondo; Veronica Gómez Leiva; Martha Abigail Reyes de Vasquez; Fredis Molina; Iris Rodriguez Cermeño; Omar Alonzo Villagran; Jose Carlos Penagos Cordón; Marco Antonio Rodas Estrada; Allan Roberto Rivera Juárez; Jose Tarton Sisimit; Carlos Eduardo Ramos Midence; Brenda Zúniga Rodriguez; Ramon Bueso Cruz; Nuria Zobeida Granados Moreno; Julio Cesar Altamirano Osorio; Rolando Jiron Toruño; Julio Effio Rodriguez; Antonio Rodriguez Ramos; Elaine Nuñez Ayala; Yovanka Abreu Feliz; Ana Noelia Vergés Castro; Alfonso Corral Danna; Fernando Vidal Bett; Reynie Leonel Reinoso Gonell; Persio Jose Lopez Contreras; Wistremundo Dones Figueroa; and Edmundo Jordán Morey. The authors also acknowledge Mefasa Farma (Grupo Profarmaco) for the economic support for the development of this project and GOC Health Consulting for their methodological and medical writing support.
Conflicts of Interest
Fernando Wyss has not received honoraria for participating in research, lecturing, and other financial benefits from Mefasa Farma (Grupo Profarmaco). No potential conflict of interest. Vivencio Barrios has received funding for participating in research, lecturing, and consultancy from Bayer, Boehringer Ingelheim, Daiichi Sankyo, Bristol, Myers Squibb, and Pfizer. Máxima Méndez has no potential conflict of interest. Samuel Ramos has not received honoraria for participating in research, lecturing, and other financial benefit from Mefasa Farma (Grupo Profarmaco). No potential conflict of interest. Osiris Valdez has not received honoraria for participating in research, lecturing, and other financial benefit from Mefasa Farma (Grupo Profarmaco). No potential conflict of interest. Ángel Gonzalez has not received honoraria for participating in research, lecturing, and other financial benefit from Mefasa Farma (Grupo Profarmaco). No potential conflict of interest. Hector Ortiz has no potential conflict of interest. Marco Rodas Díaz has no potential conflict of interest. Gabriela Castillo has no potential conflict of interest. Daniel Quesada has received honorarium for lecturing, research, and advisory boards from Astra Zeneca, Bayer, Pfizer, Servier, and Asofarma. Carlos Enrique Franco has no potential conflict of interest. Jaime Ventura has no potential conflict of interest. Emilio Samael Peralta López has not received honoraria for participating in research, lecturing, and other financial benefit from Mefasa Farma (Grupo Profarmaco). He has received honoraria for lecturing from Pfizer and Bayer. Francisco Somoza has no potential conflict of interest. Ariel Arguello Montealegre has no potential conflict of interest. Daniel Meneses has no potential conflict of interest. Daniel Pichel has received honorarium for lecturing from Novartis, AstraZeneca, Sanofi, Asofarma, Abbott, Pfizer, Sandoz, Eurofarma, Boehringer, and for advisory boards from Novartis, AstraZeneca, Merck Sharpe, and Dohme.
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [PubMed]
- Jame, S.; Barnes, G. Stroke and thromboembolism prevention in atrial fibrillation. Heart 2020, 106, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Beaser, A.; Cifu, A. Management of Patients with Atrial Fibrillation. JAMA Clin. Guidel. Synop. 2019, 321, 1100–1101. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.B.; Yin, X.; Gona, P.; Larson, M.G.; Beiser, A.S.; McManus, D.D.; Newton-Cheh, C.; A Lubitz, S.; Magnani, J.W.; Ellinor, P.T.; et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet 2015, 386, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Soldevila, J.G.; Martínez Ruíz, M.D.; Robert, I.D.; Tornos, P.; Martínez-Rubio, A. Evaluación de riesgo tromboembólico y hemorrágico de los pacientes con fibrilación auricular. Rev. Esp. Cardiol. 2013, 13, 9–13. [Google Scholar] [CrossRef]
- Wolfes, J.; Ellermann, C.; Frommeyer, G.; Eckardt, L. Evidence-based treatment of atrial fibrillation around the globe: Comparison of the latest ESC, AHA/ACC/HRS, and CCS guidelines on the management of atrial fibrillation. Rev. Cardiovasc. Med. 2022, 23, 56. [Google Scholar] [CrossRef] [PubMed]
- Pastori, D.; Farcomeni, A.; Pignatelli, P.; Violi, F.; Lip, G.Y. ABC (Atrial fibrillation Better Care) Pathway and Healthcare Costs in Atrial Fibrillation: The ATHERO-AF Study. Am. J. Med. 2019, 132, 856–861. [Google Scholar] [CrossRef]
- Yoon, M.; Yang, P.S.; Jang, E.; Yu, H.T.; Kim, T.H.; Uhm, J.S.; Kim, J.-Y.; Sung, J.-H.; Pak, H.-N.; Lee, M.-H.; et al. Improved Population-Based Clinical Outcomes of Patients with Atrial Fibrillation by Compliance with the Simple ABC (Atrial Fibrillation Better Care) Pathway for Integrated Care Management: A Nationwide Cohort Study. Thromb. Haemost. 2019, 119, 1695–1703. [Google Scholar] [CrossRef]
- Proietti, M.; Romiti, G.F.; Olshansky, B.; Lane, D.A.; Lip, G.Y.H. Improved Outcomes by Integrated Care of Anticoagulated Patients with Atrial Fibrillation Using the Simple ABC (Atrial Fibrillation Better Care) Pathway. Am. J. Med. 2018, 131, 1359–1366.e6. [Google Scholar] [CrossRef]
- Hart, R.G.; Pearce, L.A.; Aguilar, M.I. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann. Intern. Med. 2007, 146, 857–867. [Google Scholar] [CrossRef]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Franco Moreno, A.I.; Martín Díaz, R.M.; García Navarro, M.J. Direct oral anticoagulants: An update. Med. Clin. 2018, 151, 198–206. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration with the Society of Thoracic Surgeons. Circulation 2019, 140, e125–e151. [Google Scholar]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.-C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016, 18, 1609–1678. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.H.; Banerjee, A.; Boriani, G.; Chiang, C.E.; Fargo, R.; Freedman, B.; Lane, D.A.; Ruff, C.T.; Turakhia, M.; Werring, D.; et al. Antithrombotic Therapy for Atrial Fibrillation: CHEST Guideline and Expert Panel Report. Chest 2018, 154, 1121–1201. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Xie, Q.; Ma, L.; Hu, K.; Zhang, Z.; Mu, G.; Cui, Y. Accuracy of HAS-BLED and other bleeding risk assessment tools in predicting major bleeding events in atrial fibrillation: A network meta-analysis. J. Thromb. Haemost. 2020, 18, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Pisters, R.; Lane, D.A.; Nieuwlaat, R.; de Vos, C.B.; Crijns, H.J.; Lip, G.Y. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest 2010, 138, 1093–1100. [Google Scholar] [CrossRef]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The well-built clinical question: A key to evidence-based decisions. ACP J. Club 1995, 123, A12–A13. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Collet, J.P.; Haude, M.; Byrne, R.; Chung, E.H.; Fauchier, L.; Halvorsen, S.; Lau, D.; Lopez-Cabanillas, N.; Lettino, M.; et al. 2018 Joint European consensus document on the management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous cardiovascular interventions: A joint consensus document of the European Heart Rhythm Association (EHRA), European Society of Cardiology Working Group on Thrombosis, European Association of Percutaneous Cardiovascular Interventions (EAPCI), and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA). Europace 2019, 21, 192–193. [Google Scholar]
- Task Force for the Management of COVID-19 of the European Society of Cardiology. ESC guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: Part 2-care pathways, treatment, and follow-up. Eur. Heart J. 2022, 43, 1059–1103. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Estes, N.A.M., 3rd; Fonarow, G.C.; Jurgens, C.Y.; Kittleson, M.M.; Marine, J.E.; McManus, D.D.; McNamara, R.L. 2020 Update to the 2016 ACC/AHA Clinical Performance and Quality Measures for Adults with Atrial Fibrillation or Atrial Flutter: A Report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J. Am. Coll. Cardiol. 2021, 77, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Tomaselli, G.F.; Mahaffey, K.W.; Cuker, A.; Dobesh, P.P.; Doherty, J.U.; Eikelboom, J.W.; Florido, R.; Gluckman, T.J.; Hucker, W.J.; Mehran, R.; et al. 2020 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2020, 76, 594–622. [Google Scholar] [CrossRef] [PubMed]
- Vivas, D.; Roldan, I.; Ferrandis, R.; Marin, F.; Roldan, V.; Tello-Montoliu, A.; Ruiz-Nodar, J.M.; Gómez-Doblas, J.J.; Martín, A.; Llau, J.V.; et al. Perioperative and Periprocedural Management of Antithrombotic Therapy: Consensus Document of SEC, SEDAR, SEACV, SECTCV, AEC, SECPRE, SEPD, SEGO, SEHH, SETH, SEMERGEN, SEMFYC, SEMG, SEMICYUC, SEMI, SEMES, SEPAR, SENEC, SEO, SEPA, SERVEI, SECOT and AEU. Rev. Esp. Cardiol. (Engl. Ed.) 2018, 71, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Doherty, J.U.; Gluckman, T.J.; Hucker, W.J.; Januzzi, J.L.; Ortel, T.L., Jr.; Saxonhouse, S.J.; Spinler, S.A. 2017 ACC Expert Consensus Decision Pathway for Periprocedural Management of Anticoagulation in Patients with Nonvalvular Atrial Fibrillation: A Report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J. Am. Coll. Cardiol. 2017, 69, 871–898. [Google Scholar] [CrossRef] [PubMed]
- Dizon, J.M.; Machingaidze, S.; Grimmer, K. To adopt, to adapt, or to contextualise? The big question in clinical practice guideline development. BMC Res. Notes 2016, 9, 442. [Google Scholar] [CrossRef]
- Schünemann, H.J.; Wiercioch, W.; Brozek, J.; Etxeandia-Ikobaltzeta, I.; Mustafa, R.A.; Manja, V.; Brignardello-Petersen, R.; Neumann, I.; Falavigna, M.; Alhazzani, W.; et al. GRADE Evidence to Decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. J. Clin. Epidemiol. 2017, 81, 101–110. [Google Scholar] [CrossRef]
- Dalkey, N.C. The Delphi Method: An Experimental Study of Group Opinion; Rand Corp Santa Monica Calif: Santa Monica, CA, USA, 1969. [Google Scholar]
- Darzi, A.; Harfouche, M.; Arayssi, T.; Alemadi, S.; Alnaqbi, K.A.; Badsha, H.; Al Balushi, F.; Elzorkany, B.; Halabi, H.; Hamoudeh, M.; et al. Adaptation of the 2015 American College of Rheumatology treatment guideline for rheumatoid arthritis for the Eastern Mediterranean Region: An exemplar of the GRADE Adolopment. Health Qual Life Outcomes 2017, 15, 183. [Google Scholar] [CrossRef]
- Barrios, V.; Cinza-Sanjurjo, S.; Gavín, O.; Egocheaga, I.; Burgos-Pol, R.; Soto, J.; Polanco, C.; Suárez, J.; Casado, M.Á. Cost and burden of poor anticoagulation control with vitamin K antagonists in patients with nonvalvular atrial fibrillation in Spain. Rev. Esp. Cardiol. (Engl. Ed.) 2021, 74, 773–780. [Google Scholar] [CrossRef]
- Lee, J.L.; Fischer, M.A.; Shrank, W.H.; Polinski, J.M.; Choudhry, N.K. A systematic review of reference pricing: Implications for US prescription drug spending. Am. J. Manag. Care 2012, 18, e429–e437. [Google Scholar]
- Miller, S. Generic Drugs: A Treatment for High-Cost Health Care. Mo. Med. 2020, 117, 12–13. [Google Scholar] [PubMed]
- Birkett, D. Generics—Equal or not? Aust. Prescr. 2003, 26, 85–87. [Google Scholar] [CrossRef]
- Hayıroğlu, M.İ.; Altay, S. The Role of Artificial Intelligence in Coronary Artery Disease and Atrial Fibrillation. Balk. Med. J. 2023, 40, 151–152. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).