Navigating the ICI Combination Treatment Journey: Patterns of Response and Progression to First-Line ICI-Based Combination Treatment in Metastatic Renal Cell Carcinoma
Abstract
1. Introduction
2. First-Line Therapy for Clear Cell Histology
| CheckMate 214: Nivolumab + Ipilimumab [26] | KEYNOTE-426: Pembrolizumab + Axitinib [27,28,29,30] | CheckMate 9ER: Nivolumab + Cabozantinib [31] | CLEAR: Lenvatinib + Pembrolizumab [32,33] | |
|---|---|---|---|---|
| POPULATION AND FOLLOW-UP | ||||
| Median follow-up for OS | 67.7 months | 30.6 months ¤ | 32.9 months | 33.7 months † |
| Number of patients in the ITT population in the experimental arm versus sunitinib arm (safety population) | 550 (547) versus 546 (535) | 432 (429) versus 429 (425) | 323 (320) versus 328 (320) | 355 (352) versus 357 (340) |
| Pts characteristics according to IMDC score: Favourable/Intermediate/Poor (% *) ^ | 125 (22.7)/ 334 (60.7)/ 91 (16.5) | 138 (31.9)/ 238 (55.1)/ 56 (12.7) | 74 (22.9%)/ 188 (58.2%)/ 61 (18.9%) | 110 (31.0)/ 210 (59.2)/ 33 (9.3)/ 2 pts could not be evaluated (0.6) |
| Number of patients that discontinued therapy at data cut-off (% *) | 516 (93.8) | 312 (72.2) | 228 (70.6) | 238 (67.7) |
| OUTCOMES | ||||
| Discontinuation of one or both experimental drugs for drug-related AEs # | 148 (27.0). (148 discontinued both drugs; single drug interruption was not possible) | 148 (34.5). (92 discontinued at least pembrolizumab, 56 axitinib, 28 both) | 87 (27.2). (34 discontinued nivolumab only, 29 dc cabozantinib only, 20 both) | 131 (37.2) reported as any Aes leading to discontinuation (90 discontinued at least lenvatinib, 101 pembrolizumab, 47 both drugs) † |
| Discontinuation of one or both experimental drugs for AEs unrelated to treatment (% #) | 40 (7.3) | Not reported | 24 (7.4) | See above. † |
| Discontinuation for disease progression (% #) | 266 (48.6) | 181 (42.2) | 129 (40.3) | 116 (33.0) † |
| Completion of ICI and continuing TKI where applicable (% #) | Not applicable | 19 (4.4) | Not reported. | 101 (28.9) |
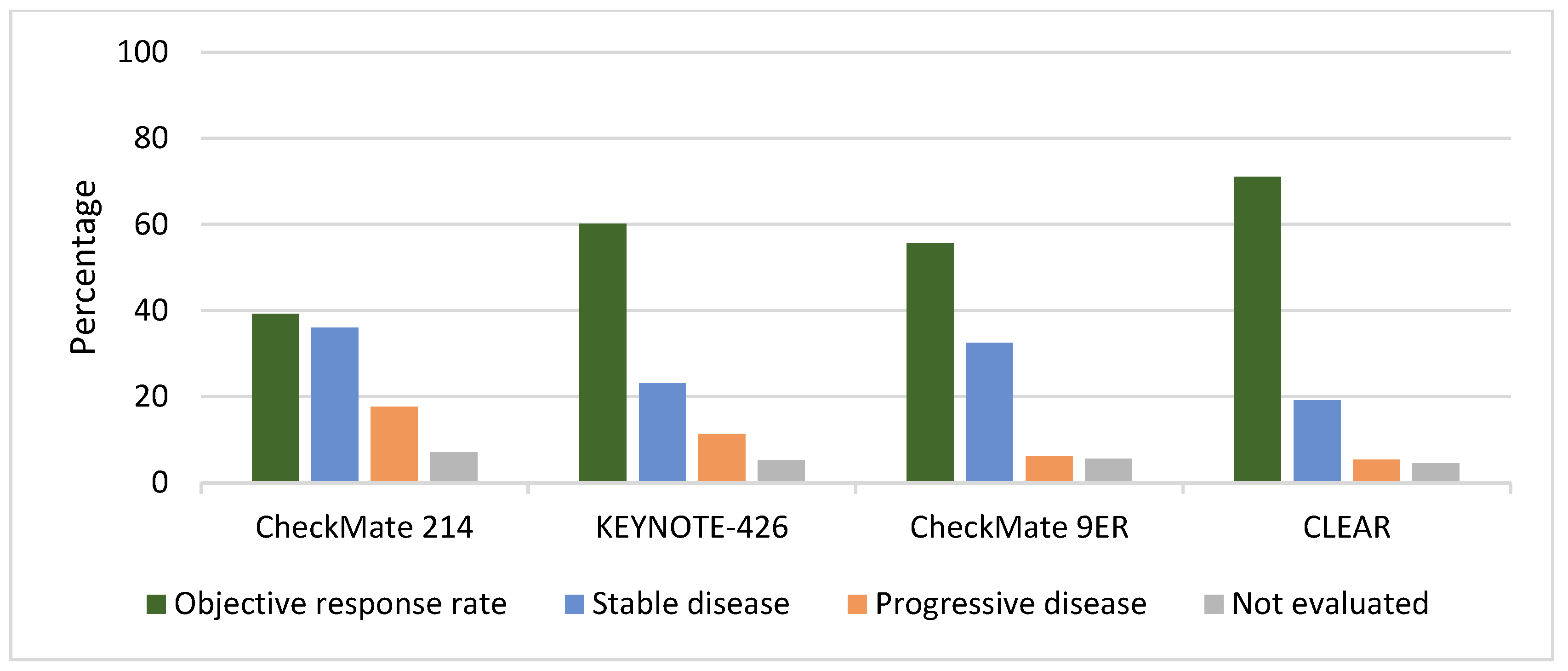
| Number of patients that had the best overall response to experimental drug: CR/PR/SD/PD/not evaluated (% *) | 64 (11.6) 152 (27.6) 198 (36.0) 97 (17.6) 39 (7.1%) | 38 (8.8)/CR increased to 10% over time § 222 (51.4) 100 (23.1) 49 (11.3) 23 (5.3) | 40 (12.4) 140 (43.3) 105 (32.5) 20 (6.2) 18 (5.6) | 61 (17.2) 191 (53.8) 68 (19.2) 19 (5.4) 16 (4.5) |
| Number of patients that had the best overall response to sunitinib: CR/PR/SD/PD/not evaluated or unable to determine (% *) | 14 (2.6) 163 (29.9) 230 (42.1) 77 (14.1) 62 (11.3) | 13 (3.0) 158 (36.8) 150 (35.0) 74 (17.2) 34 (7.9) | 17 (5.2) 76 (23.2) 134 (40.9) 45 (13.7) 56 (17.1) | 15 (4.2) 114 (31.9) 136 (38.1) 50 (14.0) 42 (11.8) |
| Median duration of response (DoR) in the experimental treatment group versus the sunitinib group (95% CI) | Not reached (59.0–not estimable) versus 24.8 months (19.7–30.1) | 23.5 months (19.4–29.0) versus 15.9 months (13.8–20.4) | 23.1 months (20.2–27.9) versus 15·1 months (9.9–20.5) | 26.0 (22.2–41.4) versus 14.7 (9.4–16.8) |
| Ongoing responses (as % of responders; as % of ITT population) at median follow-up time reported in the first row | 136 (47.7; 24.9) | 62 (23.9; 14.4) § | 88 (48.9; 27.2) | Not reported |
| SAFETY | ||||
| Any G3-4 AEs (exp arm versus sunitinib arm) (% #) | 373 (68.2) versus 417 (77.9) | Not reported as all G3-4 AEs | Not reported | 74.1% versus 60.3% † |
| Treatment-related G3-4 AEs (exp arm versus sunitinib arm) (% #) | 263 (48.1) versus 344 (64.3) | 283 (66.0) versus 259 (61.0) | 208 (65.0) vs. 172 (53.8) | 252 (71.6) versus 200 (58.8) (includes only trAEs occurring in 10% or more of the patients) † |
| Treatment-related deaths (exp arm versus sunitinib arm) (% #) | 8 (1.5) versus 5 (0.9) | 4 (0.9) versus 6 (1.4) | 1 (0.3) versus 3 (0.9) | 4 (1.1) versus 1 (0.3) † |
| Any death not related to PD or treatment (exp arm versus sunitinib arm) (% #) | Not reported | 19 (4.4) versus 17 (4.0) | 3 (0.9 vs. 3 (0.9) | Not reported |
| SUBSEQUENT THERAPIES | ||||
| Number of patients that received subsequent systemic therapies after discontinuation for any reason (as % of patients that discontinued treatment #) | 305 (55.7) | 204 (47.2) § | 70 (30.7) | 132 (37.5) |
| Type of subsequent systemic therapies after discontinuation (total number of patients) ~ |
|
|
|
|
3. Possible Outcomes in mccRCC following a Combination Treatment
3.1. Progression While on Treatment
3.1.1. Pseudoprogression
3.1.2. Hyperprogressive Disease
3.1.3. True Progression
3.1.4. Oligoprogression
3.1.5. Systemic Progression
3.1.6. Therapy beyond Progression
3.1.7. Changing Systemic Therapy
3.2. Discontinuation for Adverse Events
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Cimadamore, A.; Cheng, L.; Scarpelli, M.; Massari, F.; Mollica, V.; Santoni, M.; Lopez-Beltran, A.; Montironi, R.; Moch, H. Towards a new WHO classification of renal cell tumor: What the clinician needs to know—A narrative review. Transl. Androl. Urol. 2021, 10, 1506–1520. [Google Scholar] [CrossRef] [PubMed]
- Santoni, M.; Massari, F.; Myint, Z.W.; Iacovelli, R.; Pichler, M.; Basso, U.; Kopecky, J.; Kucharz, J.; Buti, S.; Rizzo, M.; et al. Global Real-World Outcomes of Patients Receiving Immuno-Oncology Combinations for Advanced Renal Cell Carcinoma: The ARON-1 Study. Target. Oncol. 2023, 18, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Klapper, J.A.; Downey, S.G.; Smith, F.O.; Yang, J.C.; Hughes, M.S.; Kammula, U.S.; Sherry, R.M.; Royal, R.E.; Steinberg, S.M.; Rosenberg, S. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma. Cancer 2008, 113, 293–301. [Google Scholar] [CrossRef]
- Rowena, N.; Schwartz, P.; Lori Stover, R.N.; Janice, P.; Dutcher, M.D. Managing Toxicities of High-Dose Interleukin-2. Oncology 2002, 16, 11–20. [Google Scholar]
- Minasian, L.M.; Motzer, R.J.; Gluck, L.; Mazumdar, M.; Vlamis, V.; Krown, S.E. Interferon alfa-2a in advanced renal cell carcinoma: Treatment results and survival in 159 patients with long-term follow-up. J. Clin. Oncol. 1993, 11, 1368–1375. [Google Scholar] [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Tomczak, P.; Michaelson, M.D.; Bukowski, R.M.; Rixe, O.; Oudard, S.; Negrier, S.; Szczylik, C.; Kim, S.T.; et al. Sunitinib versus Interferon Alfa in Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 2007, 356, 115–124. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Yang, J.C.; Topalian, S.L.; Schwartzentruber, D.J.; Weber, J.S.; Parkinson, D.R.; Seipp, C.A.; Einhorn, J.H.; White, D.E. Treatment of 283 Consecutive Patients With Metastatic Melanoma or Renal Cell Cancer Using High-Dose Bolus Interleukin 2. JAMA 1994, 271, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Zambrana, F.; Carril-Ajuria, L.; Gómez de Liaño, A.; Martinez Chanza, N.; Manneh, R.; Castellano, D.; de Velasco, G. Complete response and renal cell carcinoma in the immunotherapy era: The paradox of good news. Cancer Treat. Rev. 2021, 99, 102239. [Google Scholar] [CrossRef] [PubMed]
- Buchler, T.; Bortlicek, Z.; Poprach, A.; Pavlik, T.; Veskrnova, V.; Honzirkova, M.; Zemanova, M.; Fiala, O.; Kubackova, K.; Slaby, O.; et al. Outcomes for Patients with Metastatic Renal Cell Carcinoma Achieving a Complete Response on Targeted Therapy: A Registry-based Analysis. Eur. Urol. 2016, 70, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Hessel, C.; Halabi, S.; Sanford, B.; Michaelson, M.D.; Hahn, O.; Walsh, M.; Olencki, T.; Picus, J.; Small, E.J.; et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): Progression-free survival by independent review and overall survival update. Eur. J. Cancer 2018, 94, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Plimack, E.R.; Procopio, G.; McDermott, D.F.; et al. Nivolumab versus Everolimus in Patients with Advanced Renal Cell Carcinoma: Updated Results with Long-Term Follow-Up of the Randomized, Open-Label, Phase 3 CheckMate 025 Trial. Cancer 2020, 126, 4156–4167. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Burotto, M.; Choueiri, T.K.; Hammers, H.J.; Plimack, E.R.; Porta, C.G.; George, S.; Powles, T.B.; et al. 661P Conditional survival and 5-year follow-up in CheckMate 214: First-line nivolumab + ipilimumab (N+I) versus sunitinib (S) in advanced renal cell carcinoma (aRCC). Ann. Oncol. 2021, 32, S685–S687. [Google Scholar] [CrossRef]
- Lee, D.; Gittleman, H.; Weinstock, C.; Suzman, D.L.; Bloomquist, E.; Agrawal, S.; Brave, M.H.; Brewer, J.R.; Singh, H.; Tang, S.; et al. An FDA-pooled analysis of frontline combination treatment benefits by risk groups in metastatic renal cell carcinoma (mRCC). J. Clin. Oncol. 2021, 39, 4559. [Google Scholar] [CrossRef]
- Lee, D.; Gittleman, H.; Weinstock, C.; Suzman, D.; Bloomquist, E.; Agrawal, S.; Brave, M.; Brewer, J.; Fallah, J.; Singh, H.; et al. A U.S. Food and Drug Administration–pooled Analysis of Frontline Combination Treatment Survival Benefits by Risk Groups in Metastatic Renal Cell Carcinoma. Eur. Urol. 2023, 84, 373–378. [Google Scholar] [CrossRef]
- Heng, D.Y.C.; Xie, W.; Regan, M.M.; Warren, M.A.; Golshayan, A.R.; Sahi, C.; Eigl, B.J.; Ruether, J.D.; Cheng, T.; North, S.; et al. Prognostic Factors for Overall Survival in Patients with Metastatic Renal Cell Carcinoma Treated with Vascular Endothelial Growth Factor–Targeted Agents: Results from a Large, Multicenter Study. J. Clin. Oncol. 2009, 27, 5794–5799. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Motzer, R.J.; Choueiri, T.K.; Rini, B.I.; Miyake, H.; Uemura, H.; Albiges, L.; Fujii, Y.; Umeyama, Y.; Wang, J.; et al. Efficacy and Safety of Avelumab plus Axitinib in Elderly Patients with Advanced Renal Cell Carcinoma: Extended Follow-Up Results from JAVELIN Renal 101. ESMO Open 2022, 2, 100450. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Powles, T.; Atkins, M.B.; Escudier, B.; McDermott, D.F.; Alekseev, B.Y.; Lee, J.-L.; Suarez, C.; Stroyakovskiy, D.; De Giorgi, U.; et al. Final Overall Survival and Molecular Analysis in IMmotion151, a Phase 3 Trial Comparing Atezolizumab Plus Bevacizumab vs Sunitinib in Patients with Previously Untreated Metastatic Renal Cell Carcinoma. JAMA Oncol. 2022, 8, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Powles, T.; Albiges, L.; Burotto, M.; Szczylik, C.; Zurawski, B.; Yanez Ruiz, E.; Maruzzo, M.; Suarez Zaizar, A.; Fein, L.E.; et al. Cabozantinib plus Nivolumab and Ipilimumab in Renal-Cell Carcinoma. N. Engl. J. Med. 2023, 388, 1767–1778. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.Q.; Ye, M.J.; Zou, Q.; Chen, P.; He, Z.S.; Wu, B.; He, D.L.; He, C.H.; Xue, X.Y.; Ji, Z.G.; et al. Toripalimab plus Axitinib versus Sunitinib as First-Line Treatment for Advanced Renal Cell Carcinoma: RENOTORCH, a Randomized, Open-Label, Phase III Study. Ann. Oncol. 2023, in press. [CrossRef] [PubMed]
- Motzer, R.J.; McDermott, D.F.; Escudier, B.; Burotto, M.; Choueiri, T.K.; Hammers, H.J.; Barthélémy, P.; Plimack, E.R.; Porta, C.; George, S.; et al. Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma. Cancer 2022, 128, 2085–2097. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Plimack, E.R.; Soulières, D.; Waddell, T.; Stus, V.; Gafanov, R.; Nosov, D.; Pouliot, F.; Melichar, B.; Vynnychenko, I.; et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): Extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020, 21, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Waddell, T.; Gafanov, R.; Pouliot, F.; Nosov, D.; Melichar, B.; Soulieres, D.; Borchiellini, D.; et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for advanced clear cell renal cell carcinoma (ccRCC): Results from 42-month follow-up of KEYNOTE-426. J. Clin. Oncol. 2021, 39, 4500. [Google Scholar] [CrossRef]
- Gafanov, R.; Powles, T.; Bedke, J.; Stus, V.; Waddell, T.; Nosov, D.; Pouliot, F.; Soulieres, D.; Melichar, B.; Azevedo, S.; et al. Subsequent Therapy Following Pembrolizumab Plus Axitinib or Sunitinib Treatment for Advanced Renal Cell Carcinoma in the Phase 3 KEYNOTE-426 Study. Ann. Oncol. 2021, 32 (Suppl. S5), S678–S724. [Google Scholar] [CrossRef]
- Powles, T.; Plimack, E.R.; Stus, V.; Waddell, T.; Gafanov, R.; Pouliot, F.; Nosov, D.; Melichar, B.; Soulieres, D.; Borchiellini, D.; et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for advanced clear cell renal cell carcinoma (ccRCC): Analysis of progression after first subsequent therapy in KEYNOTE-426. J. Clin. Oncol. 2022, 40, 4513. [Google Scholar] [CrossRef]
- Motzer, R.J.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Shah, A.Y.; Suárez, C.; Hamzaj, A.; Porta, C.; Hocking, C.M.; et al. Nivolumab plus cabozantinib versus sunitinib in first-line treatment for advanced renal cell carcinoma (CheckMate 9ER): Long-Term follow-up results from an open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Eto, M.; Motzer, R.; De Giorgi, U.; Buchler, T.; Basappa, N.S.; Méndez-Vidal, M.J.; Tjulandin, S.; Hoon Park, S.; Melichar, B.; et al. Lenvatinib plus pembrolizumab versus sunitinib as first-line treatment of patients with advanced renal cell carcinoma (CLEAR): Extended follow-up from the phase 3, randomised, open-label study. Lancet Oncol. 2023, 24, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.; Alekseev, B.; Rha, S.-Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Kopyltsov, E.; Méndez-Vidal, M.J.; et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Waddell, T.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulieres, D.; Melichar, B.; et al. Pembrolizumab plus axitinib versus sunitinib as first-line therapy for advanced clear cell renal cell carcinoma: 5-year analysis of KEYNOTE-426. J. Clin. Oncol. 2023, 41, LBA4501. [Google Scholar] [CrossRef]
- Motzer, R.J.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Alekseev, B.; Rha, S.Y.; Merchan, J.R.; Goh, J.C.; et al. Final prespecified overall survival (OS) analysis of CLEAR: 4-Year follow-up of lenvatinib plus pembrolizumab (L+P) vs sunitinib (S) in patients (pts) with advanced renal cell carcinoma (aRCC). J. Clin. Oncol. 2023, 41, 4502. [Google Scholar] [CrossRef]
- Wei, H.; Miao, J.; Cui, J.; Zheng, W.; Chen, X.; Zhang, Q.; Liu, F.; Mao, Z.; Qiu, S.; Zhang, D. The prognosis and clinicopathological features of different distant metastases patterns in renal cell carcinoma: Analysis based on the SEER database. Sci. Rep. 2021, 11, 17822. [Google Scholar] [CrossRef]
- Desai, K.; Brown, L.; Wei, W.; Tucker, M.; Kao, C.; Kinsey, E.; Rini, B.; Beckermann, K.; Zhang, T.; Ornstein, M.C. A Multi-institutional, Retrospective Analysis of Patients with Metastatic Renal Cell Carcinoma to Bone Treated with Combination Ipilimumab and Nivolumab. Target. Oncol. 2021, 16, 633–642. [Google Scholar] [CrossRef]
- Quhal, F.; Mori, K.; Bruchbacher, A.; Resch, I.; Mostafaei, H.; Pradere, B.; Schuettfort, V.M.; Laukhtina, E.; Egawa, S.; Fajkovic, H.; et al. First-line Immunotherapy-based Combinations for Metastatic Renal Cell Carcinoma: A Systematic Review and Network Meta-analysis. Eur. Urol. Oncol. 2021, 4, 755–765. [Google Scholar] [CrossRef]
- Rini, B.I.; Signoretti, S.; Choueiri, T.K.; McDermott, D.F.; Motzer, R.J.; George, S.; Powles, T.; Donskov, F.; Tykodi, S.S.; Pal, S.K.; et al. Long-term outcomes with nivolumab plus ipilimumab versus sunitinib in first-line treatment of patients with advanced sarcomatoid renal cell carcinoma. J. Immunother. Cancer 2022, 10, e005445. [Google Scholar] [CrossRef]
- Rocha, P.; Hardy-Werbin, M.; Naranjo, D.; Taus, Á.; Rodrigo, M.; Zuccarino, F.; Roth, R.; Wood, O.; Ottensmeier, C.H.; Arriola, E. CD103+CD8+ Lymphocytes Characterize the Immune Infiltration in a Case with Pseudoprogression in Squamous NSCLC. J. Thorac. Oncol. 2018, 13, e193–e196. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Q.; Dong, Q.; Zhan, L.; Zhang, J. How to differentiate pseudoprogression from true progression in cancer patients treated with immunotherapy. Am. J. Cancer Res. 2019, 9, 1546–1553. [Google Scholar] [PubMed]
- Park, H.J.; Kim, K.W.; Pyo, J.; Suh, C.H.; Yoon, S.; Hatabu, H.; Nishino, M. Incidence of Pseudoprogression during Immune Checkpoint Inhibitor Therapy for Solid Tumors: A Systematic Review and Meta-Analysis. Radiology 2020, 297, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Giobbie-Hurder, A.; Gargano, M.; Suda, M.; Ramaiya, N.H.; Hodi, F.S. Developing a Common Language for Tumor Response to Immunotherapy: Immune-Related Response Criteria Using Unidimensional Measurements. Clin. Cancer Res. 2013, 19, 3936–3943. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Hoos, A.; O’Day, S.; Weber, J.S.; Hamid, O.; Lebbé, C.; Maio, M.; Binder, M.; Bohnsack, O.; Nichol, G.; et al. Guidelines for the Evaluation of Immune Therapy Activity in Solid Tumors: Immune-Related Response Criteria. Clin. Cancer Res. 2009, 15, 7412–7420. [Google Scholar] [CrossRef] [PubMed]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, Y.; Hirano, K. Which criteria should we use to evaluate the efficacy of immune-checkpoint inhibitors? Ann. Transl. Med. 2018, 6, 222. [Google Scholar] [CrossRef] [PubMed]
- Chiou, V.L.; Burotto, M. Pseudoprogression and Immune-Related Response in Solid Tumors. J. Clin. Oncol. 2015, 33, 3541–3543. [Google Scholar] [CrossRef]
- Soria, F.; Beleni, A.I.; D’Andrea, D.; Resch, I.; Gust, K.M.; Gontero, P.; Shariat, S.F. Pseudoprogression and hyperprogression during immune checkpoint inhibitor therapy for urothelial and kidney cancer. World J. Urol. 2018, 36, 1703–1709. [Google Scholar] [CrossRef]
- Escudier, B.; Motzer, R.J.; Sharma, P.; Wagstaff, J.; Plimack, E.R.; Hammers, H.J.; Donskov, F.; Gurney, H.; Sosman, J.A.; Zalewski, P.G.; et al. Treatment Beyond Progression in Patients with Advanced Renal Cell Carcinoma Treated with Nivolumab in CheckMate 025. Eur. Urol. 2017, 72, 368–376. [Google Scholar] [CrossRef]
- Melian, M.; Lorente, D.; Aparici, F.; Juan, O. Lung brain metastasis pseudoprogression after nivolumab and ipilimumab combination treatment. Thorac. Cancer 2018, 9, 1770–1773. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Larribere, L.; Pan, L.; Haberkorn, U.; Dimitrakopoulou-Strauss, A.; Hassel, J.C. Predictive value of early 18F-FDG PET/CT studies for treatment response evaluation to ipilimumab in metastatic melanoma: Preliminary results of an ongoing study. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, A.M.; Danielli, R.; Guidoboni, M.; Calabrò, L.; Carlucci, D.; Miracco, C.; Volterrani, L.; Mazzei, M.A.; Biagioli, M.; Altomonte, M.; et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: Clinical and immunological evidence from three patient cases. Cancer Immunol. Immunother. CII 2009, 58, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Manitz, J.; D’Angelo, S.P.; Apolo, A.B.; Eggleton, S.P.; Bajars, M.; Bohnsack, O.; Gulley, J.L. Comparison of tumor assessments using RECIST 1.1 and irRECIST, and association with overall survival. J. Immunother. Cancer 2022, 10, e003302. [Google Scholar] [CrossRef] [PubMed]
- Kurra, V.; Sullivan, R.J.; Gainor, J.F.; Hodi, F.S.; Gandhi, L.; Sadow, C.A.; Harris, G.J.; Flaherty, K.; Lee, S. Pseudoprogression in cancer immunotherapy: Rates, time course and patient outcomes. J. Clin. Oncol. 2016, 34, 6580. [Google Scholar] [CrossRef]
- Tazdait, M.; Mezquita, L.; Lahmar, J.; Ferrara, R.; Bidault, F.; Ammari, S.; Balleyguier, C.; Planchard, D.; Gazzah, A.; Soria, J.C.; et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: Comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur. J. Cancer 2018, 88, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Colle, R.; Radzik, A.; Cohen, R.; Pellat, A.; Lopez-Tabada, D.; Cachanado, M.; Duval, A.; Svrcek, M.; Menu, Y.; André, T. Pseudoprogression in patients treated with immune checkpoint inhibitors for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer. Eur. J. Cancer 2021, 144, 9–16. [Google Scholar] [CrossRef]
- Lee, C.-H.; Shah, A.Y.; Hsieh, J.J.; Rao, A.; Pinto, A.; Bilen, M.A.; Cohn, A.L.; Di Simone, C.; Shaffer, D.R.; Girones Sarrio, R.; et al. 710P Phase II trial of lenvatinib (LEN) + pembrolizumab (PEMBRO) for progressive disease after PD-1/PD-L1 immune checkpoint inhibitor (ICI) in metastatic clear cell (mcc) renal cell carcinoma (RCC): Results by independent imaging review and subgroup analyses. Ann. Oncol. 2020, 31, S558–S559. [Google Scholar] [CrossRef]
- Gomez-Roca, C.; Koscielny, S.; Ribrag, V.; Dromain, C.; Marzouk, I.; Bidault, F.; Bahleda, R.; Ferté, C.; Massard, C.; Soria, J.-C. Tumour growth rates and RECIST criteria in early drug development. Eur. J. Cancer 2011, 47, 2512–2516. [Google Scholar] [CrossRef]
- Ferrara, R.; Mezquita, L.; Texier, M.; Lahmar, J.; Audigier-Valette, C.; Tessonnier, L.; Mazieres, J.; Zalcman, G.; Brosseau, S.; Le Moulec, S.; et al. Hyperprogressive Disease in Patients With Advanced Non–Small Cell Lung Cancer Treated With PD-1/PD-L1 Inhibitors or With Single-Agent Chemotherapy. JAMA Oncol. 2018, 4, 1543–1552. [Google Scholar] [CrossRef]
- Matos Garcia, I.; Azaro, A.; Viaplana, C.; Hierro, C.; Martin-Liberal, J.; Brana, I.; Gardeazabal, I.; Gomila, P.; Vieito Villar, M.; Ochoa De Olza Amat, M.; et al. Immune prognostic index (IPI) and hyper-progressive disease (HPD) in patients (pts) exposed to targeted agents (TAs) in phase I trials (Ph1T): Can lessons from immune checkpoint inhibitors (ICIs) be translated to other scenarios? Ann. Oncol. 2018, 29, viii35. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, K.H.; Kang, J.; Borcoman, E.; Saada-Bouzid, E.; Kronbichler, A.; Hong, S.H.; de Rezende, L.F.M.; Ogino, S.; Keum, N.; et al. Hyperprogressive Disease during Anti-PD-1 (PDCD1)/PD-L1 (CD274) Therapy: A Systematic Review and Meta-Analysis. Cancers 2019, 11, 1699. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, K.W.; Won, S.E.; Yoon, S.; Chae, Y.K.; Tirumani, S.H.; Ramaiya, N.H. Definition, Incidence, and Challenges for Assessment of Hyperprogressive Disease during Cancer Treatment with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2021, 4, e211136. [Google Scholar] [CrossRef] [PubMed]
- Ferté, C.; Fernandez, M.; Hollebecque, A.; Koscielny, S.; Levy, A.; Massard, C.; Balheda, R.; Bot, B.; Gomez-Roca, C.; Dromain, C.; et al. Tumor growth rate is an early indicator of antitumor drug activity in phase I clinical trials. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Adashek, J.J.; Kato, S.; Ferrara, R.; Lo Russo, G.; Kurzrock, R. Hyperprogression and Immune Checkpoint Inhibitors: Hype or Progress? Oncologist 2020, 25, 94–98. [Google Scholar] [CrossRef]
- Remon, J.; Esteller, L.; Taus, Á. Nivolumab plus ipilimumab combination therapy for the first-line treatment NSCLC: Evidence to date. Cancer Manag. Res. 2019, 11, 4893–4904. [Google Scholar] [CrossRef] [PubMed]
- Gandara, D.; Reck, M.; Moro-Sibilot, D.; Mazieres, J.; Gadgeel, S.; Morris, S.; Cardona, A.; Mendus, D.; Ballinger, M.; Rittmeyer, A.; et al. Fast progression in non–small cell lung cancer: Results from the randomized phase III OAK study evaluating second-line atezolizumab versus docetaxel. J. Immunother. Cancer 2021, 9, e001882. [Google Scholar] [CrossRef]
- Champiat, S.; Dercle, L.; Ammari, S.; Massard, C.; Hollebecque, A.; Postel-Vinay, S.; Chaput, N.; Eggermont, A.; Marabelle, A.; Soria, J.-C.; et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin. Cancer Res. 2017, 23, 1920–1928. [Google Scholar] [CrossRef]
- Kang, Y.-K.; Reck, M.; Nghiem, P.; Feng, Y.; Plautz, G.; Kim, H.R.; Owonikoko, T.K.; Boku, N.; Chen, L.-T.; Lei, M.; et al. Assessment of hyperprogression versus the natural course of disease development with nivolumab with or without ipilimumab versus placebo in phase III, randomized, controlled trials. J. Immunother. Cancer 2022, 10, e004273. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, J.; Hu, D.; Wang, H.; Huang, C.; Luo, R.; Zhou, Z.; Huang, X.; Xie, T.; Lou, J. Hyperprogression, a challenge of PD-1/PD-L1 inhibitors treatments: Potential mechanisms and coping strategies. Biomed. Pharmacother. 2022, 150, 112949. [Google Scholar] [CrossRef]
- Hwang, I.; Park, I.; Yoon, S.; Lee, J.L. Hyperprogressive Disease in Patients with Urothelial Carcinoma or Renal Cell Carcinoma Treated With PD-1/PD-L1 Inhibitors. Clin. Genitourin. Cancer 2020, 18, e122–e133. [Google Scholar] [CrossRef]
- Palma, D. Stereotactic Radiotherapy for Oligo-Progressive Metastatic Cancer (The STOP Trial): A Randomized Phase II Trial. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02756793 (accessed on 1 January 2024).
- Centre Francois Baclesse A Randomized Phase II Study Assessing Stereotactic Radiotherapy in Therapeutic Strategy of Oligoprogressive Renal Cell Carcinoma Metastases. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04299646 (accessed on 1 January 2024).
- Yale University Phase II Trial Of Stereotactic Body Radiation Therapy (SBRT) for Oligoprogression on Immune Checkpoint Inhibitors (ICI) in Metastatic Renal Cell Carcinoma. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04974671 (accessed on 1 January 2024).
- Tannir, N.M.; Motzer, R.J.; Albiges, L.; Plimack, E.R.; George, S.; Powles, T.; Donskov, F.; Rini, B.I.; Grünwald, V.; Hammers, H.J.; et al. Patterns of progression in patients treated with nivolumab plus ipilimumab (NIVO+IPI) versus sunitinib (SUN) for first-line treatment of advanced renal cell carcinoma (aRCC) in CheckMate 214. J. Clin. Oncol. 2021, 39, 313. [Google Scholar] [CrossRef]
- Buttigliero, C.; Allis, S.; Tucci, M.; Zichi, C.; Leone, G.; Di Stefano, R.F.; Ruo Redda, M.G.; Ricardi, U.; Scagliotti, G.V.; Di Maio, M.; et al. Role of radiotherapy in improving activity of immune-modulating drugs in advanced renal cancer: Biological rationale and clinical evidences. Cancer Treat. Rev. 2018, 69, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, Y.; Kong, L.; Shi, F.; Zhu, H.; Yu, J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Masini, C.; Iotti, C.; De Giorgi, U.; Bellia, R.S.; Buti, S.; Salaroli, F.; Zampiva, I.; Mazzarotto, R.; Mucciarini, C.; Baldessari, C.; et al. Nivolumab (NIVO) in combination with stereotactic body radiotherapy (SBRT) in pretreated patients (pts) with metastatic renal cell carcinoma (mRCC): First results of phase II NIVES study. J. Clin. Oncol. 2020, 38, 613. [Google Scholar] [CrossRef]
- McBride, S.; Sherman, E.; Tsai, C.J.; Baxi, S.; Aghalar, J.; Eng, J.; Zhi, W.I.; McFarland, D.; Michel, L.S.; Young, R.; et al. Randomized Phase II Trial of Nivolumab With Stereotactic Body Radiotherapy Versus Nivolumab Alone in Metastatic Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2021, 39, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Theelen, W.S.M.E.; Peulen, H.M.U.; Lalezari, F.; van der Noort, V.; de Vries, J.F.; Aerts, J.G.J.V.; Dumoulin, D.W.; Bahce, I.; Niemeijer, A.-L.N.; de Langen, A.J.; et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients with Advanced Non–Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1276–1282. [Google Scholar] [CrossRef]
- Siva, S.; Bressel, M.; Wood, S.T.; Shaw, M.G.; Loi, S.; Sandhu, S.K.; Tran, B.; Azad, A.A.; Lewin, J.H.; Cuff, K.E.; et al. Stereotactic Radiotherapy and Short-course Pembrolizumab for Oligometastatic Renal Cell Carcinoma—The RAPPORT Trial. Eur. Urol. 2022, 81, 364–372. [Google Scholar] [CrossRef]
- Stenman, M.; Sinclair, G.; Paavola, P.; Wersäll, P.; Harmenberg, U.; Lindskog, M. Overall survival after stereotactic radiotherapy or surgical metastasectomy in oligometastatic renal cell carcinoma patients treated at two Swedish centres 2005–2014. Radiother. Oncol. 2018, 127, 501–506. [Google Scholar] [CrossRef]
- Dabestani, S.; Marconi, L.; Hofmann, F.; Stewart, F.; Lam, T.B.L.; Canfield, S.E.; Staehler, M.; Powles, T.; Ljungberg, B.; Bex, A. Local treatments for metastases of renal cell carcinoma: A systematic review. Lancet Oncol. 2014, 15, e549–e561. [Google Scholar] [CrossRef]
- Franzese, C.; Marvaso, G.; Francolini, G.; Borghetti, P.; Trodella, L.E.; Sepulcri, M.; Matrone, F.; Nicosia, L.; Timon, G.; Ognibene, L.; et al. The role of stereotactic body radiation therapy and its integration with systemic therapies in metastatic kidney cancer: A multicenter study on behalf of the AIRO (Italian Association of Radiotherapy and Clinical Oncology) genitourinary study group. Clin. Exp. Metastasis 2021, 38, 527–537. [Google Scholar] [CrossRef]
- Meyer, E.; Pasquier, D.; Bernadou, G.; Calais, G.; Maroun, P.; Bossi, A.; Theodore, C.; Albiges, L.; Stefan, D.; de Crevoisier, R.; et al. Stereotactic radiation therapy in the strategy of treatment of metastatic renal cell carcinoma: A study of the Getug group. Eur. J. Cancer 2018, 98, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Barata, P.C.; Mendiratta, P.; Kotecha, R.; Gopalakrishnan, D.; Juloori, A.; Chao, S.T.; Koshkin, V.; Ornstein, M.; Gilligan, T.D.; Wood, L.S.; et al. Effect of Switching Systemic Treatment After Stereotactic Radiosurgery for Oligoprogressive, Metastatic Renal Cell Carcinoma. Clin. Genitourin. Cancer 2018, 16, 413–419.e1. [Google Scholar] [CrossRef] [PubMed]
- Schoenhals, J.E.; Mohamad, O.; Christie, A.; Zhang, Y.; Li, D.; Singla, N.; Bowman, I.; Arafat, W.; Hammers, H.; Courtney, K.; et al. Stereotactic Ablative Radiation Therapy for Oligoprogressive Renal Cell Carcinoma. Adv. Radiat. Oncol. 2021, 6, 100692. [Google Scholar] [CrossRef] [PubMed]
- Santini, D.; Ratta, R.; Pantano, F.; Lisi, D.D.; Maruzzo, M.; Galli, L.; Biasco, E.; Farnesi, A.; Buti, S.; Sternberg, C.N.; et al. Outcome of oligoprogressing metastatic renal cell carcinoma patients treated with locoregional therapy: A multicenter retrospective analysis. Oncotarget 2017, 8, 100708–100716. [Google Scholar] [CrossRef] [PubMed]
- De, B.; Venkatesan, A.M.; Msaouel, P.; Ghia, A.J.; Li, J.; Yeboa, D.N.; Nguyen, Q.-N.; Bishop, A.J.; Jonasch, E.; Shah, A.Y.; et al. Definitive radiotherapy for extracranial oligoprogressive metastatic renal cell carcinoma as a strategy to defer systemic therapy escalation. BJU Int. 2022, 129, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.; Patel, S.; North, S.A.; Sahgal, A.; Chu, W.; Soliman, H.; Ahmad, B.; Winquist, E.; Niazi, T.; Patenaude, F.; et al. Stereotactic Radiotherapy for Oligoprogression in Metastatic Renal Cell Cancer Patients Receiving Tyrosine Kinase Inhibitor Therapy: A Phase 2 Prospective Multicenter Study. Eur. Urol. 2021, 80, 693–700. [Google Scholar] [CrossRef]
- Hannan, R.; Christensen, M.; Hammers, H.; Christie, A.; Paulman, B.; Lin, D.; Garant, A.; Arafat, W.; Courtney, K.; Bowman, I.; et al. Phase II Trial of Stereotactic Ablative Radiation for Oligoprogressive Metastatic Kidney Cancer. Eur. Urol. Oncol. 2022, 5, 216–224. [Google Scholar] [CrossRef]
- Cella, D.; Grünwald, V.; Escudier, B.; Hammers, H.J.; George, S.; Nathan, P.; Grimm, M.-O.; Rini, B.I.; Doan, J.; Ivanescu, C.; et al. Patient-reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): A randomised, phase 3 trial. Lancet Oncol. 2019, 20, 297–310. [Google Scholar] [CrossRef]
- Albiges, L.; Tannir, N.M.; Burotto, M.; McDermott, D.; Plimack, E.R.; Barthélémy, P.; Porta, C.; Powles, T.; Donskov, F.; George, S.; et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: Extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 2020, 5, e001079. [Google Scholar] [CrossRef]
- Tamada, S.; Kondoh, C.; Matsubara, N.; Mizuno, R.; Kimura, G.; Anai, S.; Tomita, Y.; Oyama, M.; Masumori, N.; Kojima, T.; et al. Pembrolizumab plus axitinib versus sunitinib in metastatic renal cell carcinoma: Outcomes of Japanese patients enrolled in the randomized, phase III, open-label KEYNOTE-426 study. Int. J. Clin. Oncol. 2022, 27, 154–164. [Google Scholar] [CrossRef]
- Voss, M.H.; Powles, T.; McGregor, B.A.; Porta, C.; Grünwald, V.; Merchan, J.R.; Rolland, F.; Maroto-Rey, P.; Goh, J.C.; Xing, D.; et al. Impact of subsequent therapies in patients (pts) with advanced renal cell carcinoma (aRCC) receiving lenvatinib plus pembrolizumab (LEN + PEMBRO) or sunitinib (SUN) in the CLEAR study. J. Clin. Oncol. 2022, 40, 4514. [Google Scholar] [CrossRef]
- Tomita, Y.; Kimura, G.; Fukasawa, S.; Numakura, K.; Sugiyama, Y.; Yamana, K.; Naito, S.; Kaneko, H.; Tajima, Y.; Oya, M. Subgroup analysis of the AFTER I-O study: A retrospective study on the efficacy and safety of subsequent molecular targeted therapy after immune-oncology therapy in Japanese patients with metastatic renal cell carcinoma. Jpn. J. Clin. Oncol. 2021, 51, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Motzer, R.J.; Rini, B.I.; Haanen, J.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Gravis-Mescam, G.; Uemura, M.; Lee, J.L.; et al. Updated efficacy results from the JAVELIN Renal 101 trial: First-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann. Oncol. 2020, 31, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Albiges, L.; Tomczak, P.; Suárez, C.; Voss, M.H.; de Velasco, G.; Chahoud, J.; Procopio, G.; Mahammedi, H.; Zengerling, F.; et al. Efficacy and safety of atezolizumab plus cabozantinib vs cabozantinib alone after progression with prior immune checkpoint inhibitor (ICI) treatment in metastatic renal cell carcinoma (RCC): Primary PFS analysis from the phase 3, randomized, open-label CONTACT-03 study. J. Clin. Oncol. 2023, 41, LBA4500. [Google Scholar] [CrossRef]
- McKay, R.R.; McGregor, B.A.; Xie, W.; Braun, D.A.; Wei, X.; Kyriakopoulos, C.E.; Zakharia, Y.; Maughan, B.L.; Rose, T.L.; Stadler, W.M.; et al. Optimized Management of Nivolumab and Ipilimumab in Advanced Renal Cell Carcinoma: A Response-Based Phase II Study (OMNIVORE). J. Clin. Oncol. 2020, 38, 4240–4248. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Kluger, H.; George, S.; Tykodi, S.S.; Kuzel, T.M.; Perets, R.; Nair, S.; Procopio, G.; Carducci, M.A.; Castonguay, V.; et al. FRACTION-RCC: Nivolumab plus ipilimumab for advanced renal cell carcinoma after progression on immuno-oncology therapy. J. Immunother. Cancer 2022, 10, e005780. [Google Scholar] [CrossRef]
- Atkins, M.B.; Jegede, O.; Haas, N.B.; McDermott, D.F.; Bilen, M.A.; Drake, C.G.; Sosman, J.A.; Alter, R.S.; Plimack, E.R.; Rini, B.I.; et al. Phase II study of nivolumab and salvage nivolumab + ipilimumab in treatment-naïve patients (pts) with advanced renal cell carcinoma (RCC) (HCRN GU16-260). J. Clin. Oncol. 2020, 38, 5006. [Google Scholar] [CrossRef]
- Grimm, M.-O.; Esteban, E.; Barthélémy, P.; Schmidinger, M.; Busch, J.; Valderrama, B.P.; Schmitz, M.; Schumacher, U.; Baretton, G.B.; Duran, I.; et al. Efficacy of nivolumab/ipilimumab in patients with initial or late progression with nivolumab: Updated analysis of a tailored approach in advanced renal cell carcinoma (TITAN-RCC). J. Clin. Oncol. 2021, 39, 4576. [Google Scholar] [CrossRef]
- Carril-Ajuria, L.; Lora, D.; Carretero-González, A.; Martín-Soberón, M.; Rioja-Viera, P.; Castellano, D.; de Velasco, G. Systemic Analysis and Review of Nivolumab-ipilimumab Combination as a Rescue Strategy for Renal Cell Carcinoma After Treatment With Anti–PD-1/PD-L1 Therapy. Clin. Genitourin. Cancer 2021, 19, 95–102. [Google Scholar] [CrossRef]
- Dizman, N.; Austin, M.; Considine, B.; Jessel, S.; Schoenfeld, D.; Merl, M.Y.; Hurwitz, M.; Sznol, M.; Kluger, H. Outcomes with combination pembrolizumab and axitinib in second and further line treatment of metastatic renal cell carcinoma. Clin. Genitourin. Cancer 2023, 21, 221–229. [Google Scholar] [CrossRef]
- Auvray, M.; Auclin, E.; Barthelemy, P.; Bono, P.; Kellokumpu-Lehtinen, P.; Gross-Goupil, M.; De Velasco, G.; Powles, T.; Mouillet, G.; Vano, Y.-A.; et al. Second-line targeted therapies after nivolumab-ipilimumab failure in metastatic renal cell carcinoma. Eur. J. Cancer 2019, 108, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Powles, T.; Donskov, F.; Plimack, E.R.; Barthélémy, P.; Hammers, H.J.; et al. Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. J. Immunother. Cancer 2021, 8, e000891. [Google Scholar] [CrossRef] [PubMed]
- Tannir, N.M.; Motzer, R.J.; Plimack, E.R.; McDermott, D.F.; Barthelemy, P.; Porta, C.; George, S.; Powles, T.; Donskov, F.; Kollmannsberger, C.K.; et al. Outcomes in patients (pts) with advanced renal cell carcinoma (aRCC) who discontinued (DC) first-line nivolumab + ipilimumab (N+I) or sunitinib (S) due to treatment-related adverse events (TRAEs) in CheckMate 214. J. Clin. Oncol. 2019, 37, 581. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, J.; Xu, L.; Yang, H.; Liang, N.; Zhang, L.; Zhang, F.; Zhang, X. Safety and Efficacy of the Rechallenge of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients With Cancer: A Systemic Review and Meta-Analysis. Front. Immunol. 2021, 12, 730320. [Google Scholar] [CrossRef] [PubMed]
- Dolladille, C.; Ederhy, S.; Sassier, M.; Cautela, J.; Thuny, F.; Cohen, A.A.; Fedrizzi, S.; Chrétien, B.; Da-Silva, A.; Plane, A.-F.; et al. Immune Checkpoint Inhibitor Rechallenge after Immune-Related Adverse Events in Patients with Cancer. JAMA Oncol. 2020, 6, 865–871. [Google Scholar] [CrossRef]
- Haanen, J.; Ernstoff, M.; Wang, Y.; Menzies, A.; Puzanov, I.; Grivas, P.; Larkin, J.; Peters, S.; Thompson, J.; Obeid, M. Rechallenge patients with immune checkpoint inhibitors following severe immune-related adverse events: Review of the literature and suggested prophylactic strategy. J. Immunother. Cancer 2020, 8, e000604. [Google Scholar] [CrossRef]
- Alaiwi, S.A.; Xie, W.; Nassar, A.H.; Dudani, S.; Martini, D.; Bakouny, Z.; Steinharter, J.A.; Nuzzo, P.V.; Flippot, R.; Martinez-Chanza, N.; et al. Safety and efficacy of restarting immune checkpoint inhibitors after clinically significant immune-related adverse events in metastatic renal cell carcinoma. J. Immunother. Cancer 2020, 8, e000144. [Google Scholar] [CrossRef]
- Fitzgerald, K.N.; Duzgol, C.; Knezevic, A.; Shapnik, N.; Kotecha, R.; Aggen, D.H.; Carlo, M.I.; Shah, N.J.; Voss, M.H.; Feldman, D.R.; et al. Progression-free Survival After Second Line of Therapy for Metastatic Clear Cell Renal Cell Carcinoma in Patients Treated with First-line Immunotherapy Combinations. Eur. Urol. 2023, 83, 195–199. [Google Scholar] [CrossRef]

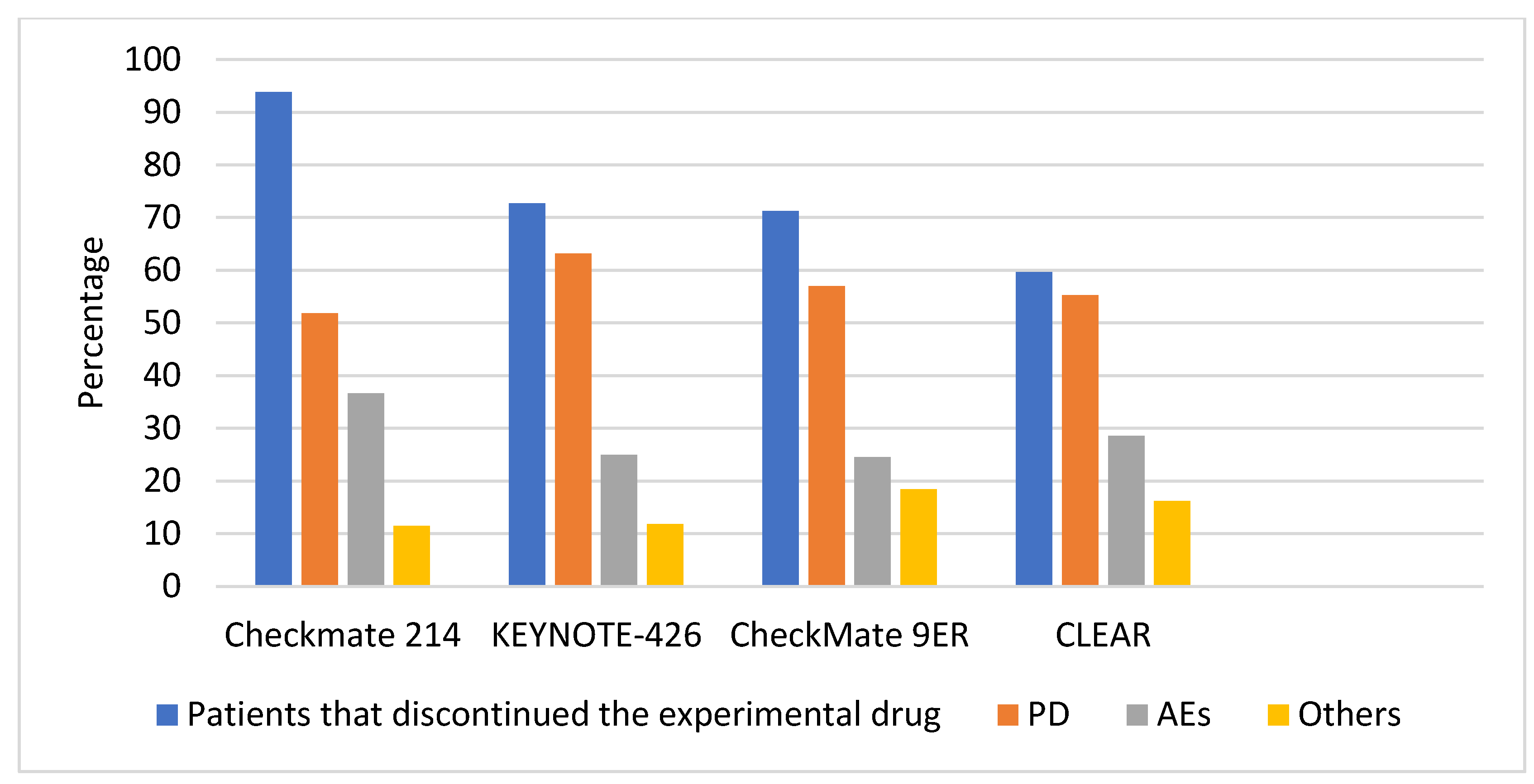
| CheckMate 214: Nivolumab + Ipilimumab [26] | KEYNOTE-426: Pembrolizumab + Axitinib [27] | CheckMate 9ER: Nivolumab + Cabozantinib [31] | CLEAR: Lenvatinib + Pembrolizumab [33] | |
|---|---|---|---|---|
| Median follow-up for OS at CONSORT diagram | 67.7 months | 30.6 months | 32.9 months | 26.6 months |
| Number of pts that discontinued therapy at last follow-up (% #) | 513 (547) | 312 (429) | 228 (320) | 210 (352) |
| Disease progression (% #) | 266 (48.6) | 197 (45.9) (181 radiologic + 16 clinical) | 130 (40.6) (of which 1 per physician PET/CT confirmed PD, listed in others) | 116 (33.0) |
| Treatment-related AEs (% #) | 148 (27.1) | 78 (18.2) | 32 (10) | Total discontinuations of both drugs for trAEs + unrelated AEs = 60 (17.0) |
| AEs unrelated to treatment (% #) | 40 (7.3) | / | 24 (7.5) | Total discontinuations of both drugs for trAEs + unrelated AEs = 60 (17.0) |
| Maximum clinical benefit (% #) | 18 (3.3) | 7 (1.6) | 2 (0.6) | / |
| Withdrew consent or requested to discontinue treatment (% #) | 27 (4.9) | 18 (4.2) | 10 (3.1) | 21 (4 withdrew consent, 17 patient choice) (6.0) |
| Died (% #) | 1 (1.8) ^ | / | 3 (0.9) | / |
| Lost to follow-up (% #) | 1 (1.8) | / | 2 (0.6) | / |
| Other (% #) | 11 (2.0) | 11 (2.6) various reasons: + 8 physician decision + 2 non-study anticancer therapy + 1 excluded medication | 10 (3.1) various reasons: + 1 investigator decision + 2 underwent treatment for a new primary + 3 underwent local therapy/resection + 2 for poor status + 1 no longer measurable disease + 1 no target lesion | 13 (3.7) |
| Poor compliance (% #) | 1 (1.8) | 1 (0.2) | 1 (0.3) (in others: patient refused to do anything) | / |
| Discontinued ICI per protocol completion (% #) | Single drug discontinuation not allowed | 19 (4.4) | Reported 14 (4.4) completion of treatment for both study drugs † | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samuelly, A.; Di Stefano, R.F.; Turco, F.; Delcuratolo, M.D.; Pisano, C.; Saporita, I.; Calabrese, M.; Carfì, F.M.; Tucci, M.; Buttigliero, C. Navigating the ICI Combination Treatment Journey: Patterns of Response and Progression to First-Line ICI-Based Combination Treatment in Metastatic Renal Cell Carcinoma. J. Clin. Med. 2024, 13, 307. https://doi.org/10.3390/jcm13020307
Samuelly A, Di Stefano RF, Turco F, Delcuratolo MD, Pisano C, Saporita I, Calabrese M, Carfì FM, Tucci M, Buttigliero C. Navigating the ICI Combination Treatment Journey: Patterns of Response and Progression to First-Line ICI-Based Combination Treatment in Metastatic Renal Cell Carcinoma. Journal of Clinical Medicine. 2024; 13(2):307. https://doi.org/10.3390/jcm13020307
Chicago/Turabian StyleSamuelly, Alessandro, Rosario Francesco Di Stefano, Fabio Turco, Marco Donatello Delcuratolo, Chiara Pisano, Isabella Saporita, Mariangela Calabrese, Federica Maria Carfì, Marcello Tucci, and Consuelo Buttigliero. 2024. "Navigating the ICI Combination Treatment Journey: Patterns of Response and Progression to First-Line ICI-Based Combination Treatment in Metastatic Renal Cell Carcinoma" Journal of Clinical Medicine 13, no. 2: 307. https://doi.org/10.3390/jcm13020307
APA StyleSamuelly, A., Di Stefano, R. F., Turco, F., Delcuratolo, M. D., Pisano, C., Saporita, I., Calabrese, M., Carfì, F. M., Tucci, M., & Buttigliero, C. (2024). Navigating the ICI Combination Treatment Journey: Patterns of Response and Progression to First-Line ICI-Based Combination Treatment in Metastatic Renal Cell Carcinoma. Journal of Clinical Medicine, 13(2), 307. https://doi.org/10.3390/jcm13020307






