Post-Intensive Care Syndrome as a Burden for Patients and Their Caregivers: A Narrative Review
Abstract
1. Introduction
2. History of PICS and the Identification of Patients at Risk
- -
- Temporally antecedent to the ICU stay itself and linked to the patients themselves (age, gender, pre-existing comorbidities, psychological impairment, etc.);
- -
- Related to the cause of admission for critical illness (sepsis or shock);
- -
- Associated with the development of complications during the ICU stay (i.e., prolonged and deeper sedation, protracted mechanical ventilation, or delirium);
- -
- Subsequent to the ICU discharge (early symptoms of anxiety or depression) [8].
3. Clinical Manifestations of Pics: Multi-Dimensional Impairments
3.1. Physical Dysfunction
3.2. Cognitive Dysfunction
3.3. Psychological Dysfunction
3.4. Further Challenges for the ICU Survivors
4. Post-Intensive Care Syndrome Family
5. Diagnostic Evaluation
- Mental health: Patient Health Questionnaire-4;
- Cognition: MiniCog, Animal Naming;
- Physical function: Timed Up-and-Go, handgrip strength;
- HRQOL: EQ-5D-5L questionnaire.
- Mental health: Patient Health Questionnaire-8, Generalized Anxiety Disorder Scale-7, Impact of Event Scale—revised;
- Cognition: Repeatable Battery for the Assessment of Neuropsychological Status, Trail Making Test A and B;
- Physical function: 2-Minute Walk Test, handgrip strength, Short Physical Performance Battery;
- HRQOL: EQ-5D-5L, 12-Item WHO Disability Assessment Schedule [85].
- A limited number of tools covering all the three domains of PICS;
- The unclear validity, and often limited feasibility, of these tools (i.e., translation);
- A low degree of evidence on the efficacy of these assessment tools on psychological health;
- Only two tools address the issue of PICS-F [86].
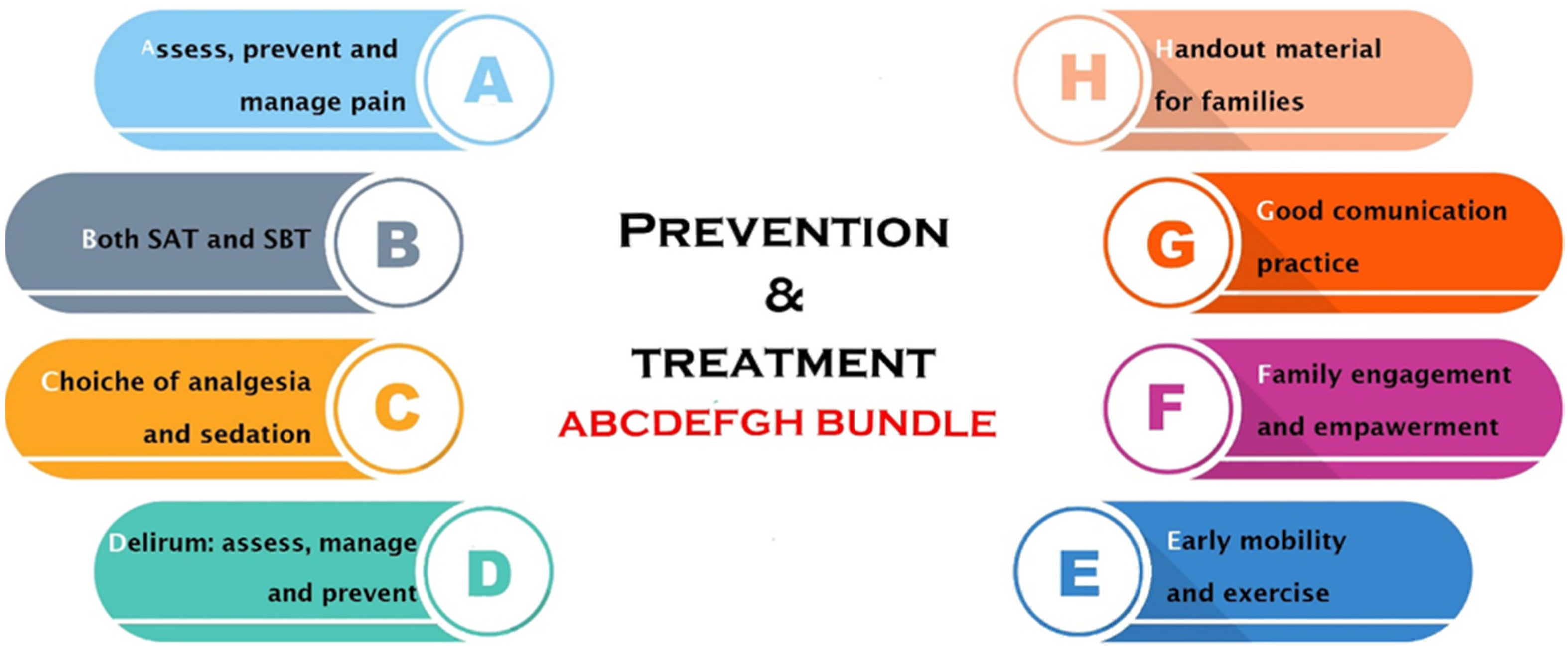
6. PICS Prevention and Treatment
- A.
- Assessing and managing pain by using validated tools; pain management can be either pharmacological or non-pharmacological.
- B.
- Breathing spontaneously should be encouraged with spontaneous breathing trials implemented unless contraindicated whilst correcting communication barriers.
- C.
- Choice of the sedation, avoiding as much as possible benzodiazepines, using the allowed minimum dose of sedatives to avoid deep levels of sedation, and performing sedation holds as much as possible.
- D.
- Delirium assessment on a daily basis in order to intervene as soon as possible both pharmacologically and non-pharmacologically.
- E.
- F.
- Family involvement by the healthcare providers, enhancing communication strategies.
- G.
- Good communication practices with the family members from the medical team to prevent the PICS-F.
- H.
- Hand out material provided to families to allow a better understanding of the ICU environment.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dunn, H.P.; Balas, M.C.P.; Hetland, B.P.; Krupp, A.P. Post-intensive care syndrome: A review for the primary care. Nurse Pract. 2022, 47, 15–22. [Google Scholar] [CrossRef]
- Herridge, M.S.; Azoulay, E. Outcomes after Critical Illness. N. Engl. J. Med. 2023, 388, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Vrettou, C.S.; Mantziou, V.; Vassiliou, A.G.; Orfanos, S.E.; Kotanidou, A.; Dimopoulou, I. Post-Intensive Care Syndrome in Survivors from Critical Illness including COVID-19 Patients: A Narrative Review. Life 2022, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Patsaki, I.; Dimopoulos, S. Increasing role of post-intensive care syndrome in quality of life of intensive care unit survivors. World J. Crit. Care Med. 2024, 13, 90428. [Google Scholar] [CrossRef] [PubMed]
- Shirasaki, K.; Hifumi, T.; Nakanishi, N.; Nosaka, N.; Miyamoto, K.; Komachi, M.H.; Haruna, J.; Inoue, S.; Otani, N. Postintensive care syndrome family: A comprehensive review. Acute Med. Surg. 2024, 11, e939. [Google Scholar] [CrossRef]
- Needham, D.M.; Davidson, J.; Cohen, H.; Hopkins, R.O.; Weinert, C.; Wunsch, H.; Zawistowski, C.; Bemis-Dougherty, A.; Berney, S.C.; Bienvenu, O.J.; et al. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Crit. Care Med. 2012, 40, 502–509. [Google Scholar] [CrossRef]
- Yuan, C.; Timmins, F.; Thompson, D.R. Post-intensive care syndrome: A concept analysis. Int. J. Nurs. Stud. 2021, 114, 103814. [Google Scholar] [CrossRef]
- Mikkelsen, M.E.M.; Still, M.M.; Anderson, B.J.M.; Bienvenu, O.J.; Brodsky, M.B.M.; Brummel, N.; Butcher, B.; Clay, A.S.; Felt, H.; Ferrante, L.E.; et al. Society of Critical Care Medicine’s International Consensus Conference on Prediction and Identification of Long-Term Impairments After Critical Illness. Crit. Care Med. 2020, 48, 1670–1679. [Google Scholar] [CrossRef]
- Lee, M.; Kang, J.; Jeong, Y.J. Risk factors for post–intensive care syndrome: A systematic review and meta-analysis. Aust. Crit. Care 2020, 33, 287–294. [Google Scholar] [CrossRef]
- Davydow, D.S.; Gifford, J.M.; Desai, S.V.; Needham, D.M.; Bienvenu, O.J. Posttraumatic stress disorder in general intensive care unit survivors: A systematic review. Gen. Hosp. Psychiatry 2008, 30, 421–434. [Google Scholar] [CrossRef]
- Marra, A.; Pandharipande, P.P.M.; Girard, T.D.M.; Patel, M.B.; Hughes, C.G.; Jackson, J.C.P.; Thompson, J.L.; Chandrasekhar, R.M.; Ely, E.W.; Brummel, N.E.M. Co-Occurrence of Post-Intensive Care Syndrome Problems Among 406 Survivors of Critical Illness. Crit. Care Med. 2018, 46, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.C.; Pandharipande, P.P.; Girard, T.D.; Brummel, N.E.; Thompson, J.L.; Hughes, C.G.; Pun, B.T.; E Vasilevskis, E.; Morandi, A.; Shintani, A.K.; et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: A longitudinal cohort study. Lancet Respir. Med. 2014, 2, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Herridge, M.S.; Chu, L.M.; Matte, A.; Tomlinson, G.; Chan, L.; Thomas, C.; Friedrich, J.O.; Mehta, S.; Lamontagne, F.; Levasseur, M.; et al. The RECOVER Program: Disability Risk Groups and 1-Year Outcome after 7 or More Days of Mechanical Ventilation. Am. J. Respir. Crit. Care Med. 2016, 194, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Needham, D.M.; Wozniak, A.W.; Hough, C.L.; Morris, P.E.; Dinglas, V.D.; Jackson, J.C.; Mendez-Tellez, P.A.; Shanholtz, C.; Ely, E.W.; Colantuoni, E.; et al. Risk factors for physical impairment after acute lung injury in a national, multicenter study. Am. J. Respir. Crit. Care Med. 2014, 189, 1214–1224. [Google Scholar] [CrossRef]
- Farley, K.J.; Eastwood, G.M.; Bellomo, R. A feasibility study of functional status and follow-up clinic preferences of patients at high risk of post intensive care syndrome. Anaesth. Intensiv. Care 2016, 44, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Griffiths, R.D.; Humphris, G.; Skirrow, P.M. Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Crit. Care Med. 2001, 29, 573–580. [Google Scholar] [CrossRef]
- Chung, C.R.; Yoo, H.J.; Park, J.; Ryu, S. Cognitive Impairment and Psychological Distress at Discharge from Intensive Care Unit. Psychiatry Investig. 2017, 14, 376–379. [Google Scholar] [CrossRef]
- Riegel, B.; Huang, L.; Mikkelsen, M.E.; Kutney-Lee, A.; Hanlon, A.L.; Murtaugh, C.M.; Bowles, K.H. Early Post-Intensive Care Syndrome among Older Adult Sepsis Survivors Receiving Home Care. J. Am. Geriatr. Soc. 2019, 67, 520–526. [Google Scholar] [CrossRef]
- Unoki, T.; Sakuramoto, H.; Uemura, S.; Tsujimoto, T.; Yamaguchi, T.; Shiba, Y.; Hino, M.; Kuribara, T.; Fukuda, Y.; Nagao, T.; et al. Prevalence of and risk factors for post-intensive care syndrome: Multicenter study of patients living at home after treatment in 12 Japanese intensive care units, SMAP-HoPe study. PLoS ONE 2021, 16, e0252167. [Google Scholar] [CrossRef]
- Voiriot, G.; Oualha, M.; Pierre, A.; Salmon-Gandonnière, C.; Gaudet, A.; Jouan, Y.; Kallel, H.; Radermacher, P.; Vodovar, D.; Sarton, B.; et al. Chronic critical illness and post-intensive care syndrome: From pathophysiology to clinical challenges. Ann. Intensiv. Care 2022, 12, 58. [Google Scholar] [CrossRef]
- Stevens, R.D.; Marshall, S.A.; Cornblath, D.R.; Hoke, A.; Needham, D.M.; de Jonghe, B.; Ali, N.A.; Sharshar, T. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit. Care Med. 2009, 37, S299–S308. [Google Scholar] [CrossRef] [PubMed]
- Jolley, S.E.; Bunnell, A.E.; Hough, C.L. ICU-Acquired Weakness. Chest 2016, 150, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Van Aerde, N.; Meersseman, P.; Debaveye, Y.; Wilmer, A.; Gunst, J.; Casaer, M.P.; Bruyninckx, F.; Wouters, P.J.; Gosselink, R.; Berghe, G.V.D.; et al. Five-year impact of ICU-acquired neuromuscular complications: A prospective, observational study. Intensiv. Care Med. 2020, 46, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Yende, S.; Austin, S.; Rhodes, A.; Finfer, S.; Opal, S.; Thompson, T.; Bozza, F.A.; LaRosa, S.P.; Ranieri, V.M.; Angus, D.C. Long-Term Quality of Life Among Survivors of Severe Sepsis: Analyses of Two International Trials. Crit. Care Med. 2016, 44, 1461–1467. [Google Scholar] [CrossRef]
- Solverson, K.J.; Grant, C.; Doig, C.J. Assessment and predictors of physical functioning post-hospital discharge in survivors of critical illness. Ann. Intensiv. Care 2016, 6, 92. [Google Scholar] [CrossRef]
- Herridge, M.S.; Batt, J.; Dos Santos, C. ICU-acquired weakness, morbidity, and death. Am. J. Respir. Crit. Care Med. 2014, 190, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Van Aerde, N.; Meersseman, P.; Debaveye, Y.; Wilmer, A.; Casaer, M.P.; Gunst, J.; Wauters, J.; Wouters, P.J.; Goetschalckx, K.; Gosselink, R.; et al. Aerobic exercise capacity in long-term survivors of critical illness: Secondary analysis of the post-EPaNIC follow-up study. Intensiv. Care Med. 2021, 47, 1462–1471. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, X.; Wang, F.; Peng, Z.; Fan, Y. A systematic review and meta-analysis of risk factors for intensive care unit acquired weakness. Medicine 2022, 101, e31405. [Google Scholar] [CrossRef]
- Vassiliou, A.G.; Jahaj, E.; Mastora, Z.; Karnezis, I.; Dimopoulou, I.; Orfanos, S.E.; Kotanidou, A. Prognostic Value of Bone Formation and Resorption Proteins in Heterotopic Ossification in Critically-Ill Patients. A Single-Cent. Study. J. Crit. Care Med. 2021, 7, 37–45. [Google Scholar] [CrossRef]
- Zuercher, P.; Moret, C.S.; Dziewas, R.; Schefold, J.C. Dysphagia in the intensive care unit: Epidemiology, mechanisms, and clinical management. Crit. Care 2019, 28, 103. [Google Scholar] [CrossRef]
- Dinglas, V.D.; Friedman, L.S.A.; Colantuoni, E.; Mendez-Tellez, P.A.; Shanholtz, C.B.; Ciesla, N.D.; Pronovost, P.J.; Needham, D.M.F. Muscle Weakness and 5-Year Survival in Acute Respiratory Distress Syndrome Survivors. Crit. Care Med. 2017, 45, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Ulvik, A.; Kvåle, R.; Wentzel-Larsen, T.; Flaatten, H. Sexual function in ICU survivors more than 3 years after major trauma. Intensiv. Care Med. 2008, 34, 447–453. [Google Scholar] [CrossRef]
- Sansone, A.; Mollaioli, D.; Ciocca, G.; Limoncin, E.; Colonnello, E.; Vena, W.; Jannini, E.A. Addressing male sexual and reproductive health in the wake of COVID-19 outbreak. J. Endocrinol. Investig. 2021, 44, 223–231. [Google Scholar] [CrossRef]
- Abedinzadeh, M.; Vahidi, S.; Rahavian, A.; Lojje, H.; Abouei, S. Effect of COVID-19 On the Sexual Activity of Men. Am. J. Mens Health 2023, 17, 15579883231193913. [Google Scholar] [CrossRef]
- Pandharipande, P.; Girard, T.; Jackson, J.; Morandi, A.; Thompson, J.; Pun, B.; Brummel, N.; Hughes, C.; Vasilevskis, E.; Shintani, A.; et al. Long-term cognitive impairment after critical illness. N. Engl. J. Med. 2013, 369, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Iwashyna, T.J.; Ely, E.W.; Smith, D.M.; Langa, K.M. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010, 304, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.G.; Morandi, A.; Girard, T.D.; Riedel, B.; Thompson, J.L.; Shintani, A.K.; Pun, B.T.; Ely, E.W.; Pandharipande, P.P. Association between endothelial dysfunction and acute brain dysfunction during critical illness. Anesthesiology 2013, 118, 631–639. [Google Scholar] [CrossRef]
- Sakusic, A.; O’HOro, J.C.; Dziadzko, M.; Volha, D.; Ali, R.; Singh, T.D.; Kashyap, R.; Farrell, A.M.; Fryer, J.D.; Petersen, R.; et al. Potentially Modifiable Risk Factors for Long-Term Cognitive Impairment after Critical Illness: A Systematic Review. Mayo Clin. Proc. 2018, 93, 68–82. [Google Scholar] [CrossRef]
- Bulic, D.; Bennett, M.; Georgousopoulou, E.N.; Shehabi, Y.; Pham, T.; Looi, J.C.L.; van Haren, F.M.P. Cognitive and psychosocial outcomes of mechanically ventilated intensive care patients with and without delirium. Ann. Intensiv. Care 2020, 10, 104. [Google Scholar] [CrossRef]
- Girard, T.D.M.; Jackson, J.C.P.; Pandharipande, P.P.M.; Pun, B.T.M.; Thompson, J.L.; Shintani, A.K.; Gordon, S.M.P.; Canonico, A.E.; Dittus, R.S.; Bernard, G.R.; et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit. Care Med. 2010, 38, 1513–1520. [Google Scholar] [CrossRef]
- Morandi, A.; Rogers, B.P.; Gunther, M.L.; Merkle, K.B.; Pandharipande, P.M.; Girard, T.D.M.; Jackson, J.C.P.; Thompson, J.; Shintani, A.K.; Geevarghese, S.M.; et al. The relationship between delirium duration, white matter integrity, and cognitive impairment in intensive care unit survivors as determined by diffusion tensor imaging: The Visions prospective cohort magnetic resonance imaging study. Crit. Care Med. 2012, 40, 2182–2189. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, C.B.; Andersen-Ranberg, N.C.; Poulsen, L.M.; Granholm, A.; Rasmussen, B.S.; Kjær, M.-B.N.; Lange, T.; Ebdrup, B.H.; Collet, M.O.; Andreasen, A.S.; et al. Long-term outcomes with haloperidol versus placebo in acutely admitted adult ICU patients with delirium. Intensiv. Care Med. 2024, 50, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Aiello, G.; Cuocina, M.; La Via, L.; Messina, S.; Attaguile, G.A.; Cantarella, G.; Sanfilippo, F.; Bernardini, R. Melatonin or Ramelteon for Delirium Prevention in the Intensive Care Unit: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2023, 12, 435. [Google Scholar] [CrossRef] [PubMed]
- Vrettou, C.S.; Mantziou, V.; Ilias, I.; Vassiliou, A.G.; Orfanos, S.E.; Kotanidou, A.; Dimopoulou, I. Quality of Life, Depression, and Anxiety in Survivors of Critical Illness from a Greek ICU. A Prospective Observational Study. Healthcare 2021, 9, 849. [Google Scholar] [CrossRef]
- Angus, D.C.; Musthafa, A.A.; Clermont, G.; Griffin, M.F.; Linde-Zwirble, W.T.; Dremsizov, T.T.; Pinsky, M.R. Quality-adjusted survival in the first year after the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2001, 163, 1389–1394. [Google Scholar] [CrossRef]
- Cheung, A.M.; Tansey, C.M.; Tomlinson, G.; Diaz-Granados, N.; Matté, A.; Barr, A.; Mehta, S.; Mazer, C.D.; Guest, C.B.; Stewart, T.E.; et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2006, 174, 538–544. [Google Scholar] [CrossRef]
- Dowdy, D.W.; Dinglas, V.; Mendez-Tellez, P.A.; Bienvenu, O.J.; Sevransky, J.; Dennison, C.R.; Shanholtz, C.; Needham, D.M. Intensive care unit hypoglycemia predicts depression during early recovery from acute lung injury. Crit. Care Med. 2008, 36, 2726–2733. [Google Scholar] [CrossRef]
- Weinert, C.; Meller, W. Epidemiology of depression and antidepressant therapy after acute respiratory failure. Psychosomatics 2006, 47, 399–407. [Google Scholar] [CrossRef]
- Rattray, J.E.; Johnston, M.; Wildsmith, J.A.W. Predictors of emotional outcomes of intensive care. Anaesthesia 2005, 60, 1085–1092. [Google Scholar] [CrossRef]
- Nikayin, S.; Rabiee, A.; Hashem, M.D.; Huang, M.; Bienvenu, O.J.; Turnbull, A.E.; Needham, D.M. Anxiety symptoms in survivors of critical illness: A systematic review and meta-analysis. Gen. Hosp. Psychiatry 2016, 43, 23–29. [Google Scholar] [CrossRef]
- Hatch, R.; Young, D.; Barber, V.; Griffiths, J.; Harrison, D.A.; Watkinson, P. Anxiety, Depression and Post Traumatic Stress Disorder after critical illness: A UK-wide prospective cohort study. Crit. Care 2018, 22, 310. [Google Scholar] [CrossRef] [PubMed]
- Myhren, H.; Ekeberg, O.; Tøien, K.; Karlsson, S.; Stokland, O. Posttraumatic stress, anxiety and depression symptoms in patients during the first year post intensive care unit discharge. Crit. Care 2010, 14, R14. [Google Scholar] [CrossRef] [PubMed]
- Righy, C.; Rosa, R.G.; da Silva, R.T.A.; Kochhann, R.; Migliavaca, C.B.; Robinson, C.C.; Teche, S.P.; Teixeira, C.; Bozza, F.A.; Falavigna, M. Prevalence of post-traumatic stress disorder symptoms in adult critical care survivors: A systematic review and meta-analysis. Crit. Care 2019, 23, 213. [Google Scholar] [CrossRef] [PubMed]
- Bienvenu, O.J.; Colantuoni, E.; Mendez-Tellez, P.A.; Shanholtz, C.; Dennison-Himmelfarb, C.R.R.; Pronovost, P.J.; Needham, D.M. Cooccurrence of and remission from general anxiety, depression, and posttraumatic stress disorder symptoms after acute lung injury: A 2-year longitudinal study. Crit. Care Med. 2015, 43, 642–653. [Google Scholar] [CrossRef]
- Schmidt, K.F.R.; Gensichen, J.S.; Schroevers, M.; Kaufmann, M.; Mueller, F.; Schelling, G.; Gehrke-Beck, S.; Boede, M.; Heintze, C.; Wensing, M.; et al. Trajectories of post-traumatic stress in sepsis survivors two years after ICU discharge: A secondary analysis of a randomized controlled trial. Crit. Care 2024, 28, 35. [Google Scholar] [CrossRef]
- Wintermann, G.-B.; Rosendahl, J.; Weidner, K.; Strauß, B.; Petrowski, K. Risk Factors of Delayed Onset Posttraumatic Stress Disorder in Chronically Critically Ill Patients. J. Nerv. Ment. Dis. 2017, 205, 780–787. [Google Scholar] [CrossRef]
- Bienvenu, O.J.; Gellar, J.; Althouse, B.M.; Colantuoni, E.; Sricharoenchai, T.; Mendez-Tellez, P.A.; Shanholtz, C.; Dennison, C.R.; Pronovost, P.J.; Needham, D.M. Post-traumatic stress disorder symptoms after acute lung injury: A 2-year prospective longitudinal study. Psychol. Med. 2013, 43, 2657–2671. [Google Scholar] [CrossRef]
- van Zuiden, M.; Engel, S.; Karchoud, J.F.; Wise, T.J.; Sijbrandij, M.; Mouthaan, J.; Olff, M.; van de Schoot, R. Sex-differential PTSD symptom trajectories across one year following suspected serious injury. Eur. J. Psychotraumatol. 2022, 13, 2031593. [Google Scholar] [CrossRef]
- Lowe, S.R.; Ratanatharathorn, A.; Lai, B.S.; van der Mei, W.; Barbano, A.C.; Bryant, R.A.; Delahanty, D.L.; Matsuoka, Y.J.; Olff, M.; Schnyder, U.; et al. Posttraumatic stress disorder symptom trajectories within the first year following emergency department admissions: Pooled results from the International Consortium to predict PTSD. Psychol. Med. 2021, 51, 1129–1139. [Google Scholar] [CrossRef]
- Bienvenu, O.J.; Friedman, L.A.; Colantuoni, E.; Dinglas, V.D.; Sepulveda, K.A.; Mendez-Tellez, P.; Shanholz, C.; Pronovost, P.J.; Needham, D.M. Psychiatric symptoms after acute respiratory distress syndrome: A 5-year longitudinal study. Intensiv. Care Med. 2018, 44, 38–47. [Google Scholar] [CrossRef]
- Shaffer, K.M.; Riklin, E.B.; Jacobs, J.M.; Rosand, J.M.; Vranceanu, A.-M. Mindfulness and Coping Are Inversely Related to Psychiatric Symptoms in Patients and Informal Caregivers in the Neuroscience ICU: Implications for Clinical Care. Crit. Care Med. 2016, 44, 2028–2036. [Google Scholar] [CrossRef] [PubMed]
- Elbert, T.; Schauer, M.; Neuner, F. Narrative Exposure Therapy (NET): Reorganizing Memories of Traumatic Stress, Fear, and Violence. In Evidence Based Treatments for Trauma-Related Psychological Disorders; Schnyder, U., Cloitre, M., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar]
- McIlroy, P.A.; King, R.S.; Garrouste-Orgeas, M.; Tabah, A.; Ramanan, M. The Effect of ICU Diaries on Psychological Outcomes and Quality of Life of Survivors of Critical Illness and Their Relatives: A Systematic Review and Meta-Analysis. Crit. Care Med. 2019, 47, 273–279. [Google Scholar] [CrossRef]
- Norman, B.C.; Jackson, J.C.P.; Graves, J.A.; Girard, T.D.M.; Pandharipande, P.P.M.M.; Brummel, N.E.M.M.; Wang, L.; Thompson, J.L.; Chandrasekhar, R.; Ely, E.W. Employment Outcomes After Critical Illness: An Analysis of the Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors Cohort. Crit. Care Med. 2016, 44, 2003–2009. [Google Scholar] [CrossRef]
- Rothenhäusler, H.-B.; Ehrentraut, S.; Stoll, C.; Schelling, G.; Kapfhammer, H.-P. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: Results of an exploratory study. Gen. Hosp. Psychiatry 2001, 23, 90–96. [Google Scholar] [CrossRef]
- Mattioni, M.F.; Dietrich, C.; Sganzerla, D.; Rosa, R.G.; Teixeira, C. Return to work after discharge from the intensive care unit: A Brazilian multicenter cohort. Rev. Bras. De Ter. Intensiv. 2022, 34, 492–498. [Google Scholar] [CrossRef]
- Skei, N.V.; Moe, K.; Nilsen, T.I.L.; Aasdahl, L.; Prescott, H.C.; Damås, J.K.; Gustad, L.T. Return to work after hospitalization for sepsis: A nationwide, registry-based cohort study. Crit. Care 2023, 27, 443. [Google Scholar] [CrossRef] [PubMed]
- Kamdar, B.B.; Suri, R.; Suchyta, M.R.; Digrande, K.F.; Sherwood, K.D.; Colantuoni, E.; Dinglas, V.D.; Needham, D.M.; O Hopkins, R. Return to work after critical illness: A systematic review and meta-analysis. Thorax 2020, 75, 17–27. [Google Scholar] [CrossRef]
- Chelluri, L.; Im, K.A.; Belle, S.H.; Schulz, R.; Rotondi, A.J.; Donahoe, M.P.; Sirio, C.A.; Mendelsohn, A.B.; Pinsky, M.R. Long-term mortality and quality of life after prolonged mechanical ventilation. Crit. Care Med. 2004, 32, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Van Pelt, D.C.; Milbrandt, E.B.; Qin, L.; Weissfeld, L.A.; Rotondi, A.J.; Schulz, R.; Chelluri, L.; Angus, D.C.; Pinsky, M.R. Informal caregiver burden among survivors of prolonged mechanical ventilation. Am. J. Respir. Crit. Care Med. 2007, 175, 167–173. [Google Scholar] [CrossRef]
- Davidson, J.E.; Jones, C.; Bienvenu, O.J. Family response to critical illness: Postintensive care syndrome-family. Crit. Care Med. 2012, 40, 618–624. [Google Scholar] [CrossRef]
- Piccinelli, M.; Wilkinson, G. Gender differences in depression. Br. J. Psychiatry 2000, 177, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Spruytte, N.; Van Audenhove, C.; Lammertyn, F.; Storms, G. The quality of the caregiving relationship in informal care for older adults with dementia and chronic psychiatric patients. Psychol. Psychother. Theory Res. Pract. 2002, 75 Pt 3, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Haines, K.J.; Denehy, L.; Skinner, E.H.; Warrillow, S.; Berney, S. Psychosocial outcomes in informal caregivers of the critically ill: A systematic review. Crit. Care Med. 2015, 43, 1112–1120. [Google Scholar] [CrossRef]
- Szuhany, K.L.; Malgaroli, M.; Miron, C.D.; Simon, N.M. Prolonged Grief Disorder: Course, Diagnosis, Assessment, and Treatment. FOCUS 2021, 19, 161–172. [Google Scholar] [CrossRef]
- Kotfis, K.; Maj, P.; Szylińska, A.; Pankowiak, M.; Reszka, E.; Ely, E.W.; Marra, A. The spectrum of psychological disorders in family members of patients suffering from delirium associated with critical illness: A prospective, observational study. Sci. Rep. 2024, 14, 4562. [Google Scholar] [CrossRef] [PubMed]
- Dupont, T.; Kentish-Barnes, N.; Pochard, F.; Duchesnay, E.; Azoulay, E. Prediction of post-traumatic stress disorder in family members of ICU patients: A machine learning approach. Intensiv. Care Med. 2024, 50, 114–124. [Google Scholar] [CrossRef]
- Azoulay, E.; Pochard, F.; Chevret, S.; Jourdain, M.; Bornstain, C.; Wernet, A.; Cattaneo, I.; Annane, D.; Brun, F.; Bollaert, P.-E.; et al. Impact of a family information leaflet on effectiveness of information provided to family members of intensive care unit patients: A multicenter, prospective, randomized, controlled trial. Am. J. Respir. Crit. Care Med. 2002, 165, 438–442. [Google Scholar] [CrossRef]
- Jabre, P.; Belpomme, V.; Azoulay, E.; Jacob, L.; Bertrand, L.; Lapostolle, F.; Tazarourte, K.; Bouilleau, G.; Pinaud, V.; Broche, C.; et al. Family presence during cardiopulmonary resuscitation. N. Engl. J. Med. 2013, 368, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Lautrette, A.; Darmon, M.; Megarbane, B.; Joly, L.M.; Chevret, S.; Adrie, C.; Barnoud, D.; Bleichner, G.; Bruel, C.; Choukroun, G.; et al. A communication strategy and brochure for relatives of patients dying in the ICU. N. Engl. J. Med. 2007, 356, 469–478. [Google Scholar] [CrossRef]
- Rosa, R.G.; Falavigna, M.; da Silva, D.B.; Sganzerla, D.; Santos, M.M.S.; Kochhann, R.; de Moura, R.M.; Eugênio, C.S.; Haack, T.d.S.R.; Barbosa, M.G.; et al. Effect of Flexible Family Visitation on Delirium Among Patients in the Intensive Care Unit: The ICU Visits Randomized Clinical Trial. JAMA 2019, 322, 216–228. [Google Scholar] [CrossRef]
- Curtis, J.R.; Treece, P.D.; Nielsen, E.L.; Gold, J.; Ciechanowski, P.S.; Shannon, S.E.; Khandelwal, N.; Young, J.P.; Engelberg, R.A. Randomized Trial of Communication Facilitators to Reduce Family Distress and Intensity of End-of-Life Care. Am. J. Respir. Crit. Care Med. 2016, 193, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.E.; Hough, C.L.; Carson, S.S.; White, D.B.; Kahn, J.M.; Olsen, M.K.; Jones, D.M.; Somers, T.J.; Kelleher, S.A.; Porter, L.S. Effects of a Telephone- and Web-based Coping Skills Training Program Compared with an Education Program for Survivors of Critical Illness and Their Family Members. A Randomized Clinical Trial. Am. J. Respir. Crit. Care Med. 2018, 197, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.H.; Angel, S.; Egerod, I.; Lund, T.H.; Renberg, M.; Hansen, T.B. The effect of family-authored diaries on posttraumatic stress disorder in intensive care unit patients and their relatives: A randomised controlled trial (DRIP-study). Aust. Crit. Care 2020, 33, 123–129. [Google Scholar] [CrossRef]
- Spies, C.D.; Krampe, H.; Paul, N.; Denke, C.; Kiselev, J.; Piper, S.K.; Kruppa, J.; Grunow, J.J.; Steinecke, K.; Gülmez, T.; et al. Instruments to measure outcomes of post-intensive care syndrome in outpatient care settings—Results of an expert consensus and feasibility field test. J. Intensiv. Care Soc. 2021, 22, 159–174. [Google Scholar] [CrossRef]
- Pant, U.; Vyas, K.; Meghani, S.; Park, T.; Norris, C.M.; Papathanassoglou, E. Screening tools for post–intensive care syndrome and post-traumatic symptoms in intensive care unit survivors: A scoping review. Aust. Crit. Care 2023, 36, 863–871. [Google Scholar] [CrossRef]
- Pun, B.T.; Balas, M.C.; Barnes-Daly, M.A.; Thompson, J.L.; Aldrich, J.M.; Barr, J.; Byrum, D.; Carson, S.S.; Devlin, J.W.; Engel, H.J.; et al. Caring for Critically Ill Patients with the ABCDEF Bundle: Results of the ICU Liberation Collaborative in Over 15,000 Adults. Crit. Care Med. 2019, 47, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Fuke, R.; Hifumi, T.; Kondo, Y.; Hatakeyama, J.; Takei, T.; Yamakawa, K.; Inoue, S.; Nishida, O. Early rehabilitation to prevent postintensive care syndrome in patients with critical illness: A systematic review and meta-analysis. BMJ Open 2018, 8, e019998. [Google Scholar] [CrossRef]
- Routsi, C.; Gerovasili, V.; Vasileiadis, I.; Karatzanos, E.; Pitsolis, T.; Tripodaki, E.S.; Markaki, V.; Zervakis, D.; Nanas, S. Electrical muscle stimulation prevents critical illness polyneuromyopathy: A randomized parallel intervention trial. Crit. Care 2010, 14, R74. [Google Scholar] [CrossRef]
- Garrouste-Orgeas, M.; Périer, A.; Mouricou, P.; Grégoire, C.; Bruel, C.; Brochon, S.; Philippart, F.; Max, A.; Misset, B. Writing in and reading ICU diaries: Qualitative study of families’ experience in the ICU. PLoS ONE 2014, 9, e110146. [Google Scholar] [CrossRef]
- Mehlhorn, J.; Freytag, A.; Schmidt, K.; Brunkhorst, F.M.; Graf, J.; Troitzsch, U.; Schlattmann, P.; Wensing, M.; Gensichen, J. Rehabilitation interventions for postintensive care syndrome: A systematic review. Crit. Care Med. 2014, 42, 1263–1271. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, X.-Y.; He, X.; Zhou, C.-M.; Shen, J.-C.; Tong, J.-H. Parvalbumin interneuron-mediated neural disruption in an animal model of postintensive care syndrome: Prevention by fluoxetine. Aging 2021, 13, 8720–8736. [Google Scholar] [CrossRef] [PubMed]
- Knapp, P.; Beck, A.T. Cognitive therapy: Foundations, conceptual models, applications and research. Braz. J. Psychiatry 2008, 30 (Suppl. S2), s54–s64. [Google Scholar] [CrossRef] [PubMed]
- Aljuhani, O. The Role of Critical Care Pharmacists Beyond Intensive Care Units: A Narrative Review on Medication Management for ICU Survivors. Braz. J. Pharm. Sci. 2022, 58, e21012. [Google Scholar]
- Mohammad, R.A.; Eze, C.; Marshall, V.D.; Coe, A.B.; Costa, D.K.; Thompson, A.; Pitcher, M.; Haezebrouck, E.; McSparron, J.I. The impact of a clinical pharmacist in an interprofessional intensive care unit recovery clinic providing care to intensive care unit survivors. JACCP J. Am. College Clin. Pharm. 2022, 5, 1027–1038. [Google Scholar] [CrossRef]
- Stollings, J.L.; Poyant, J.O.; Groth, C.M.; Rappaport, S.H.; Kruer, R.M.; Miller, E.; Whitten, J.A.; Mcintire, A.M.; McDaniel, C.M.; Betthauser, K.D.; et al. An International, Multicenter Evaluation of Comprehensive Medication Management by Pharmacists in ICU Recovery Centers. J. Intensiv. Care Med. 2023, 38, 957–965. [Google Scholar] [CrossRef]
- Renner, C.; Jeitziner, M.-M.; Albert, M.; Brinkmann, S.; Diserens, K.; Dzialowski, I.; Heidler, M.-D.; Lück, M.; Nusser-Müller-Busch, R.; Sandor, P.S.; et al. Guideline on multimodal rehabilitation for patients with post-intensive care syndrome. Crit. Care 2023, 27, 301. [Google Scholar] [CrossRef]
- Peris, A.; Bonizzoli, M.; Iozzelli, D.; Migliaccio, M.L.; Zagli, G.; Bacchereti, A.; Debolini, M.; Vannini, E.; Solaro, M.; Bendoni, E.; et al. Early intra-intensive care unit psychological intervention promotes recovery from post traumatic stress disorders, anxiety and depression symptoms in critically ill patients. Crit. Care 2011, 15, R41. [Google Scholar] [CrossRef]
- Yasaka, T.; Ohbe, H.; Igarashi, A.; Yamamoto-Mitani, N.; Yasunaga, H. Impact of the health policy for interdisciplinary collaborative rehabilitation practices in intensive care units: A difference-in-differences analysis in Japan. Intensiv. Crit. Care Nurs. 2024, 83, 103625. [Google Scholar] [CrossRef]
- Schweickert, W.D.; Pohlman, M.C.; Pohlman, A.S.; Nigos, C.; Pawlik, A.J.; Esbrook, C.L.; Spears, L.; Miller, M.; Franczyk, M.; Deprizio, D.; et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet 2009, 373, 1874–1882. [Google Scholar] [CrossRef]
- Liu, M.; Luo, J.; Zhou, J.; Zhu, X. Intervention effect of neuromuscular electrical stimulation on ICU acquired weakness: A meta-analysis. Int. J. Nurs. Sci. 2020, 7, 228–237. [Google Scholar] [CrossRef]
- Xu, C.; Yang, F.; Wang, Q.; Gao, W. Effect of neuromuscular electrical stimulation in critically ill adults with mechanical ventilation: A systematic review and network meta-analysis. BMC Pulm. Med. 2024, 24, 56. [Google Scholar] [CrossRef] [PubMed]
- Patsaki, I.; Kouvarakos, A.; Vasileiadis, I.; Koumantakis, G.A.; Ischaki, E.; Grammatopoulou, E.; Kotanidou, A.; Magira, E.E. Low-Medium and High-Intensity Inspiratory Muscle Training in Critically Ill Patients: A Systematic Review and Meta-Analysis. Medicina 2024, 60, 869. [Google Scholar] [CrossRef] [PubMed]
- Lew, C.C.H.; Lee, Z.-Y.; Day, A.G.; Jiang, X.; Bear, D.; Jensen, G.L.; Ng, P.Y.; Tweel, L.; Parillo, A.; Heyland, D.K.; et al. The Association Between Malnutrition and High Protein Treatment on Outcomes in Critically Ill Patients: A Post Hoc Analysis of the EFFORT Protein Randomized Trial. Chest 2024, 165, 1380–1391. [Google Scholar] [CrossRef]
- Bels, J.L.M.; Thiessen, S.; van Gassel, R.J.J.; Beishuizen, A.; Dekker, A.D.B.; Fraipont, V.; Lamote, S.; Ledoux, D.; Scheeren, C.; De Waele, E.; et al. Effect of high versus standard protein provision on functional recovery in people with critical illness (PRECISe): An investigator-initiated, double-blinded, multicentre, parallel-group, randomised controlled trial in Belgium and the Netherlands. Lancet 2024, 404, 659–669. [Google Scholar] [CrossRef]
- van Zanten, A.R.H.; De Waele, E.; Wischmeyer, P.E.; van Zanten, A.R.H.; De Waele, E.; Wischmeyer, P.E.; van Zanten, A.R.H.; De Waele, E.; Wischmeyer, P.E.; van Zanten, A.R.H.; et al. Nutrition therapy and critical illness: Practical guidance for the ICU, post-ICU, and long-term convalescence phases. Crit. Care 2019, 23, 368. [Google Scholar] [CrossRef] [PubMed]
- Macht, M.; Wimbish, T.; Bodine, C.; Moss, M. ICU-acquired swallowing disorders. Crit. Care Med. 2013, 41, 2396–2405. [Google Scholar] [CrossRef]
- Salisbury, L.; Merriweather, J.; Walsh, T. The development and feasibility of a ward-based physiotherapy and nutritional rehabilitation package for people experiencing critical illness. Clin. Rehabil. 2010, 24, 489–500. [Google Scholar] [CrossRef]
- Fadeur, M.; Preiser, J.-C.; Verbrugge, A.-M.; Misset, B.; Rousseau, A.-F. Oral Nutrition during and after Critical Illness: SPICES for Quality of Care! Nutrients 2020, 12, 3509. [Google Scholar] [CrossRef]
| ICU related | Admission | Emergency admission, ICU type, hospital type, ICU LOS, and Hospital LOS |
| Experience | ICU mentality, delirium, restraint, bed rest, device self-removal, pain | |
| Treatment and therapies | Diagnosis, comorbidity, surgery, complications, disease severity, type of support (cardiovascular, respiratory, renal), no. of organs supported, analgesics, drugs administered (muscle relaxants, sedatives, steroids, inotropic drugs), no. of drug groups, laboratory data, vital signs | |
| Patient’s related | Personality traits | Illness awareness, mindfulness, optimism, coping skill, self-efficacy, and trait anxiety |
| Previous health conditions | BMI, hearing or visual impairment, previous ICU admission, pre-ICU sleep quality, frailty, trauma event, mental health problem, cognitive function, physical status | |
| Social and demographics | Age, sex, ethnicity, living situation, marital status, younger children, education, employment, socioeconomic status, caregiver, social support, social issue, alcohol, smoking, illicit drug, and physical activity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schembari, G.; Santonocito, C.; Messina, S.; Caruso, A.; Cardia, L.; Rubulotta, F.; Noto, A.; Bignami, E.G.; Sanfilippo, F. Post-Intensive Care Syndrome as a Burden for Patients and Their Caregivers: A Narrative Review. J. Clin. Med. 2024, 13, 5881. https://doi.org/10.3390/jcm13195881
Schembari G, Santonocito C, Messina S, Caruso A, Cardia L, Rubulotta F, Noto A, Bignami EG, Sanfilippo F. Post-Intensive Care Syndrome as a Burden for Patients and Their Caregivers: A Narrative Review. Journal of Clinical Medicine. 2024; 13(19):5881. https://doi.org/10.3390/jcm13195881
Chicago/Turabian StyleSchembari, Giovanni, Cristina Santonocito, Simone Messina, Alessandro Caruso, Luigi Cardia, Francesca Rubulotta, Alberto Noto, Elena G. Bignami, and Filippo Sanfilippo. 2024. "Post-Intensive Care Syndrome as a Burden for Patients and Their Caregivers: A Narrative Review" Journal of Clinical Medicine 13, no. 19: 5881. https://doi.org/10.3390/jcm13195881
APA StyleSchembari, G., Santonocito, C., Messina, S., Caruso, A., Cardia, L., Rubulotta, F., Noto, A., Bignami, E. G., & Sanfilippo, F. (2024). Post-Intensive Care Syndrome as a Burden for Patients and Their Caregivers: A Narrative Review. Journal of Clinical Medicine, 13(19), 5881. https://doi.org/10.3390/jcm13195881








