Bacterial Infection Features in Alcohol-Associated Hepatitis: Review of a 2016–2021 Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Definitions
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Infections
3.3. Bacteria and Sites of Infection
3.4. Infections upon Admission
3.5. Infections during Follow-Up
3.6. Liver-Related Decompensations and ACLF in Infected Patients Compared with Noninfected Patients
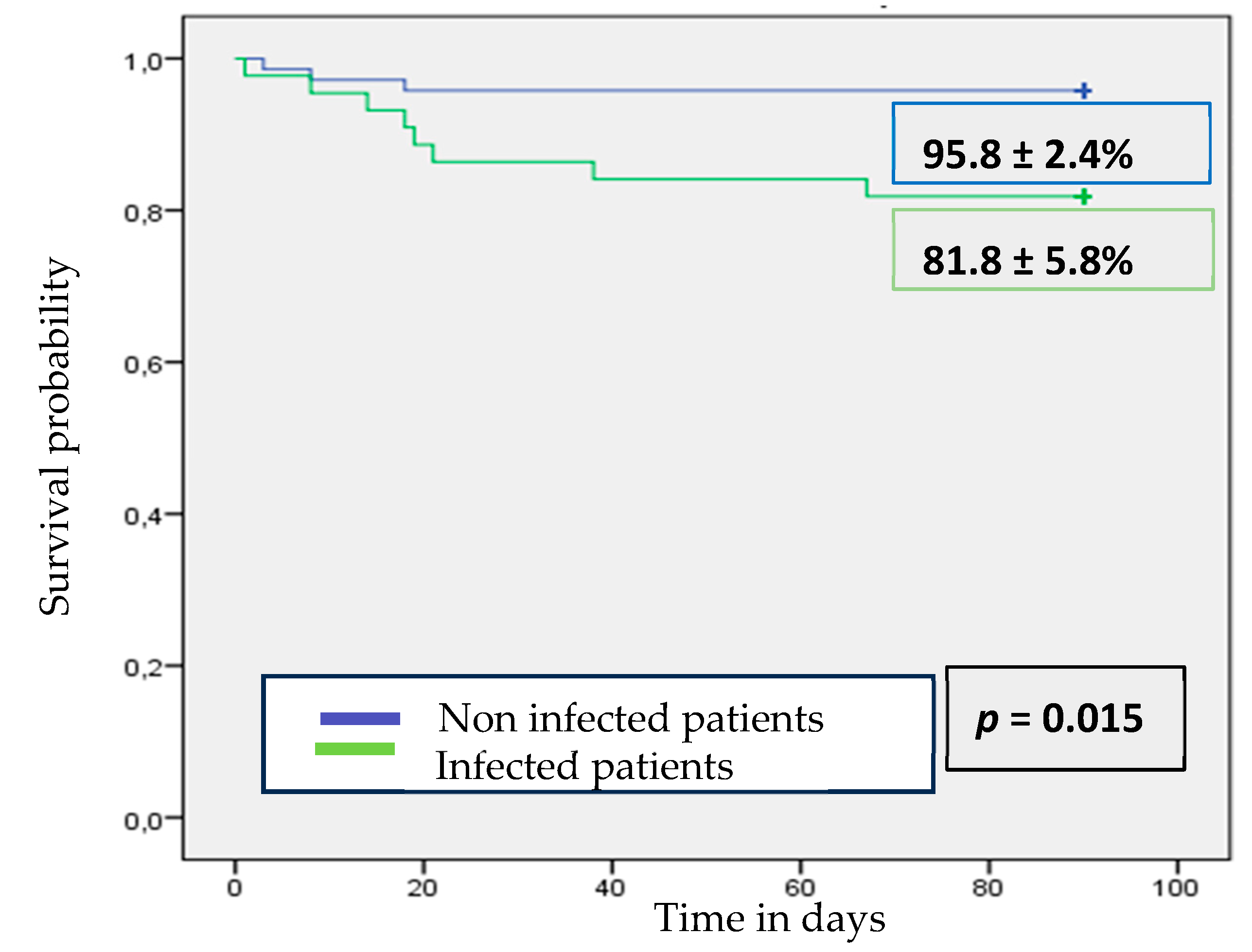
3.7. Mortality and Predictors of Mortality in Infected Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jepsen, P.; Younossi, Z.M. The global burden of cirrhosis: A review of disability adjusted life-years lost and unmet needs. J. Hepatol. 2021, 75, S3–S13. [Google Scholar] [CrossRef] [PubMed]
- Kasper, P.; Lang, S.; Steffen, H.M.; Demir, M. Management of alcoholic hepatitis: A clinical perspective. Liver Int. 2023, 43, 2078–2095. [Google Scholar] [CrossRef] [PubMed]
- Prado, V.; Caballería, J.; Vargas, V.; Bataller, R.; Altamirano, J. Alcoholic hepatitis: How far are we and where are we going? Ann. Hepatol. 2016, 15, 463–473. [Google Scholar] [PubMed]
- Vergis, N.; Atkinson, S.R.; Thursz, M.R. Assessment and management of infection in alcoholic hepatitis. Semin. Liver Dis. 2020, 40, 11–19. [Google Scholar] [CrossRef]
- Albillos, A.; Martin-Mateos, R.; Van der Merwe, S.; Wiest, R.; Jalan, R.; Álvarez-Mon, M. Cirrhosis-associated immune dysfunction. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 112–134. [Google Scholar] [CrossRef]
- Parker, R.; Im, G.; Jones, F.; Hernández, O.P.; Nahas, J.; Kumar, A.; Wheatley, D.; Sinha, A.; Gonzalez-Reimers, E.; Sanchez-Pérez, M.; et al. Clinical and microbiological features of infection in alcoholic hepatitis: An international cohort study. J. Gastroenterol. 2017, 52, 1192–1200. [Google Scholar] [CrossRef]
- Bataller, R.; Arab, J.P.; Shah, V.H. Alcohol-associated hepatitis. N. Engl. J. Med. 2022, 387, 2436–2448. [Google Scholar] [CrossRef]
- Singal, A.K.; Bataller, R.; Ahn, J.; Kamath, P.S.; Shah, V.H. ACG Clinical Guideline: Alcoholic liver disease. Am. J. Gastroenterol. 2018, 113, 175–194. [Google Scholar] [CrossRef]
- Louvet, A.; Wartel, F.; Castel, H.; Dharancy, S.; Hollebecque, A.; Canva-Delcambre, V.; Deltenre, P.; Mathurin, P. Infection in patients with severe alcoholic hepatitis treated with steroids: Early response to therapy is the key factor. Gastroenterology 2009, 137, 541–548. [Google Scholar] [CrossRef]
- Michelena, J.; Altamirano, J.; Abraldes, J.G.; Affò, S.; Morales-Ibanez, O.; Sancho-Bru, P.; Dominguez, M.; García-Pagán, J.C.; Fernández, J.; Arroyo, V.; et al. Systemic inflammatory response and serum lipopolysaccharide levels predict multiple organ failure and death in alcoholic hepatitis. Hepatology 2015, 62, 762–772. [Google Scholar] [CrossRef]
- Thursz, M.R.; Richardson, P.; Allison, M.; Austin, A.; Bowers, M.; Day, C.P.; Downs, N.; Gleeson, D.; MacGilchrist, A.; Grant, A.; et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N. Engl. J. Med. 2015, 372, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Vergis, N.; Atkinson, S.R.; Knapp, S.; Maurice, J.; Allison, M.; Austin, A.; Forrest, E.H.; Masson, S.; McCune, A.; Patch, D.; et al. In patients with severe alcoholic hepatitis, prednisolone increases susceptibility to infection and infection-related mortality, and is associated with high circulating levels of bacterial DNA. Gastroenterology 2017, 152, 1068–1077.e4. [Google Scholar] [CrossRef] [PubMed]
- Hmoud, B.S.; Patel, K.; Bataller, R.; Singal, A.K. Corticosteroids and occurrence of and mortality from infections in severe alcoholic hepatitis: A meta-analysis of randomized trials. Liver Int. 2016, 36, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, C.; Ventura-Cots, M.; Sala, M.; Calafat, M.; Garcia-Retortillo, M.; Cirera, I.; Cañete, N.; Soriano, G.; Poca, M.; Simón-Talero, M.; et al. Effect of rifaximin on infections, acute-on-chronic liver failure and mortality in alcoholic hepatitis: A pilot study (RIFA-AH). Liver Int. 2022, 42, 1109–1120. [Google Scholar] [CrossRef]
- Sersté, T.; Cornillie, A.; Njimi, H.; Pavesi, M.; Arroyo, V.; Putignano, A.; Weichselbaum, L.; Deltenre, P.; Degré, D.; Trépo, E.; et al. The prognostic value of acute-on-chronic liver failure during the course of severe alcoholic hepatitis. J. Hepatol. 2018, 69, 318–324. [Google Scholar] [CrossRef]
- Crabb, D.W.; Bataller, R.; Chalasani, N.P.; Kamath, P.S.; Lucey, M.; Mathurin, P.; McClain, C.; McCullough, A.; Mitchell, M.C.; Morgan, T.R.; et al. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: Recommendation from the NIAAA alcoholic hepatitis consortia. Gastroenterology 2016, 150, 785–790. [Google Scholar] [CrossRef]
- Angeli, P.; Bernardi, M.; Villanueva, C.; Francoz, C.; Mookerjee, R.P.; Trebicka, J.; Krag, A.; Laleman, W.; Gines, P. EASL Clinical Practice Guidelines for management of patients with decompensated cirrosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef]
- Arroyo, V.; Moreau, R.; Jalan, R.; Ginès, P.; EASL-CLIF Consortium CANONIC Study. Acute-on-chronic liver failure: A new syndrome that will re-classify cirrhosis. J. Hepatol. 2015, 62, S131–S143. [Google Scholar] [CrossRef]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef]
- Dhanda, A.D.; Sinha, A.; Hunt, V.; Saleem, S.; Cramp, M.E.; Collins, P. L Infection does not increase long-term mortality in patients with acute severe alcoholic hepatitis treated with CS. World J. Gastroenterol. 2017, 23, 2052–2059. [Google Scholar] [CrossRef]
- Beisel, C.; Blessin, U.; Schulze Zur Wiesch, J.; Wehmeyer, M.H.; Lohse, A.W.; Benten, D.; Kluwe, J. Infections complicating severe alcoholic hepatitis: Enterococcus species represent the most frequently identified pathogen. Scand. J. Gastroenterol. 2016, 51, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Piano, S.; Singh, V.; Caraceni, P.; Maiwall, R.; Alessandria, C.; Fernandez, J.; Soares, E.C.; Kim, D.J.; Kim, S.E.; Marino, M.; et al. Epidemiology and effects of bacterial infections in patients with cirrhosis worldwide. Gastroenterology 2019, 156, 1368–1380.e10. [Google Scholar] [CrossRef] [PubMed]
- Soriano, A.; Carmeli, Y.; Omrani, A.S.; Moore, L.S.P.; Tawadrous, M.; Irani, P. Ceftazidime-avibactam for the treatment of serious Gram-negative infections with limited treatment options: A systematic literature review. Infect. Dis. Ther. 2021, 10, 1989–2034. [Google Scholar] [CrossRef] [PubMed]
- Sipeki, N.; Antal-Szalmas, P.; Lakatos, P.L.; Papp, M. Immune dysfunction in cirrhosis. World J. Gastroenterol. 2014, 20, 2564–2577. [Google Scholar] [CrossRef]
- Chan, C.; Levitsky, J. Infection and alcoholic liver disease. Clin. Liver Dis. 2016, 20, 595–606. [Google Scholar] [CrossRef]
- Szabo, G.; Saha, B. Alcohol’s effect on host defense. Alcohol. Res. 2015, 37, 159–170. [Google Scholar]
- Karakike, E.; Moreno, C.; Gustot, T. Infections in severe alcoholic hepatitis. Ann. Gastroenterol. 2017, 30, 152–160. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Kamath, P.S.; Reddy, K.R. The evolving challenge of infections in cirrhosis. N. Engl. J. Med. 2021, 384, 2317–2330. [Google Scholar] [CrossRef]
- Arroyo, V.; Moreau, R.; Jalan, R. Acute-on-chronic liver failure. N. Engl. J. Med. 2020, 382, 2137–2145. [Google Scholar] [CrossRef]
- Engelmann, C.; Sheikh, M.; Sharma, S.; Kondo, T.; Loeffler-Wirth, H.; Zheng, Y.B.; Novelli, S.; Hall, A.; Kerbert, A.J.C.; Macnaughtan, J.; et al. Toll-like receptor 4 is a therapeutic target for prevention and treatment of liver failure. J. Hepatol. 2020, 73, 102–112. [Google Scholar] [CrossRef]
- Trebicka, J.; Fernandez, J.; Papp, M.; Caraceni, P.; Laleman, W.; Gambino, C.; Giovo, I.; Uschner, F.E.; Jimenez, C.; Mookerjee, R.; et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J. Hepatol. 2020, 73, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Trebicka, J.; Fernandez, J.; Papp, M.; Caraceni, P.; Laleman, W.; Gambino, C.; Giovo, I.; Uschner, F.E.; Jansen, C.; Jimenez, C.; et al. PREDICT identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. J. Hepatol. 2021, 74, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Forrest, E.; Bernal, W. The role of prophylactic antibiotics for patients with severe alcohol-related hepatitis. JAMA 2023, 329, 1552–1553. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Badal, J.; Nawras, M.; Battepati, D.; Farooq, U.; Arif, S.F.; Lee-Smith, W.; Aziz, M.; Iqbal, U.; Nawaz, A.; et al. Role of rifaximin in the management of alcohol-associated hepatitis: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2023, 38, 703–709. [Google Scholar] [CrossRef]
- Quek, J.W.E.; Loo, J.H.; Jaroenlapnopparat, A.; Jimenez, C.; Al-Karaghouli, M.; Vargas, V.; Arab, J.P.; Abraldes, J.G.; Wong, Y.J. Prophylactic antibiotics in patients with alcohol-associated hepatitis receiving steroids: A systematic review and meta-analysis. Liver Int. 2024, 44, 2469–2476. [Google Scholar] [CrossRef]


| n = 115 | |
|---|---|
| Hospital Admission (BASELINE) | |
| Sex (male), n (%) | 88 (76.5%) |
| Age, median (P 25–75) | 50 (44–58) |
| Race, n (%) | Caucasian 98 (85%) |
| BMI, median (P 25–75) | 27 (24–31) |
| Hepatic cirrhosis, n (%) | 81 (70%) |
| Hepatic decompensation, n (%) | 64 (56%) |
| Infection, n (%) | 18 (16%) |
| Ascites, n (%) | 59 (51%) |
| Hepatic encephalopathy, n (%) | 15 (13%) |
| Gastrointestinal bleeding, n (%) | 7 (6%) |
| Acute kidney injury, n (%) | 13 (11%) |
| Acute-on-chronic liver failure, n (%) | 7 (6%) |
| Maddrey score, median (P 25–75) | 40 (20–50) |
| MELD score, median (P 25–75) | 19 (16–22) |
| MELD Na score, median (P 25–75) | 22 (19–22) |
| MELD 3.0 score, median (P 25–75) | 23 (20–26) |
| Child–Pugh score, median (P 25–75) | 10 (9–11) |
| Bilirubin (mg/dL), median (P 25–75) | 7.4 (4.8–12) |
| INR, median (P 25–75) | 1.5 (1.2–1.8) |
| Albumin (g/dL), median (P 25–75) | 2.7 (2.4–3.1) |
| Creatinine (mg/dL), median (P 25–75) | 0.7 (0.5–0.9) |
| AST (UI/L), median (P 25–75) | 147 (102–264) |
| ALT (UI/L), median (P 25–75) | 61 (35–89) |
| GGT (UI/L), median (P 25–75) | 593 (223–1461) |
| ALP (UI/L), median (P 25–75) | 204 (150–328) |
| CRP (mg/dL), median (P 25–75) | 2.5 (1–5.5) |
| Leucocytes (109/L), median (P 25–75) | 8.2 (6.1–11.6) |
| Platelets 109/L (P 25–75) | 104 (64–153) |
| Site | Number of Infections, (%) | Positive Cultures | Bacteria, (n) | Gram Positive or Gram Negative, (n) |
|---|---|---|---|---|
| Chest | 24 (35%) | 3 | Streptoccocus pneumoniae, Staphylococcus aureus, Clamydia pneumoniae | Gram positive (2) gram negative (1) |
| Skin | 14 (20%) | 3 | Staphylococcus aureus (2) Staphylococcus aureus * | Gram positive (3) |
| Blood | 11 (16%) | 11 | Acinetobacter baumannii Staphylococcus hemolyticus (2) Staphylococcus hemolyticus * Staphylococcus epidermidis (4) Enterococcus faecalis *, Klebsiella oxytoca, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Enterococcus faecium | Gram positive (10) gram negative (4) |
| Abdominal (Ascites) | 11 (16%) | 10 | Staphylococcus aureus (2) Acinetobacter baumannii Staphylococcus hemolyticus Escherichia coli, Enterobacter cloacae, Klebsiella pneumoniae * Enterococcus faecium, Serratia marcenses, Acinetobacter pittii | Gram positive (4) gram negative (6) |
| Urinary tract | 9 (13%) | 9 | Escherichia coli (5), Klebsiella pneumoniae (2), Klebsiella pneumoniae * Enterococcus faecalis, Klebsiella aerogenes | Gram positive (1) gram negative (9) |
| TOTAL | 69 | 36 | 40 | Gram positive (20) gram negative (20) |
| (a) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ID-Episode | Infection | Bacteria | SIRS | ACLF | Resolution Infection | Antibiotics | MRB | |||
| P4-1 | SBP | Staphylococcus aureus | Yes | No | Yes | Cefazolin | ||||
| P6-2 | Cellulitis | Nonisolated bacteria | Yes | No | Yes | Levofloxacin + Clindamycin | ||||
| P8-3 | Aspiration Pneumonia | Nonisolated bacteria | Yes | No | Yes | Amoxicillin-Clavulanate | ||||
| P17-4 | Cellulitis | Nonisolated bacteria | No | No | Yes | Amoxicillin-Clavulanate | ||||
| P19-5 | Pneumonia | Nonisolated bacteria | Yes | No | Yes | Amoxicillin-Clavulanate | ||||
| P33-6 | Bacteremia | Acinetobacter baumannii + Staphylococcus haemolyticus | Yes | No | Yes | Ciprofloxacin | ||||
| SBP | ||||||||||
| P62-7 | Cellulitis | Nonisolated bacteria | No | No | Yes | Amoxicillin-Clavulanate | ||||
| P69-8 | Cellulitis | Nonisolated bacteria | Yes | No | Yes | Amoxicillin-Clavulanate | ||||
| P74-9 | UTI | Escherichia coli | No | No | Yes | Ceftriaxone | ||||
| P76-10 | Pneumonia | Streptococcus pneumoniae | Yes | Yes | Yes | Cefotaxime + Azithromycin | ||||
| P79-11 | Pneumonia | Chlamydia pneumoniae | No | No | Yes | Levofloxacin | ||||
| P82-12 | UTI | Klebsiella pneumoniae | Yes | Yes | Yes | Meropenem | ||||
| P86-13 | Pneumonia | Nonisolated bacteria | No | No | Yes | Amoxicillin-Clavulanate | ||||
| P26-14 | Aspiration Pneumonia | Nonisolated bacteria | No | No | Yes | Piperacillin-Tazobactam | ||||
| P27-15 | Cellulitis | Staphylococcus aureus | No | No | Yes | Amoxicillin-Clavulanate | ||||
| P89-16 | Aspiration Pneumonia | Nonisolated bacteria | Yes | Yes | Yes | Piperacillin-Tazobactam | ||||
| Cellulitis | Nonisolated bacteria | |||||||||
| P99-17 | Cellulitis | Nonisolated bacteria | No | No | Yes | Amoxicillin-Clavulanate | ||||
| P107-18 | UTI | Escherichia coli + Enterococcus faecalis | No | No | Yes | Amoxicillin-Clavulanate | ||||
| (b) | ||||||||||
| ID-Episode | CE | Infection | Bacteria | SIRS | ACLF | Resolution Infection | Cause of Death | Antibiotic | MRB | |
| P2-1 | Yes | CRBSI | Staphylococcus epidermidis | Yes | Yes | Yes | ACLF | Meropenem | ||
| P4-2 | Yes | UTI | Klebsiella aerogenes | Yes | Yes | Yes | Cefazolin | |||
| Cellulitis | Staphylococcus aureus | Cloxacillin | ||||||||
| CRBSI | Enterococcus faecalis, Staphylococcus epidermidis, Staphylococcus haemolyticus | Piperacillin-Tazobactam | ||||||||
| P5-3 | Yes | UTI | Escherichia coli | No | No | Yes | Ceftriaxone | |||
| P6-4 | Yes | Aspiration Pneumonia | Nonisolated bacteria | No | No | Yes | Meropenem | |||
| P20-5 | No | UTI | Escherichia coli | No | No | Yes | Amoxicillin-Clavulanate | |||
| P21-6 | Yes | Aspiration Pneumonia | Nonisolated bacteria | Yes | Yes | Yes | ACLF | Ceftazidime | ||
| P28-7 | Yes | Pneumonia | Nonisolated bacteria | No | No | Yes | Amoxicillin-Clavulanate | |||
| P38-8 | No | Cellulitis | Staphylococcus aureus | No | No | Yes | Linezolid | Yes | ||
| P44-9 | No | Pneumonia | Nonisolated bacteria | No | No | Yes | Amoxicillin-Clavulanate | |||
| P50-10 | Yes | Bacteremia | Klebsiella oxytoca | Yes | No | Yes | Amoxicillin-Clavulanate | |||
| P51-11 | Yes | Pneumonia | Nonisolated bacteria | No | No | Yes | Piperacillin-Tazobactam | |||
| P25-12 | No | SBP | Staphylococcus aureus | Yes | Yes | Yes | Ceftriaxone | |||
| Bacteremia | Staphylococcus aureus | Cefazolin | ||||||||
| Aspiration Pneumonia | Nonisolated bacteria | Piperacillin-Tazobactam | ||||||||
| P61-13 | Yes | Pneumonia | Nonisolated bacteria | Yes | Yes | Yes | ACLF | Piperacillin-Tazobactam | ||
| P63-14 | No | SBP | Escherichia coli | No | No | Yes | Ceftriaxone | |||
| Pneumonia | Nonisolated bacteria | |||||||||
| P68-15 | Yes | Bacteriemia | Escherichia coli | Yes | Yes | Yes | Piperacillin-Tazobactam | |||
| P73-16 | No | Aspiration Pneumonia | Nonisolated bacteria | No | No | Yes | Amoxicillin-Clavulanate | |||
| P78-17 | Yes | SBP | Enterobacter cloacae | Yes | Yes | No | SBP, ACLF | Ceftriaxone | ||
| Aspiration Pneumonia | Nonisolated bacteria | Amoxicillin-Clavulanate | ||||||||
| SBP | Klebsiella pneumoniae | Meropenem + Daptomycin | Yes | |||||||
| P82-18 | No | Pneumonia | Nonisolated bacteria | Yes | Yes | No | ACLF | Meropenem | ||
| CRBSI | Staphylococcus hemolyticus, Staphylococcus epidermidis. Candida albicans * | Meropenem + Daptomycin, Anidulafungin * | Yes | |||||||
| P87-19 | No | Aspiration Pneumonia | Nonisolated bacteria | No | No | Yes | Amoxicillin-Clavulanate | |||
| P102-20 | No | Pneumonia | Nonisolated bacteria | No | No | Yes | Ceftriaxone | |||
| P103-21 | Yes | Pneumonia | Nonisolated bacteria | Yes | Yes | Yes | Piperacillin-Tazobactam | |||
| P106-22 | No | Aspiration Pneumonia | Nonisolated bacteria | No | No | Yes | Piperacillin-Tazobactam | |||
| (c) | ||||||||||
| ID-Episode | CE | Infection | Bacteria | SIRS | ACLF | Resolution Infection | Cause of Death | Antibiotic | MRB | |
| P5-1 | Yes | Bacteremia | Staphylococcus epidermidis | No | No | Yes | Ceftriaxone | |||
| P19-2 | Yes | Cellulitis | Nonisolated bacteria | No | No | Yes | Ceftriaxone, Teicoplanin | |||
| P28-3 | Yes | Pneumonia | Staphylococcus aureus | Yes | Yes | No | Septic Shock | Meropenem + Linezolid | ||
| Intra-abdominal | Nonisolated bacteria | |||||||||
| P33-4 | Yes | SBP | Enterococcus faecium | Yes | No | Yes | Piperacillin-Tazobactam | |||
| P39-5 | Yes | Cellulitis | Nonisolated bacteria | No | No | Yes | Amoxicillin-Clavulanate | |||
| P45-6 | Yes | Bacteriemia | Klebsiella pneumoniae | Yes | Yes | Yes | Meropenem, Teicoplanin | |||
| Bacteremia | Enterococcus faecium | Yes | ||||||||
| P47-7 | Yes | SBP | Serratia marcescens | No | No | Yes | Ceftriaxone | |||
| P26-8 | No | Bacteremia | Staphylococcus aureus | No | No | Yes | Amoxicillin-Clavulanate | |||
| P74-9 | Yes | UTI | Escherichia coli | No | No | Yes | Ceftriaxone | |||
| P76-10 | No | Cellulitis | Nonisolated bacteria | No | No | Yes | Cefadroxil | |||
| P83-11 | No | Cellulitis | Nonisolated bacteria | No | No | Yes | Amoxicillin-Clavulanate | |||
| P92-12 | Yes | SBP | Klebsiella pneumoniae | No | No | Yes | Ceftriaxone | |||
| UTI | Klebsiella pneumoniae | No | No | Yes | Ciprofloxacin | |||||
| UTI | Klebsiella pneumoniae | Yes | Yes | yes | Cefotaxime + Clindamycin | Yes | ||||
| Cellulitis | Nonisolated bacteria | No | No | Yes | Amoxicillin-Clavulanate | |||||
| P105-13 | Yes | Pneumonia | Nonisolated bacteria | No | No | Yes | Piperacillin-Tazobactam | |||
| P108-14 | Yes | SBP | Acinetobacter pitti | Yes | Yes | Yes | ACLF | Meropenem | ||
| Infected Patients n = 18 | Noninfected Patients n= 97 | p | |
|---|---|---|---|
| Sex (male), n (%) | 12 (66.6%) | 75 (77.3%) | 0.28 |
| Age, median (P 25–75) | 45 (41.7–55.7) | 51 (44–58) | 0.43 |
| BMI, median (P 25–7527) | 27.3 (23–31.2) | 27.2 (24–31.7) | 0.86 |
| Hepatic cirrhosis, n (%) | 16 (88%) | 65 (67%) | 0.062 |
| Hepatic decompensations, n (%) | 16 (88%) | 48 (49.5%) | 0.005 |
| Ascites, n (%) | 14 (78%) | 45 (46.4%) | 0.029 |
| HE, n (%) | 9 (50%) | 6 (6.2%) | 0.001 |
| GIB, n (%) | 0 | 7 (7.2%) | 0.25 |
| AKI, n (%) | 3 (16.6%) | 10 (10.3%) | 0.43 |
| ACLF, n (%) | 3 (16.6%) | 4 (4.1%) | 0.041 |
| Maddrey score, median (P 25–75) | 48 (32–68.5) | 38 (18–51) | 0.022 |
| MELD score, median (P 25–75) | 22 (19–25) | 19 (15–21) | 0.012 |
| MELD Na score, median (P 25–75) | 24 (22–29) | 22 (19–26) | 0.037 |
| MELD 3.0 score, median (P 25–75) | 25 (25–28) | 23 (20–25) | 0.013 |
| Child–Pugh score, median (P 25–75) | 11 (11–12) | 10 (9–11) | 0.011 |
| Bilirubin (mg/dL), median (P 25–75) | 9.89 (6.96–16.31) | 7.1 (4.5–11.6) | 0.084 |
| INR, median (P 25–75) | 1.8 (1.4–1.95) | 1.5 (1.15–1.8) | 0.027 |
| Creatinine (mg/dL), median (P 25–75) | 0.63 (0.55–0.87) | 0.67 (0.54–0.88) | 0.81 |
| Albumin (g/dL), median (P25–P75) | 2.5 (2.3–2.7) | 2.8 (2.4–3.2) | 0.038 |
| CRP (mg/dL), median (P 25–75) | 3 (1.5–9.5) | 2.4 (0.9–5.2) | 0.31 |
| Leucocytes (109/L), median (P 25–75) | 8.2 (5.8–10.7) | 10.3 (6.6–13.2) | 0.26 |
| Infected Patients n = 37 | No Infected Patients n = 78 | p | |
|---|---|---|---|
| Sex (male), n (%) | 25 (67.5%) | 63 (81%) | 0.12 |
| Age, median (P 25–75) | 50 (43–57) | 51 (44–58) | 0.87 |
| Race, n (%) | Caucasian, 30 (87%) | Caucasian, 68 (87%) | 0.91 |
| BASELINE | |||
| Maddrey score, median (P 25–75) | 45 (31.5–61.5) | 37 (18–51) | 0.020 |
| MELD score, median (P 25–75) | 21 (18–25) | 18 (15–21) | 0.002 |
| Child–Pugh score, median (P 25–75) | 11 (10–12) | 10 (9–11) | 0.001 |
| Bilirubin (mg/dL), median (P 25–75) | 10.4 (5.4–17.5) | 6.5 (4.4–10.5) | 0.010 |
| INR, median (P 25–75) | 1.63 (1.4–1.84) | 1.48 (1.14–1.80) | 0.047 |
| Albumin (g/dL), median (P25–P75) | 2.5 (2.3–2.75) | 2.8 (2.5–3.4) | 0.002 |
| Creatinine (mg/dL), median (P 25–75) | 0.7 (0.6–1.0) | 0.65 (0.53–0.86) | 0.45 |
| COMPLICATIONS | |||
| Hepatic decompensations, n (%) | 23 (62%) | 22 (28%) | 0.001 |
| Ascites, n (%) | 16 (43%) | 18 (23%) | 0.046 |
| HE, n (%) | 15 (41%) | 10 (13%) | 0.001 |
| GIB, n (%) | 4 (11%) | 5(6.4%) | 0.41 |
| AKI, n (%) | 6 (16%) | 5 (6.4%) | 0.095 |
| Vasoactive support | 6 (16%) | 1 (1.3%) | 0.002 |
| ICU, n (%) | 10 (27%) | 2 (2.5%) | 0.001 |
| ACLF, n (%) | 12 (32.4%) | 5 (6.4%) | 0.001 |
| Death, n (%) | 6 (16%) | 3 (4%) | 0.021 |
| No Corticosteroids n = 49 | Corticosteroids n = 66 | p | |
|---|---|---|---|
| BASELINE | |||
| Male, n (%) | 36 (73.5%) | 51 (77%) | 0.63 |
| Age, median (P 25–75) | 51 (44–58) | 48 (43–56) | 0.16 |
| Maddrey score, median (P 25–75) | 34 (18–50) | 41 (28–58) | 0.27 |
| MELD score, median (P 25–75) | 19 (15–22) | 19 (16–22) | 0.88 |
| Child–Pugh score, median (P 25–75) | 10 (9–11) | 10 (9–11) | 0.44 |
| COMPLICATIONS | |||
| Infections, n (%) | 9 (18%) | 13 (20%) | 0.76 |
| Hepatic decompensations, n (%) | 18 (36%) | 27 (40%) | 0.65 |
| Ascites, n (%) | 14 (28%) | 20 (30%) | 0.89 |
| HE, n (%) | 10 (20%) | 15 (22%) | 0.73 |
| GIB, n (%) | 2 (4%) | 7 (10%) | 0.19 |
| AKI, n (%) | 7 (14%) | 4 (6%) | 0.14 |
| Vasoactive support, n (%) | 3 (6%) | 4 (6%) | 0.98 |
| ICU, n (%) | 5 (10%) | 7 (11%) | 0.94 |
| ACLF, n (%) | 4 (8%) | 13 (20%) | 0.08 |
| Death, n (%) | 4 (8%) | 5 (7.5%) | 0.92 |
| Follow up 90 days | |||
| Infections, n (%) | 3/45 (6%) | 11/61 (18%) | 0.08 |
| Number of infections, n (%) | 3/45 (6%) | 16/61 (26%) | 0.09 |
| Death, n (%) | 1 (2%) | 1 (1.5%) | 0.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez, C.; Martí-Carretero, A.; Villagrasa, A.; Aguilar, A.; Pérez-Pérez, M.; Ventura-Cots, M.; Vargas, V. Bacterial Infection Features in Alcohol-Associated Hepatitis: Review of a 2016–2021 Cohort. J. Clin. Med. 2024, 13, 5693. https://doi.org/10.3390/jcm13195693
Jiménez C, Martí-Carretero A, Villagrasa A, Aguilar A, Pérez-Pérez M, Ventura-Cots M, Vargas V. Bacterial Infection Features in Alcohol-Associated Hepatitis: Review of a 2016–2021 Cohort. Journal of Clinical Medicine. 2024; 13(19):5693. https://doi.org/10.3390/jcm13195693
Chicago/Turabian StyleJiménez, Cesar, Aina Martí-Carretero, Ares Villagrasa, Anna Aguilar, María Pérez-Pérez, Meritxell Ventura-Cots, and Victor Vargas. 2024. "Bacterial Infection Features in Alcohol-Associated Hepatitis: Review of a 2016–2021 Cohort" Journal of Clinical Medicine 13, no. 19: 5693. https://doi.org/10.3390/jcm13195693
APA StyleJiménez, C., Martí-Carretero, A., Villagrasa, A., Aguilar, A., Pérez-Pérez, M., Ventura-Cots, M., & Vargas, V. (2024). Bacterial Infection Features in Alcohol-Associated Hepatitis: Review of a 2016–2021 Cohort. Journal of Clinical Medicine, 13(19), 5693. https://doi.org/10.3390/jcm13195693





