Enteric Nervous System and Its Relationship with Neurological Diseases
Abstract
:1. Introduction
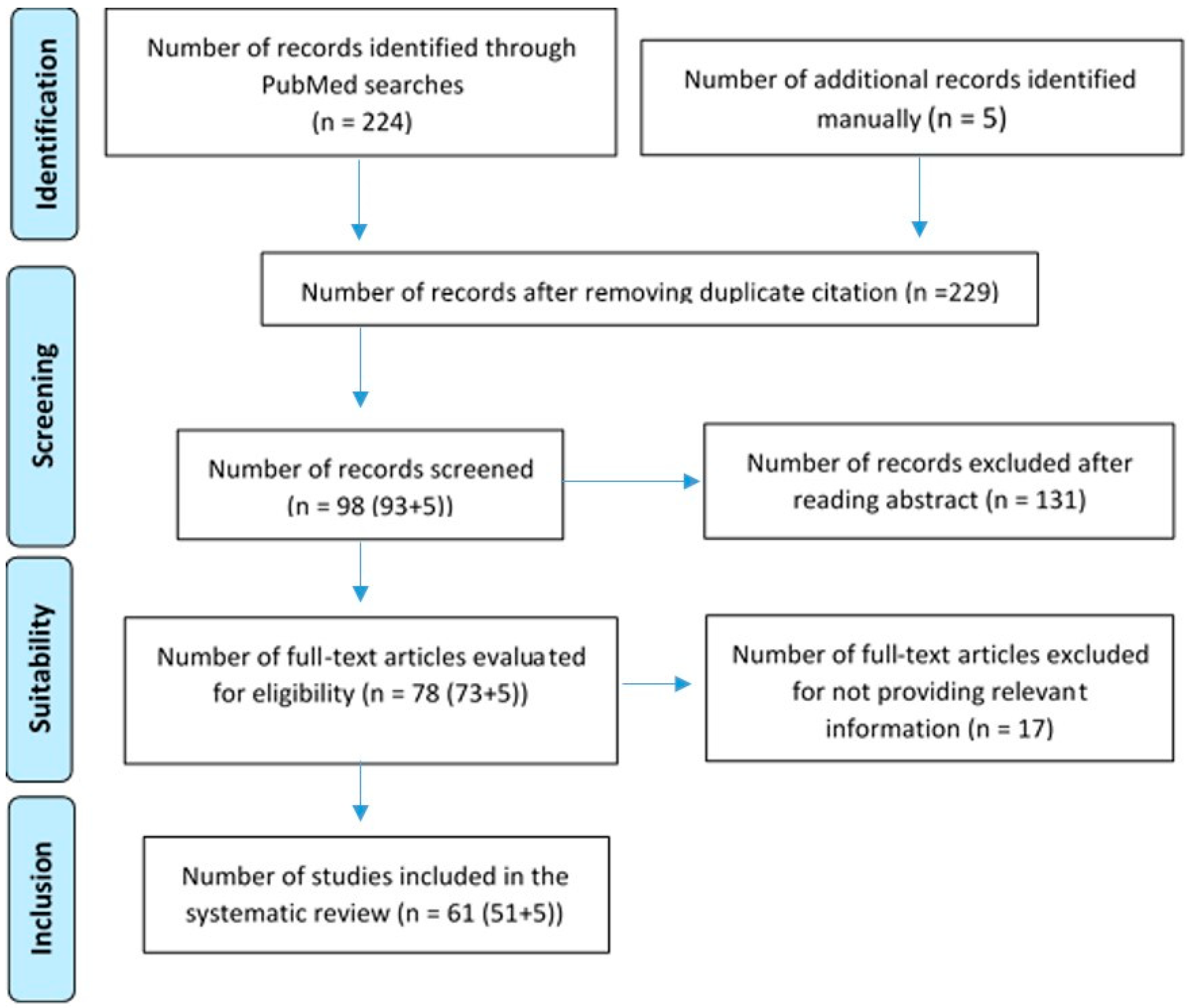
2. Materials and Methods
- 1.
- Initial Search: A general search with the term “enteric nervous system” with the “human” filter yielded 4589 publications in PubMed. Twenty articles were included for a comprehensive understanding, including general data on the embryology of the enteric nervous system, the enteric nervous system in adults, and the relationship between the enteric nervous system and the microbiota. This step was carried out between 1 December 2023 and 31 January 2024.
- 2.
- Systematic Search: To begin the study, a search was conducted on PubMed to quantify the amount of literature describing the enteric nervous system and its relationship with neurological diseases. The term “enteric nervous system AND neurological diseases” was used with the filter “Humans”. A total of 224 articles were obtained. After conducting the initial search on the enteric nervous system and the most common neurological pathologies, terms related to neurological pathologies (frontotemporal dementia, amyotrophic lateral sclerosis, spongiform encephalopathy, Parkinson disease, Alzheimer disease, multiple sclerosis, autism spectrum disorder, varicella zoster virus) were combined with ‘enteric nervous system’ in a systematic search. Using the Boolean operators AND and OR, the most appropriate combination of terms was created to yield the best results. The combination was as follows: (enteric nervous system AND neurological disease) OR (frontotemporal dementia AND amyotrophic lateral sclerosis)) OR spongiform encephalopathy) OR Parkinson disease) OR Alzheimer disease) OR multiple sclerosis) OR autism spectrum disorder) OR varicella zoster virus. A total of 93 results were obtained in PubMed. Before proceeding to the selection of articles, the inclusion and exclusion criteria were defined as follows:
- -
- Inclusion criteria: Any paper related to any article related to neurological pathology associated with the enteric nervous system in humans, including studies, reviews, case series, editorials, and guidelines published in the last 10 years in English or Spanish.
- -
- Exclusion criteria: Unusual manifestations, neurological diseases not related to enteric nervous system, those studies older than 10 years, and, finally, pathology in animals.
- 3.
- Manual Search: Based on references from the selected studies, 5 additional articles were included, bringing the total to 61 empirical articles published between 2013 and 2024. These were articles obtained from other databases such as “Google Scholar”, “Sci-Hub”, or “Elsevier”. This step took place during the month of May 2024.
3. Results
3.1. Transmissible Spongiform Encephalopathies
3.2. Parkinson’s Disease
3.3. Huntington’s Disease
3.4. Amyotrophic Lateral Sclerosis and Frontotemporal Dementia
3.5. Multiple Sclerosis
- -
- Increased intestinal permeability. Higher rate of inflammation and increased expression of haptoglobin precursor protein 2 [32].
- -
- Alterations of the ENS. There are structural (increased gliosis and decreased enteric neurons) and functional abnormalities [33].
- -
- Higher prevalence of altered microbiota. There is a difference between the intestinal microbiota of MS patients and healthy subjects [22,34,35,36,37,38,39]. Among the most notable results, the higher the frequency of polysaccharide A from the capsule of the bacterium Bacteroides fragilis, the lower the inflammatory activity. This antigen is a potent activator of immune cells, capable of inducing clonal expansion of CD4+ T cells and increased secretion of IL-10 in T and B lymphocytes, and its immunomodulatory and protective role in the development of MS has been described [37,38,40].
- -
- Elevated levels of short-chain fatty acids, bile products, and other metabolites. An increased presence of microbial metabolites has been found. Short-chain fatty acids appear to play a prominent role, being able to cross the blood–brain barrier and control neuroimmune homeostasis [41]. Another metabolite of note is acetate. It has been reported that elevated acetate levels in people with MS compared to control subjects correlated with a higher disability status scale score and a higher prevalence of CD8+ T cells [42].
3.6. Alzheimer’s Disease
3.7. Autism Spectrum
- -
- Abnormal expression of the gene encoding CHD8 leads to alterations in enteric neurogenesis together with slow gastrointestinal transit. This description indicates that gastrointestinal disturbances are not comorbidities, but part of the phenotype of autism spectrum disorder.
- -
- Transcription factor 4 (TCF4) haploinsufficiency in Pitt–Hopkins syndrome presents with alterations of rectal motility and upper gastrointestinal transit.
- -
- Disruption of the SLC6A4 gene encoding the sodium-dependent serotonin (5-HT) transporter is associated with autistic behavior disorder, together with SNE hypoplasia and slow gastrointestinal tract.
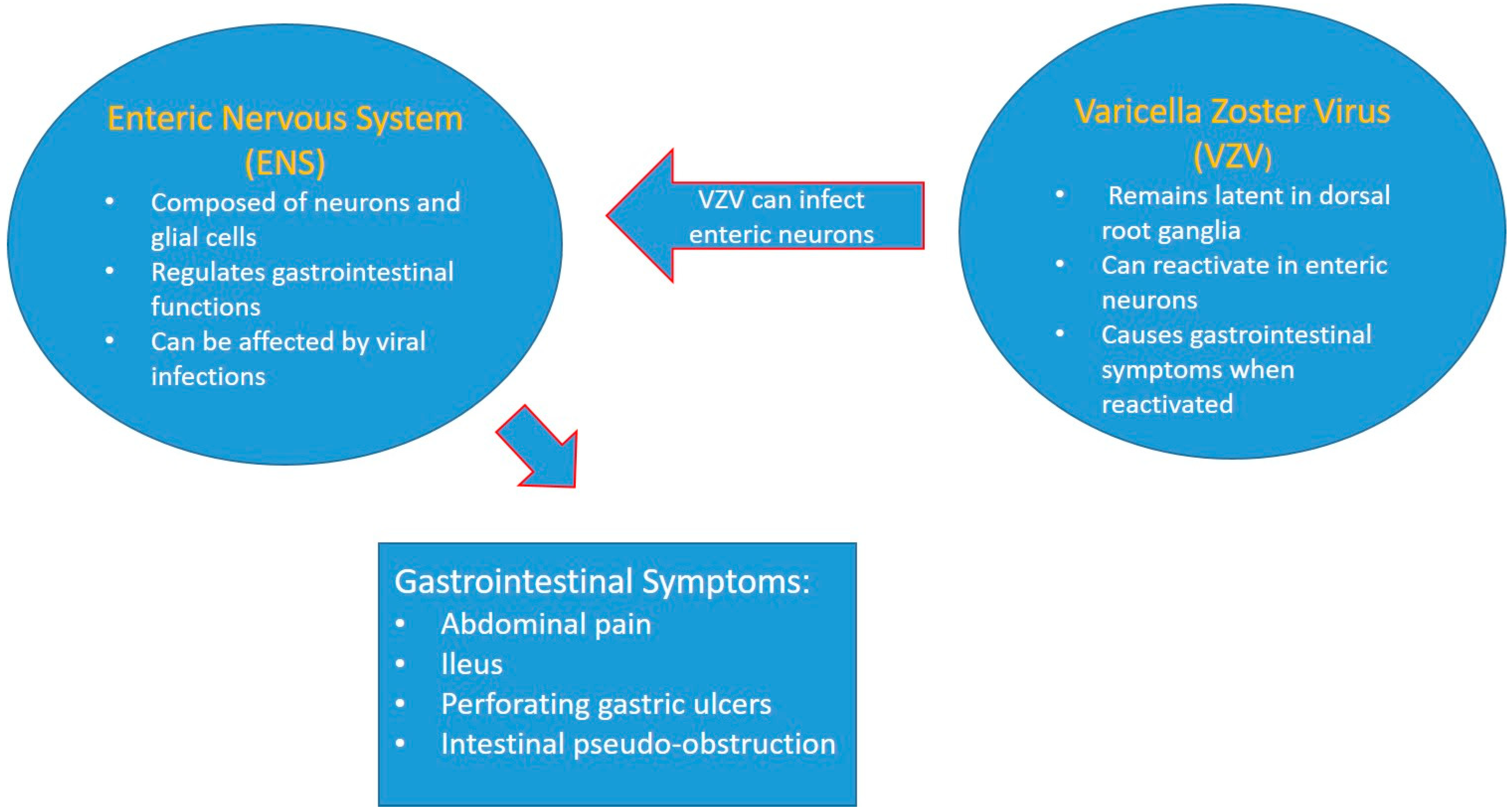
3.8. Varicella Zoster Virus ENS Viral Infection
4. Discussion
- -
- The first difficulty is to develop a protocol in which pathologists would establish histological lesion criteria suggestive of pathology. By standardizing morphological immunostaining patterns with the use of autoantibodies to improve the prognostic values of α-synucleinopathies and Lewy bodies, their presence does not always necessarily imply disease [18,19].
- -
- The second difficulty is the irregular distribution of dopaminergic neurons in the ENS, which may hinder their adequate sampling, as the organization of the ENS is currently not fully understood. If we need to biopsy those neurons, especially dopaminergic neurons because they have the best diagnostic results, we would need more certainty in localization [7,18,61].
- -
- The third difficulty is that the analysis of biopsies, although it may have a high diagnostic value, as histological lesions can be observed up to 20 years before the first symptoms of the disease, has no prognostic value for disease activity or progression [57].
- -
- Certain dietary interventions have shown beneficial results in modulating microbiota dysbiosis, reducing intestinal permeability, and decreasing oxidative stress and inflammation. One example is polyunsaturated fatty acids such as the omega-3 fatty acid docosahexaenoic acid (DHA). The results of this intervention led to a reduction in motor symptoms due to improved mitochondrial dysfunction.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cantarero, I. Aportaciones originales al conocimiento de las células intersticiales de Cajal. Univ. Zaragoza 2011, 6, 25–35. [Google Scholar]
- Pawolski, V.; Schmidt, M.H. Neuron–Glia interaction in the developing and adult Enteric nervous system. Cells 2020, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Romero-Trujillo, J.; Frank-Marquez, N.; Cervantes-Bustamante, R.; Cadena-León, J. Sistema Nervioso Entérico. Acta Pediátrica México 2012, 33, 207–214. [Google Scholar]
- Iruzubieta, P. El cilio primario: De la neurogénesis al cáncer. Univ. Zaragoza 2021, 1–234. [Google Scholar]
- Luesma, M.J.; Cantarero, I.; Álvarez-Dotu, J.M.; Santander, S.; Junquera, C. New insights into c-Ret signalling pathway in the Enteric nervous system and its relationship with ALS. Biomed. Res. Int. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Gershon, M.D.; Kirchgessner, A.L.; Wade, P.R. Anatomía funcional del sistema nervioso entérico. In La Fisiologia del Tracto Gastrointestinal, 3rd ed.; Johnson, L.R., Ed.; Raven Press: New York, NY, USA, 1994; pp. 381–422. [Google Scholar]
- Gershon, M.D. Genes y linajes en la formación del sistema nervioso entérico. Opinión Actual Neurobiol. 1997, 7, 101–109. [Google Scholar] [CrossRef]
- Forloni, G. Alpha Synuclein: Neurodegeneration and inflammation. Int. J. Mol. Sci. 2023, 24, 5914. [Google Scholar] [CrossRef]
- Niesler, B.; Kuerten, S.; Demir, I.E.; Schäfer, K.H. Disorders of the enteric nervous system—A holistic view. Nature reviews. Gastroenterol. Hepatol. 2021, 18, 393–410. [Google Scholar] [CrossRef]
- Rao, M.; Gershon, M.D. The bowel and beyond: The enteric nervous system in neurological disorders. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 517–528. [Google Scholar] [CrossRef]
- Kujawska, M.; Jodynis-Liebert, J. What is the evidence that Parkinson’s disease is a prion disorder, which originates in the gut? Int. J. Mol. Sci. 2018, 19, 3573. [Google Scholar] [CrossRef]
- Camilleri, M. Gastrointestinal motility disorders in neurologic disease. J. Clin. Investig. 2021, 131, e143771. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and metaanalyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Gravina, A.G.; Pellegrino, R.; Durante, T.; Palladino, G.; Imperio, G.; D’Amico, G.; Trotta, M.C.; Dallio, M.; Romeo, M.; D’Amico, M.; et al. The Melanocortin System in Inflammatory Bowel Diseases: Insights into Its Mechanisms and Therapeutic Potentials. Cells 2023, 12, 1889. [Google Scholar] [CrossRef] [PubMed]
- Urrútia, G.; Bonfill, X. La declaración PRISMA: Un paso adelante en la mejora de las publicaciones de la Revista Española de Salud Pública. Rev. Española Salud Pública 2013, 87, 99–102. [Google Scholar] [CrossRef]
- Prusiner, S.B. Biology and genetics of prions causing neurodegeneration. Annu. Rev. Genet. 2013, 47, 601–623. [Google Scholar] [CrossRef]
- Lambert, Z.J.; Greenlee, J.J.; Cassmann, E.D.; West Greenlee, M.H. Differential accumulation of misfolded prion strains in natural hosts of prion diseases. Viruses 2021, 13, 2453. [Google Scholar] [CrossRef]
- Liddle, R.A. Parkinson’s disease from the gut. Brain Res. 2018, 1693 Pt B, 201–206. [Google Scholar] [CrossRef]
- Miklya, I.; Pencz, N.; Hafenscher, F.; Göltl, P. The role of alpha-synuclein in Parkinson’s disease. Neuropsychopharmacol. Hung. 2014, 16, 77–84. [Google Scholar]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Kiprowska, M.; Kansara, T.; Kansara, P.; Li, P. Neuroinflammation: A Distal Consequence of Periodontitis. J. Dent. Res. 2022, 101, 1441–1449. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stolzer, I.; Scherer, E.; Süß, P.; Rothhammer, V.; Winner, B.; Neurath, M.F.; Günther, C. Impact of Microbiome-Brain Communication on Neuroinflammation and Neurodegeneration. Int. J. Mol. Sci. 2023, 24, 14925. [Google Scholar] [CrossRef] [PubMed]
- Caradonna, E.; Nemni, R.; Bifone, A.; Gandolfo, P.; Costantino, L.; Giordano, L.; Mormone, E.; Macula, A.; Cuomo, M.; Difruscolo, R.; et al. The Brain-Gut Axis, an Important Player in Alzheimer and Parkinson Disease: A Narrative Review. J. Clin. Med. 2024, 13, 4130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caputi, V.; Giron, M. Microbiome-gut-brain axis and toll-like receptors in Parkinson’s disease. Int. J. Mol. Sci. 2018, 19, 1689. [Google Scholar] [CrossRef] [PubMed]
- Gazerani, P. Probiotics for Parkinson’s disease. Int. J. Mol. Sci. 2019, 20, 4121. [Google Scholar] [CrossRef]
- Svensson, E.; Horváth-Puhó, E.; Thomsen, R.W.; Djurhuus, J.C.; Pedersen, L.; Borghammer, P.; Sørensen, H.T. Vagotomy and subsequent risk of Parkinson’s disease. Ann. Neurol. 2015, 78, 522–529. [Google Scholar] [CrossRef]
- Singh, A.; Dawson, T.M.; Kulkarni, S. Neurodegenerative disorders and gut-brain interactions. J. Clin. Investig. 2021, 131, e143775. [Google Scholar] [CrossRef]
- Kong, G.; Lê Cao, K.A.; Hannan, A.J. Alterations in the gut fungal community in a mouse model of Huntington’s disease. Microbiol. Spectr. 2022, 10, e0219221. [Google Scholar] [CrossRef]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; Van Den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17071. [Google Scholar] [CrossRef]
- Korn, T. Pathophysiology of multiple sclerosis. J. Neurol. 2008, 255, 2–6. [Google Scholar] [CrossRef]
- Ghezzi, L.; Cantoni, C.; Pinget, G.V.; Zhou, Y.; Piccio, L. Targeting the gut to treat multiple sclerosis. J. Clin. Investig. 2021, 131, e143774. [Google Scholar] [CrossRef]
- Fasano, A. Zonulin and its regulation of intestinal barrier function: The biological door to inflammation, autoimmunity, and cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Wunsch, M.; Jabari, S.; Voussen, B.; Enders, M.; Srinivasan, S.; Cossais, F.; Wedel, T.; Boettner, M.; Schwarz, A.; Weyer, L.; et al. The enteric nervous system is a potential autoimmune target in multiple sclerosis. Acta Neuropathol. 2017, 134, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Jangi, S.; Gandhi, R.; Cox, L.M.; Li, N.; von Glehn, F.; Yan, R.; Patel, B.; Mazzola, M.A.; Liu, S.; Glanz, B.L.; et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 2016, 7, 12015. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chia, N.; Kalari, K.R.; Yao, J.Z.; Novotna, M.; Paz Soldan, M.M.; Luckey, D.H.; Marietta, E.V.; Jeraldo, P.R.; Chen, X.; et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 2016, 6, 28484. [Google Scholar] [CrossRef]
- Miyake, S.; Kim, S.; Suda, W.; Oshima, K.; Nakamura, M.; Matsuoka, T.; Chihara, N.; Tomita, A.; Sato, W.; Kim, S.-W.; et al. Dysbiosis in the gut Microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to Clostridia XIVa and IV clusters. PLoS ONE 2015, 10, e0137429. [Google Scholar] [CrossRef]
- Johnson, J.L.; Jones, M.B.; Cobb, B.A. Polysaccharide A from the capsule of Bacteroides fragilis induces clonal CD4+ T cell expansion. J. Biol. Chem. 2015, 290, 5007–5014. [Google Scholar] [CrossRef]
- Ochoa-Repáraz, J.; Mielcarz, D.W.; Wang, Y.; Begum-Haque, S.; Dasgupta, S.; Kasper, D.L.; Kasper, L.H. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010, 3, 487–495. [Google Scholar] [CrossRef]
- Doroszkiewicz, J.; Groblewska, M.; Mroczko, B. The Role of Gut Microbiota and Gut-Brain Interplay in Selected Diseases of the Central Nervous System. Int. J. Mol. Sci. 2021, 22, 10028. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramakrishna, C.; Kujawski, M.; Chu, H.; Li, L.; Mazmanian, S.K.; Cantin, E.M. Bacteroides fragilis polysaccharide A induces IL-10 secreting B and T cells that prevent viral encephalitis. Nat. Commun. 2019, 10, 2153. [Google Scholar] [CrossRef]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef]
- Spielman, L.J.; Gibson, D.L.; Klegeris, A. Unhealthy gut, unhealthy brain: The role of the intestinal microbiota in neurodegenerative diseases. Neurochem. Int. 2018, 120, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Kuźniar, J.; Kozubek, P.; Czaja, M.; Leszek, J. Correlation between Alzheimer’s Disease and Gastrointestinal Tract Disorders. Nutrients 2024, 16, 2366. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kruger, G.M.; Mosher, J.T.; Bixby, S.; Joseph, N.; Iwashita, T.; Morrison, S.J. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron 2002, 35, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Felice, V.D.; Quigley, E.M.; Sullivan, A.M.; O’Keeffe, G.W.; O’Mahony, S.M. Microbiota-gut-brain signalling in Parkinson’s disease: Implications for non-motor symptoms. Park. Relat. Disord. 2016, 27, 1–8. [Google Scholar] [CrossRef]
- Liu, S.; da Cunha, A.P.; Rezende, R.M.; Cialic, R.; Wei, Z.; Bry, L.; Comstock, L.E.; Gandhi, R.; Weiner, H.L. The host shapes the gut microbiota via fecal MicroRNA. Cell Host Microbe 2016, 19, 32–43. [Google Scholar] [CrossRef]
- Rolhion, N.; Chassaing, B. When pathogenic bacteria meet the intestinal microbiota. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150504. [Google Scholar] [CrossRef]
- Winek, K.; Engel, O.; Koduah, P.; Heimesaat, M.M.; Fischer, A.; Bereswill, S.; Dames, C.; Kershaw, O.; Gruber, A.D.; Curato, C.; et al. Depletion of cultivatable gut microbiota by broad-spectrum antibiotic pretreatment worsens outcome after murine stroke. Stroke 2016, 47, 1354–1363. [Google Scholar] [CrossRef]
- Chu, F.; Shi, M.; Lang, Y.; Shen, D.; Jin, T.; Zhu, J.; Cui, L. Gut Microbiota in multiple sclerosis and experimental autoimmune encephalomyelitis: Current applications and future perspectives. Mediat. Inflamm. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Wang, X.; Tang, R.; Wei, Z.; Zhan, Y.; Lu, J.; Li, Z. The enteric nervous system deficits in autism spectrum disorder. Front. Neurosci. 2023, 17, 1101071. [Google Scholar] [CrossRef]
- Andersen-Civil, A.I.S.; Sawale, R.A.; Vanwalleghem, G.C. Zebrafish (Danio rerio) as a translational model for neuro-immune interactions in the enteric nervous system in autism spectrum disorders. Brain Behav. Immun. 2023, 112, 254–266. [Google Scholar] [CrossRef]
- Westfall, S.; Lomis, N.; Kahouli, I.; Dia, S.Y.; Singh, S.P.; Prakash, S. Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell Mol. Life Sci. 2017, 74, 3769–3787. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, F.; Cechova, K.; Amlerova, J.; Hort, J. Antibiotics, gut microbiota, and Alzheimer’s disease. J. Neuroinflammation 2019, 16, 108. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef] [PubMed]
- Hopfner, F.; Künstner, A.; Müller, S.H.; Künzel, S.; Zeuner, K.E.; Margraf, N.G.; Deuschl, G.; Baines, J.F.; Kuhlenbäumer, G. Gut microbiota in Parkinson disease in a northern German cohort. Brain Res. 2017, 1667, 41–45. [Google Scholar] [CrossRef]
- Quigley, E.M. Gut bacteria in neurodegenerative disorders. Curr. Opin. Gastroenterol. 2017, 33, 131–136. [Google Scholar]
- Romero, C. La importancia de las células intersticiales de Cajal en el estudio de las patologías digestivas. Rev. Med. Chil. 2018, 146, 741–748. [Google Scholar]
- Llorens, F.; Schmitz, M.; Ferrer, I.; Zerr, I. Cognitive impairment and altered 5-HT1A receptor in Creutzfeldt-Jakob disease. Neurobiol. Aging. 2015, 36, 2482–2489. [Google Scholar]
- Wieser, H.G. Mal de Alzheimer: ¿qué papel juega la microbiota intestinal? Rev. Neurol. 2019, 68, 114–125. [Google Scholar]
- Moser, J.J.; Fritzler, M.J. Epstein-Barr virus and the nervous system. In Current Topics in Microbiology and Immunology; Khanna, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Gagliardi, S.; Piccoli, T.; Manzoni, D.; Bellazzi, R.; Carelli, S.; Oggioni, G.D. Defining the role of gut Microbiota in the onset and development of Amyotrophic Lateral Sclerosis. Microorganisms 2021, 9, 10–27. [Google Scholar]
- Cersosimo, M.G. Neurodegenerative diseases: The role of the gut microbiota. Ann. N. Y. Acad. Sci. 2019, 1447, 5–17. [Google Scholar]
- Kumar, D.K.; Choi, S.H.; Washicosky, K.J.; Eimer, W.A.; Tucker, S.; Ghofrani, J.; Lefkowitz, A.; McColl, G.; Goldstein, L.E.; Tanzi, R.E.; et al. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci. Transl. Med. 2016, 8, 340ra72. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, T.; Martella, G.; Imbriani, P.; Di Lazzaro, G.; Franco, D.; Colona, V.L.; Lefkowitz, A.; McColl, G.; Goldstein, L.E.; Tanzi, R.E.; et al. Dietary habit changes and neurodegenerative disorders: The emerging role of gut microbiota. Int. J. Mol. Sci. 2022, 23, 2628. [Google Scholar]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef] [PubMed]
- McQuaid, C.; Merlini, M.; Duszkiewicz, A.J.; Ruggeri, F.S.; Mielke, J.; Tackenberg, C.; Lefkowitz, A.; McColl, G.; Goldstein, L.E.; Tanzi, R.E.; et al. Impact of tau pathology on the human brain and gut microbiota: Relevance to Alzheimer’s disease. Acta Neuropathol. 2021, 141, 435–457. [Google Scholar]
- Sampson, T.R.; Mazmanian, S.K. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 2015, 17, 565–576. [Google Scholar] [CrossRef]

| Diseases | Contributions of the Enteric Nervous System |
|---|---|
| Parkinson’s disease | Early diagnosis: taking biopsies by colonoscopy Treatment: dietary interventions, modulation of the TLR response, and vagotomy |
| Alzheimer’s disease | Vagotomy as a protective element against dementia |
| Transmissible spongiform encephalopathies | SNE as a possible entry point |
| Multiple sclerosis | Treatment with probiotic supplements that modulate the autoimmune response |
| Shingles virus | The use of the saliva test for the detection of virus DNA in enteric zoster cases |
| Autistic spectrum disorder | Treatment of repetitive behaviors by Bacteroides fragilis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luesma, M.J.; López-Marco, L.; Monzón, M.; Santander, S. Enteric Nervous System and Its Relationship with Neurological Diseases. J. Clin. Med. 2024, 13, 5579. https://doi.org/10.3390/jcm13185579
Luesma MJ, López-Marco L, Monzón M, Santander S. Enteric Nervous System and Its Relationship with Neurological Diseases. Journal of Clinical Medicine. 2024; 13(18):5579. https://doi.org/10.3390/jcm13185579
Chicago/Turabian StyleLuesma, María José, Liberto López-Marco, Marta Monzón, and Sonia Santander. 2024. "Enteric Nervous System and Its Relationship with Neurological Diseases" Journal of Clinical Medicine 13, no. 18: 5579. https://doi.org/10.3390/jcm13185579
APA StyleLuesma, M. J., López-Marco, L., Monzón, M., & Santander, S. (2024). Enteric Nervous System and Its Relationship with Neurological Diseases. Journal of Clinical Medicine, 13(18), 5579. https://doi.org/10.3390/jcm13185579