Selecting a Brief Cognitive Screening Test Based on Patient Profile: It Is Never Too Early to Start
Abstract
1. Introduction
2. Materials and Methods
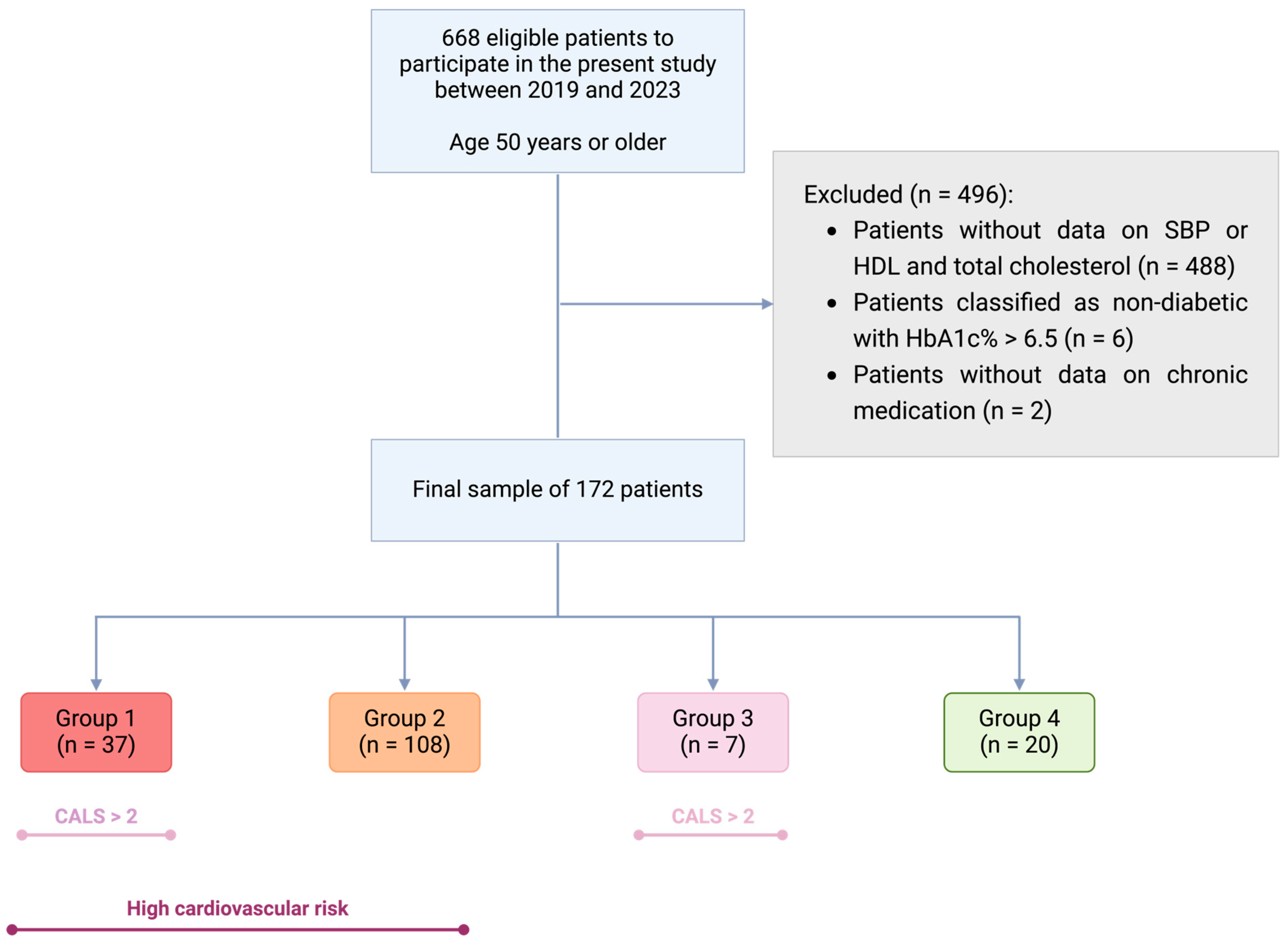
2.1. Inclusion and Exclusion Criteria
2.2. Cognitive Status Assessment
2.3. Clinical Variables
2.4. Patient Grouping
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
3.1. Demographic and Clinical Description of Participants
3.2. Increased Cardiovascular Risk and Anticholinergic Burden Are Associated with Worse Cognitive Test Performance
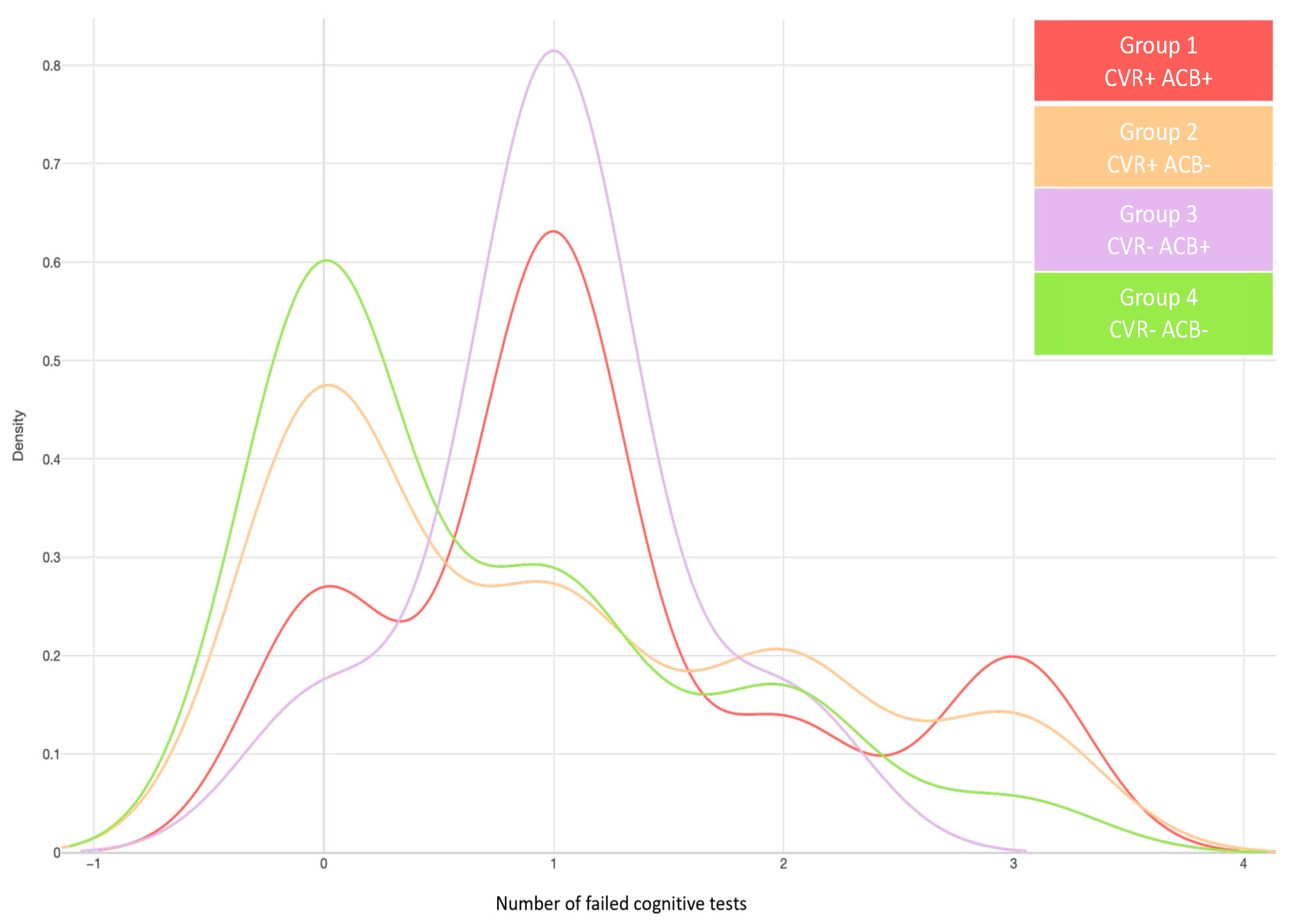
3.3. Patient Profile Based on Cardiovascular Risk and Anticholinergic Burden
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Sidhu, J.; Lui, F.; Tsao, J.W. Alzheimer Disease. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499922/ (accessed on 10 July 2024).
- 2024 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2024, 20, 3708–3821. [CrossRef] [PubMed]
- Steinberg, S.I.; Negash, S.; Sammel, M.D.; Bogner, H.; Harel, B.T.; Livney, M.G.; McCoubrey, H.; Wolk, D.A.; Kling, M.A.; Arnold, S.E. Subjective Memory Complaints, Cognitive Performance, and Psychological Factors in Healthy Older Adults. Am. J. Alzheimer’s Dis. Other Dementiasr. 2013, 28, 776–783. [Google Scholar] [CrossRef]
- Yu, J.-T.; Xu, W.; Tan, C.-C.; Andrieu, S.; Suckling, J.; Evangelou, E.; Pan, A.; Zhang, C.; Jia, J.; Feng, L.; et al. Evidence-based prevention of Alzheimer’s disease: Systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B.; Furie, K.L.; Iadecola, C.; Smith, E.E.; Waddy, S.P.; Lloyd-Jones, D.M.; Bae, H.-J.; Bauman, M.A.; Dichgans, M.; Duncan, P.W.; et al. Defining Optimal Brain Health in Adults: A Presidential Advisory From the American Heart Association/American Stroke Association. Stroke 2017, 48, E284–E303. [Google Scholar] [CrossRef] [PubMed]
- García-Lluch, G.; Pardo, J.; Moreno, L.; Peña-Bautista, C.; Baquero, M.; Cháfer-Pericás, C. Cardiovascular Risk Scales Association with Cerebrospinal Fluid Alzheimer’s Disease Biomarkers in Cardiovascular Low Cardiovascular Risk Regions. J. Prev. Alzheimer’s Dis. 2024, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, E.Y. Repositioning medication for cardiovascular and cerebrovascular disease to delay the onset and prevent progression of Alzheimer’s disease. Arch. Pharm. Res. 2020, 43, 932–960. [Google Scholar] [CrossRef]
- Frankish, H.; Horton, R. Prevention and management of dementia: A priority for public health. Lancet 2017, 390, 2614–2615. [Google Scholar] [CrossRef]
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [CrossRef]
- Ramos, H.; Moreno, L.; Pérez-Tur, J.; Cháfer-Pericás, C.; García-Lluch, G.; Pardo, J. CRIDECO Anticholinergic Load Scale: An Updated Anticholinergic Burden Scale. Comparison with the ACB Scale in Spanish Individuals with Subjective Memory Complaints. J. Pers. Med. 2022, 12, 207. [Google Scholar] [CrossRef]
- Lockery, J.E.; Broder, J.C.; Ryan, J.; Stewart, A.C.; Woods, R.L.; Chong, T.T.-J.; Cloud, G.C.; Murray, A.; Rigby, J.D.; Shah, R. A Cohort Study of Anticholinergic Medication Burden and Incident Dementia and Stroke in Older Adults. J. Gen. Intern. Med. 2021, 36, 1629–1637. [Google Scholar] [CrossRef]
- Gamble, D.T.; Clark, A.B.; Luben, R.N.; Wareham, N.J.; Khaw, K.-T.; Myint, P.K. Baseline anticholinergic burden from medications predicts incident fatal and non-fatal stroke in the EPIC-Norfolk general population. Int. J. Epidemiol. 2018, 47, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Lanctôt, K.L.; O’Regan, J.; Schwartz, Y.; Swardfager, W.; Saleem, M.; Oh, P.I.; Herrmann, N. Assessing Cognitive Effects of Anticholinergic Medications in Patients with Coronary Artery Disease. Psychosomatics 2013, 55, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Gil-Peinado, M.; Alacreu, M.; Ramos, H.; Sendra-Lillo, J.; García, C.; García-Lluch, G.; Lopez de Coca, T.; Sala, M.; Moreno, L. The A-to-Z factors associated with cognitive impairment. Results of the DeCo study. Front. Psychol. 2023, 14, 1152527. [Google Scholar] [CrossRef] [PubMed]
- Ramos, H.; Pardo, J.; Sánchez, R.; Puchades, E.; Pérez-Tur, J.; Navarro, A.; Moreno, L. Pharmacist-Physician Interprofessional Collaboration to Promote Early Detection of Cognitive Impairment: Increasing Diagnosis Rate. Front. Pharmacol. 2021, 12, 579489. [Google Scholar] [CrossRef]
- Böhm, P.; Peña-Casanova, J.; Gramunt, N.; Manero, R.M.; Terrón, C.; Quiñones-Ubeda, S. Spanish version of the Memory Impairment Screen (MIS): Normative data and discriminant validity. Neurologia 2005, 20, 402–411. [Google Scholar] [PubMed]
- López Pérez-Díaz, A.G.; Calero, M.D.; Navarro-González, E. Prediction of cognitive impairment in the elderly by analysing their performance in verbal fluency and in sustained attention. Rev. Neurol. 2013, 56, 1–7. [Google Scholar] [PubMed]
- Martínez De La Iglesia, J.; Herrero, R.D.; Vilches, M.C.O.; Taberné, C.A.; Colomer, C.A.; Luque, R.L. Adaptación y validación al castellano del cuestionario de Pfeiffer (SPMSQ) para detectar la existencia de deterioro cognitivo en personas mayores de 65 años. Med. Clin. 2001, 117, 129–134. [Google Scholar] [CrossRef]
- Schönstein, A.; Wahl, H.-W.; Katus, H.A.; Bahrmann, A. SPMSQ for risk stratification of older patients in the emergency department: An exploratory prospective cohort study. Z. Gerontol. Geriatr. 2019, 52, 222–228. [Google Scholar] [CrossRef]
- Price, S.E.; Kinsella, G.J.; Ong, B.; Storey, E.; Mullaly, E.; Phillips, M.; Pangnadasa-Fox, L.; Perre, D. Semantic verbal fluency strategies in amnestic mild cognitive impairment. Neuropsychology 2012, 26, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Carnero-Pardo, C.; Lendínez-González, A. The utility of the semantic verbal fluency test in diagnosis of dementia. Rev. Neurol. 1999, 29, 709–714. [Google Scholar] [CrossRef]
- Diagnosis | ADA. Available online: https://diabetes.org/about-diabetes/diagnosis (accessed on 9 June 2024).
- SCORE2 Working Group and ESC Cardiovascular Risk Collaboration. SCORE2 risk prediction algorithms: New models to estimate 10-year risk of cardiovascular disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [Google Scholar] [CrossRef] [PubMed]
- SCORE2-OP risk prediction algorithms: Estimating incident cardiovascular event risk in older persons in four geographical risk regions. Eur. Heart J. 2021, 42, 2455–2467. [CrossRef] [PubMed]
- Boustani, M.; Campbell, N.; Munger, S.; Maidment, I.; Fox, C. Impact of anticholinergics on the aging brain: A review and practical application. Aging Health 2008, 4, 311–320. [Google Scholar] [CrossRef]
- Gabriel, R.; Brotons, C.; Tormo, M.J.; Segura, A.; Rigo, F.; Elosua, R.; Carbayo, J.A.; Gavrila, D.; Moral, I.; Tuomilehto, J.; et al. The ERICE-score: The new native cardiovascular score for the low-risk and aged mediterranean population of Spain. Rev. Esp. De Cardiol. 2015, 68, 205–215. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbæk, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Mukadam, N.; Wolters, F.J.; Walsh, S.; Wallace, L.; Brayne, C.; Matthews, F.E.; Sacuiu, S.; Skoog, I.; Seshadri, S.; Beiser, A.; et al. Changes in prevalence and incidence of dementia and risk factors for dementia: An analysis from cohort studies. Lancet Public Health 2024, 9, e443–e460. [Google Scholar] [CrossRef]
- Rhea, E.M.; Leclerc, M.; Yassine, H.N.; Capuano, A.W.; Tong, H.; Petyuk, V.A.; Macauley, S.L.; Fioramonti, X.; Carmichael, O.; Calon, F.; et al. State of the science on brain insulin resistance and cognitive decline due to alzheimer’s disease. Aging Dis. 2023, 15, 1688–1725. [Google Scholar] [CrossRef]
- Dove, A.; Shang, Y.; Xu, W.; Grande, G.; Laukka, E.J.; Fratiglioni, L.; Marseglia, A. The impact of diabetes on cognitive impairment and its progression to dementia. Alzheimer’s Dement. 2021, 17, 1769–1778. [Google Scholar] [CrossRef]
- García-Lluch, G.; Peña-Bautista, C.; Royo, L.M.; Baquero, M.; Cañada-Martínez, A.J.; Cháfer-Pericás, C. Angiotensin II Receptor Blockers Reduce Tau/Aß42 Ratio: A cerebrospinal fluid biomarkers’ case-control study. Pharmaceutics 2023, 15, 924. [Google Scholar] [CrossRef]
- Sánchez-Benavides, G.; Gómez-Ansón, B.; Molinuevo, J.L.; Blesa, R.; Monte, G.C.; Buschke, H.; Peña-Casanova, J. medial temporal lobe correlates of memory screening measures in normal aging, MCI, and AD. J. Geriatr. Psychiatry Neurol. 2009, 23, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Yang, A.S.-H.; Tsai, D.H.-T.; Shao, S.-C.; Lin, S.-J.; Lai, E.C.-C. Association between recently raised anticholinergic burden and risk of acute cardiovascular events: Nationwide case-case-time-control study. BMJ 2023, 382, e076045. [Google Scholar] [CrossRef] [PubMed]
- López-Álvarez, J.; Sevilla-Llewellyn-Jones, J.; Agüera-Ortiz, L. Anticholinergic drugs in geriatric psychopharmacology. Front. Neurosci. 2019, 13, 1309. [Google Scholar] [CrossRef]
- Ediciones TEA, Normal NN. TFV. Test de Fluidez Verbal. 2020; pp. 1–6. Available online: https://web.teaediciones.com/Ejemplos/TFV_Caso_ilustrativo.pdf (accessed on 13 July 2024).
- Olazarán, J.; Hoyos-Alonso, M.; del Ser, T.; Barral, A.G.; Conde-Sala, J.; Bermejo-Pareja, F.; López-Pousa, S.; Pérez-Martínez, D.; Villarejo-Galende, A.; Cacho, J.; et al. Aplicación práctica de los test cognitivos breves. Neurología 2015, 31, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J. Am. Geriatr. Soc. 1975, 23, 433–441. [Google Scholar] [CrossRef]
- Carnero-Pardo, C.; Rego-García, I.; Llorente, M.M.; Ródenas, M.A.; Carrillo, R.V. Diagnostic performance of brief cognitive tests in cognitive impairment screening. Neurol. (Engl. Ed.) 2022, 37, 441–449. [Google Scholar] [CrossRef]
- Nebes, R.D.; Pollock, B.G.; Perera, S.; Halligan, E.M.; Saxton, J.A. The greater sensitivity of elderly apoe ε4 carriers to anticholinergic medications is independent of cerebrovascular disease risk. Am. J. Geriatr. Pharmacother. 2012, 10, 185–192. [Google Scholar] [CrossRef]
- Bocti, C.; Swartz, R.H.; Gao, F.-Q.; Sahlas, D.J.; Behl, P.; Black, S.E. A new visual rating scale to assess strategic white matter hyperintensities within cholinergic pathways in dementia. Stroke 2005, 36, 2126–2131. [Google Scholar] [CrossRef]
| Variable | Measure | CI+ (n = 104) | CI− (n = 68) | p-Value |
|---|---|---|---|---|
| Age | Mean (sd) | 74.61 (8.25) | 73.67 (10.54) | 0.54 |
| Sex (Female) | n (%) | 74 (71.15) | 42 (61.76) | 0.20 |
| Educational level (High) | n (%) | 22 (21.4) | 49 (73.1) | <0.01 |
| Number of concomitant drugs | Mean (sd) | 6.77 (3.40) | 5.56 (3.05) | 0.02 |
| Total anticholinergic burden | Mean (sd) | 1.95 (1.72) | 1.01 (1.33) | <0.01 |
| Significant anicholinergic burden (CALS > 2) | Yes, n (%) | 35 (33.65) | 9 (13.24) | <0.01 |
| Type 2 Diabetes features | ||||
| HbA1c | Mean (sd) | 6.68 (1.22) | 5.68 (1.12) | <0.01 |
| Glucose | Mean (sd) | 105.08 (26.86) | 106.58 (26.90) | 0.74 |
| Antidiabetic agents intake | Yes, n (%) | 26 (25.00) | 21 (29.41) | 0.52 |
| Hypertenssion-related variables | ||||
| SBP (mmHg) | Mean (sd) | 134.84 (14.85) | 129.43 (17.75) | 0.04 |
| DBP (mmHg) | Mean (sd) | 73.88 (10.63) | 75.01 (9.55) | 0.44 |
| Antihypertensive drugs intake | Yes, n (%) | 66 (63.46) | 47 (69.12) | 0.44 |
| Dyslipidemia-related variables | ||||
| Total cholesterol (mg/dL) | Mean (sd) | 192.99 (44.22) | 190.19 (40.91) | 0.67 |
| HDL cholesterol (mg/dL) | Mean (sd) | 56.64 (15.43) | 57.57 (19.57) | 0.74 |
| LDL cholesterol (mg/dL) | Mean (sd) | 110.21 (35.04) | 106.13 (35.95) | 0.46 |
| Lipid-lowering drugs intake | Yes, n (%) | 49 (60.49) | 51 (58.62) | 0.80 |
| SCORE2 (Score) | Mean (sd) | 13.63 (8.26) | 12.65 (7.55) | 0.42 |
| SCORE2 (low risk) | n (%) | 15 (14.42) | 12 (17.65) | 0.56 |
| SCORE2 (moderate risk) | n (%) | 53 (50.96) | 29 (42.65) | |
| SCORE2 (high risk) | n (%) | 36 (34.62) | 27 (39.71) | |
| CVR Alert | Yes (%) | 29 (27.88) | 22 (32.35) | 0.53 |
| MIS | Mean (sd) | 4.38 (2.27) | 7.28 (0.94) | <0.01 |
| SPMSQ | Mean (sd) | 2.91 (1.99) | 0.60 (0.74) | <0.01 |
| SVF | Mean (sd) | 10.61 (4.36) | 19.16 (6.95) | <0.01 |
| Group 1 CVR+, ACB+ | Mean (sd) | 29 (11.76) | 8 (27.88) | 0.02 |
| Group 2 CVR+, ACB− | Mean (sd) | 48 (70.59) | 60 (57.69) | |
| Group 3 CVR−, ACB+ | Mean (sd) | 1 (1.47) | 6 (5.77) | |
| Group 4 CVR−, ACB− | Mean (sd) | 11(16.18) | 9 (8.65) |
| Variable | Measure | Failed MIS (n = 60) | Passed MIS (n = 112) | p-Value | Failed SVF (n = 50) | Passed SVF (n = 122) | p-Value | Failed SPMSQ (n = 64) | Passed SPMSQ (n = 108) | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| SCORE2 (score) | Mean (sd) | 15.92 (9.12) | 11.81 (6.92) | <0.01 | 14.78 (8.82) | 12.61 (7.55) | 0.13 | 14.15 (8.93) | 12.70 (7.35) | 0.27 |
| SCORE2 (Low) | n (%) | 4 (6.67) | 23 (20.54) | 0.04 | 5 (10.00) | 22 (18.03) | 0.36 | 12 (18.75) | 15 (13.89) | 0.49 |
| SCORE2 (Moderate) | n (%) | 29 (48.33) | 53 (43.15) | 27 (54.00) | 55 (45.08) | 27 (42.19) | 55 (50.93) | |||
| SCORE2 (High) | n (%) | 27 (45.00) | 36 (40.18) | 18 (36.00) | 45 (36.89) | 25(39.06) | 38 (35.19) | |||
| CVR Alert (SCORE2) | Yes (%) | 56 (93.33) | 89 (79.46) | 0.02 | 52 (81.25) | 93 (86.11) | 0.40 | 45 (90.00) | 100 (81.97) | 0.19 |
| Total anticholinergic burden | Mean (sd) | 1.75 (1.70) | 1.49 (1.60) | 0.33 | 1.74 (1.37) | 1.52 (1.74) | 0.37 | 2.03 (1.84) | 1.31 (1.45) | <0.01 |
| CALS > 2 | n (%) | 16 (26.67) | 28 (25) | 0.81 | 14 (28) | 30 (24.59) | 0.64 | 22 (34.38) | 22 (20.37) | 0.04 |
| Group | Failed MIS OR (IC) | p-Value | Failed SVF OR (IC) | p-Value | Failed SPMSQ OR (IC) | p-Value |
|---|---|---|---|---|---|---|
| Group 1 CVR+, ACB+ | 5.47 (1.31, 37.8) | 0.04 | 2.17 (0.63, 8.78) | 0.74 | 1.42 (0.47, 4.39) | 0.53 |
| Group 2 CVR+, ACB− | 5.73 (1.54, 37.2) | 0.02 | 1.68 (0.56, 6.22) | 0.38 | 0.69 (0.26, 1.90) | 0.46 |
| Group 3 CVR−, ACB+ | 3.60 (0.36, 37.09) | 0.25 | 0.67 (0.03, 5.77) | 0.24 | 2.00 (0.35, 12.6) | 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Lluch, G.; Muedra-Moreno, A.; García-Zamora, M.; Gómez, B.; Sánchez-Roy, R.; Moreno, L. Selecting a Brief Cognitive Screening Test Based on Patient Profile: It Is Never Too Early to Start. J. Clin. Med. 2024, 13, 6009. https://doi.org/10.3390/jcm13196009
García-Lluch G, Muedra-Moreno A, García-Zamora M, Gómez B, Sánchez-Roy R, Moreno L. Selecting a Brief Cognitive Screening Test Based on Patient Profile: It Is Never Too Early to Start. Journal of Clinical Medicine. 2024; 13(19):6009. https://doi.org/10.3390/jcm13196009
Chicago/Turabian StyleGarcía-Lluch, Gemma, Ariadna Muedra-Moreno, Mar García-Zamora, Beatriz Gómez, Rafael Sánchez-Roy, and Lucrecia Moreno. 2024. "Selecting a Brief Cognitive Screening Test Based on Patient Profile: It Is Never Too Early to Start" Journal of Clinical Medicine 13, no. 19: 6009. https://doi.org/10.3390/jcm13196009
APA StyleGarcía-Lluch, G., Muedra-Moreno, A., García-Zamora, M., Gómez, B., Sánchez-Roy, R., & Moreno, L. (2024). Selecting a Brief Cognitive Screening Test Based on Patient Profile: It Is Never Too Early to Start. Journal of Clinical Medicine, 13(19), 6009. https://doi.org/10.3390/jcm13196009






