Fatigue Assessment in Patients with Hereditary Hemochromatosis: First Use of the Popular Diagnostic Tools
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Questionaries
- The Fatigue Assessment Scale (FAS) is evaluated using a scoring system where each response can be rated from 1 to 5, corresponding to the following frequency: 1 = never, 2 = sometimes, 3 = regularly, 4 = often, and 5 = always. However, for questions 4 and 10, the scoring is reversed: 5 = never, 4 = sometimes, 3 = regularly, 2 = often, and 1 = always. The cumulative score for the FAS ranges from 10 to 50 points. Based on the total score, patients are categorized into two subgroups: fatigue (scores 22–34) and extreme fatigue (scores ≥ 35).
- The Chalder Fatigue Scale (CFQ) can be evaluated using two distinct scoring methods. The first is the bimodal scoring method, where responses are scored as follows: less than usual—0, no more than usual—0, more than usual—1, and much more than usual—1. The second is the Likert scoring method, where responses are as follows: less than usual—0, no more than usual—1, more than usual—2, and much more than usual—3. The possible score range for the bimodal method is 0 to 11 points, while for the Likert method, the range is 0 to 33 points [11]. In both methods, a higher score indicates a greater level of fatigue.
- The Fatigue Severity Scale (FSS) consists of 9 items, each rated on a scale from 1 to 7, where 1 represents “strongly disagree” and 7 means “strongly agree”. The total score is calculated by summing the points received across all items, with a higher total score indicating a greater level of fatigue.
2.3. Statistical Analyses
3. Results
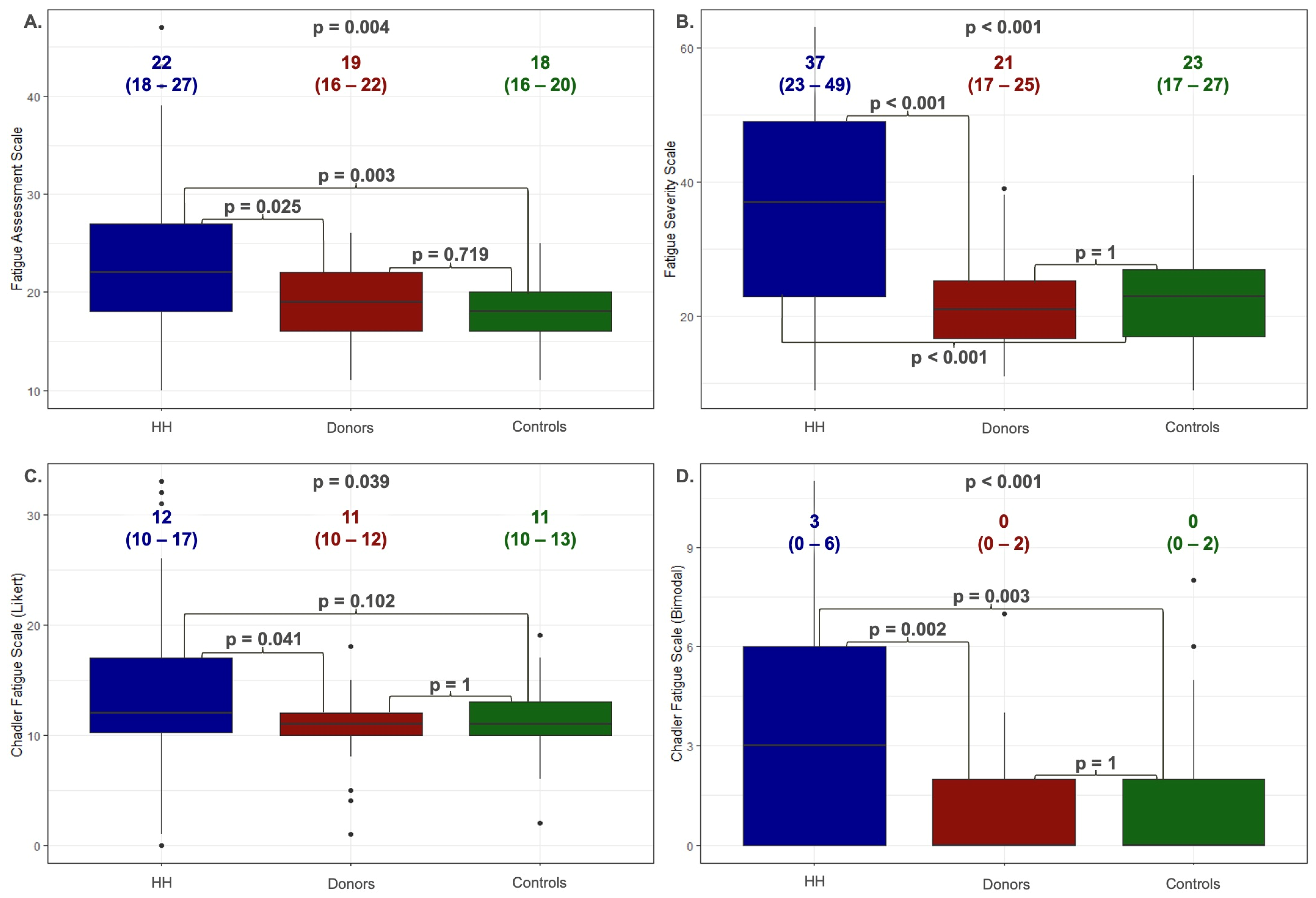
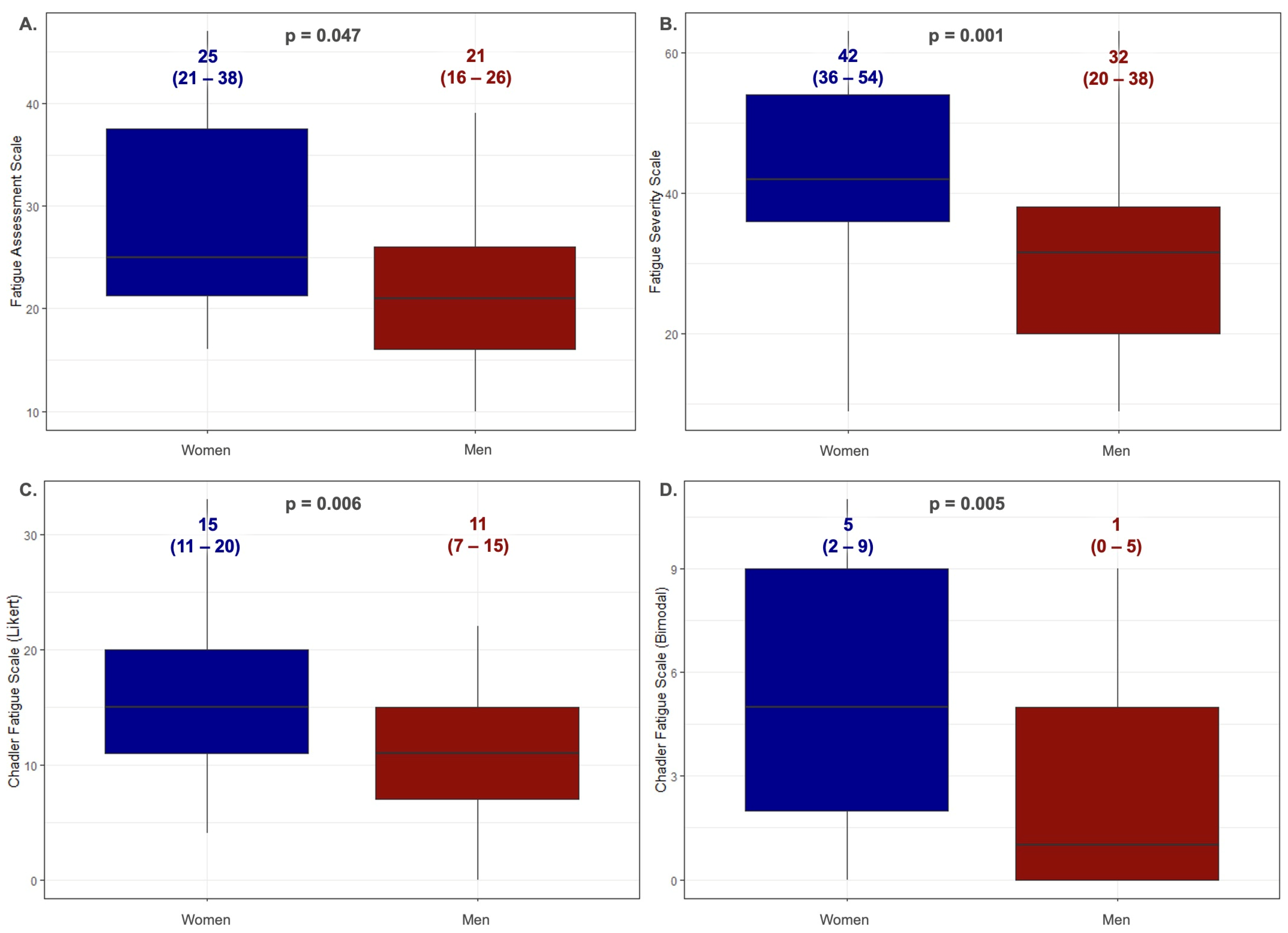
3.1. Analysis of Fatigue among Patients with Hereditary Hemochromatosis
3.2. Reliability Analysis and Validation of the Fatigue Assessment Scales Were Implemented
4. Discussion
5. Clinical Implications
6. Study Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hjollund, N.H.; Andersen, J.H.; Bech, P. Assessment of fatigue in chronic disease: A bibliographic study of fatigue measurement scales. Health Qual. Life Outcomes 2007, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Michielsen, H.J.; De Vries, J.; Drent, M.; Peros-Golubicic, T. Psychometric qualities of the Fatigue Assessment Scale in Croatian sarcoidosis patients. Sarcoidosis Vasc. Diffus. Lung Dis. 2005, 22, 133–138. [Google Scholar]
- Michielsen, H.J.; De Vries, J.; Van Heck, G.L. Psychometric qualities of a brief self-rated fatigue measure: The Fatigue Assessment Scale. J. Psychosom. Res. 2003, 54, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C. The Chalder Fatigue Scale (CFQ 11). Occup. Med. 2015, 65, 86. [Google Scholar] [CrossRef]
- Evangelista, L.S.; Moser, D.K.; Westlake, C.; Pike, N.; Ter-Galstanyan, A.; Dracup, K. Correlates of fatigue in patients with heart failure. Prog. Cardiovasc. Nurs. 2008, 23, 12–17. [Google Scholar] [CrossRef]
- Christensen, D.; Johnsen, S.P.; Watt, T.; Harder, I.; Kirkevold, M.; Andersen, G. Dimensions of post-stroke fatigue: A two-year follow-up study. Cereb. Dis. 2008, 26, 134–141. [Google Scholar] [CrossRef]
- Drent, M.; Lower, E.E.; De Vries, J. Sarcoidosis-associated fatigue. Eur. Respir. J. 2012, 40, 255–263. [Google Scholar] [CrossRef]
- Ferentinos, P.; Kontaxakis, V.; Havaki-Kontaxaki, B.; Dikeos, D.; Lykouras, L. Psychometric evaluation of the Fatigue Severity Scale in patients with major depression. Qual. Life Res. 2011, 20, 457–465. [Google Scholar] [CrossRef]
- Rosti-Otajärvi, E.; Hämäläinen, P.; Wiksten, A.; Hakkarainen, T.; Ruutiainen, J. Validity and reliability of the Fatigue Severity Scale in Finnish multiple sclerosis patients. Brain Behav. 2017, 7, e00743. [Google Scholar] [CrossRef]
- De Vries, J.; Van der Steeg, A.F.; Roukema, J.A. Psychometric properties of the Fatigue Assessment Scale (FAS) in women with breast problems. Int. J. Clin. Health Psychol. 2010, 10, 125–139. [Google Scholar]
- De Vries, J.; Michielsen, H.; Van Heck, G.L.; Drent, M. Measuring fatigue in sarcoidosis: The Fatigue Assessment Scale (FAS). Br. J. Health Psychol. 2004, 9, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, C.; Drent, M.; Elfferich, M.; De Vries, J. The Fatigue Assessment Scale: Quality and availability in sarcoidosis and other diseases. Curr. Opin. Pulm. Med. 2018, 24, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Loge, J.H.; Ekeberg, O.; Kaasa, S. Fatigue in the general Norwegian population: Normative data and associations. J. Psychosom. Res. 1998, 45, 53–65. [Google Scholar] [CrossRef]
- Valko, P.O.; Bassetti, C.L.; Bloch, K.E.; Held, U.; Baumann, C.R. Validation of the fatigue severity scale in a Swiss cohort. Sleep 2008, 31, 1601–1607. [Google Scholar] [CrossRef]
- Świątczak, M.; Młodziński, K.; Sikorska, K.; Raczak, A.; Lipiński, P.; Daniłowicz-Szymanowicz, L. Chronic Fatigue Syndrome in Patients with Deteriorated Iron Metabolism. Diagnostics 2022, 12, 2057. [Google Scholar] [CrossRef]
- Gulati, V.; Harikrishnan, P.; Palaniswamy, C.; Aronow, W.S.; Jain, D.; Frishman, W.H. Cardiac involvement in hemochromatosis. Cardiol. Rev. 2014, 22, 56–68. [Google Scholar] [CrossRef]
- Li, K.; Reichmann, H. Role of iron in neurodegenerative diseases. J. Neural. Transm. 2016, 123, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, K.; Bielawski, K.; Romanowski, T.; Stalke, P. Hemochromatoza dziedziczna-najczęstsza choroba genetyczna człowieka (Hereditary hemochromatosis: The most frequent inherited human disease). Postep. Hig. Med. Dosw. 2006, 60, 667–676. [Google Scholar]
- Strohmeyer, G.; Niederau, C.; Stremmel, W. Survival and causes of death in hemochromatosis. Observations in 163 patients. Ann. N. Y. Acad. Sci. 1988, 526, 245–257. [Google Scholar] [CrossRef]
- Niederau, C.; Fischer, R.; Sonnenberg, A.; Stremmel, W.; Trampisch, H.J.; Strohmeyer, G. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N. Engl. J. Med. 1985, 313, 1256–1262. [Google Scholar] [CrossRef]
- Vigorita, V.J.; Hutchins, G.M. Cardiac conduction system in hemochromatosis: Clinical and pathologic features of six patients. Am. J. Cardiol. 1979, 44, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Buja, L.M.; Roberts, W.C. Iron in the heart. Etiology and clinical significance. Am. J. Med. 1971, 51, 209–221. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on haemochromatosis. J. Hepatol. 2022, 77, 479–502. [Google Scholar] [CrossRef]
- Brislin, R.W. Translation and content analysis of oral and written materials. Methodology 1980, 389–444. [Google Scholar]
- Moirand, R.; Adams, P.C.; Bicheler, V.; Brissot, P.; Deugnier, Y. Clinical features of genetic hemochromatosis in women compared with men. Ann. Intern. Med. 1997, 127, 105–110. [Google Scholar] [CrossRef]
- Świątczak, M.; Rozwadowska, K.; Sikorska, K.; Młodziński, K.; Świątczak, A.; Raczak, G.; Daniłowicz-Szymanowicz, L. The potential impact of hereditary hemochromatosis on the heart considering the disease stage and patient age-the role of echocardiography. Front. Cardiovasc. Med. 2023, 10, 1202961. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.J.; Gurrin, L.C.; Constantine, C.C.; Osborne, N.J.; Delatycki, M.B.; Nicoll, A.J.; Gertig, D.M. Iron-overload-related disease in HFE hereditary hemochromatosis. N. Engl. J. Med. 2008, 358, 221–230. [Google Scholar] [CrossRef]
- Eldadah, B.A. Fatigue and fatigability in older adults. PMR 2010, 2, 406–413. [Google Scholar] [CrossRef]


| HH All n = 79 | |
|---|---|
| Age | 44 (34–55) |
| Male sex [%] | 53 |
| Patients reporting fatigue [%] | 71 |
| Hypertension [%] | 22 |
| Liver cirrhosis [%] | 18 |
| Active treatment with venesections [%] | 56 |
| Iron [ug/dL] | 171 (120–208) |
| Ferritin [ng/mL] | 151 (72–358) |
| Haemoglobin [mg/dL] | 15.3 (14.3–16) |
| ASPAT [U/L] | 27 (20–49) |
| ALAT [U/L] | 32 (19–51) |
| Fatigue Assessment Scale | Fatigue Severity Scale | Chadler Fatigue Scale (Likert) | Chadler Fatigue Scale (Bimodal) | |
|---|---|---|---|---|
| BMI | r = −0.055 (p = 0.598) | r = 0.075 (p = 0.405) | r = 0.002 (p = 0.979) | r = −0.042 (p = 0.645) |
| Age | r = −0.151 (p = 0.140) | r = 0.122 (p = 0.164) | r = 0.042 (p = 0.635) | r = 0.061 (p = 0.489) |
| Iron ug/dL | r = −0.168 (p = 0.344) | r = −0.102 (p = 0.411) | r = −0.016 (p = 0.901) | r = −0.033 (p = 0.797) |
| Ferritin ng/mL | r = 0.321 (p = 0.073) | r = 0.274 (p = 0.030) | r = 0.135 (p = 0.303) | r = 0.180 (p = 0.170) |
| Year from diagnosis | r = 0.109 (p = 0.478) | r = 0.204 (p = 0.075) | r = 0.312 (p = 0.006) | r = 0.250 (p = 0.033) |
| Scale | C282Y/C282Y n = 49 | C282Y/H63D n = 16 | H63D/H63D n = 12 | p-Value |
|---|---|---|---|---|
| Fatigue Assessment Scale | 21 (18–29) | 21 (20–26) | 24 (23–27) | 0.666 |
| Fatigue Severity Scale | 38 (26–51) | 28 (17–47) | 36 (29–44) | 0.4336 |
| Chadler Fatigue Scale (Likert) | 12 (11–19) | 11 (19–15) | 15 (11–16) | 0.5587 |
| Chadler Fatigue Scale (Bimodal) | 3 (1–7) | 1 (0–5) | 4 (1–6) | 0.4241 |
| Scale | Age 18–25 n = 6 | Age 26–45 n = 38 | Age 46–65 n = 12 | Age >65 n = 6 | p-Value |
|---|---|---|---|---|---|
| Fatigue Assessment Scale | 21 (21–24) | 25.5 (20–37) | 21 (16–25) | 21 (16–22) | 0.2016 |
| Fatigue Severity Scale | 35 (31–46) | 37 (23–51) | 36 (26–46) | 37 (36–53) | 0.8914 |
| Chadler Fatigue Scale (Likert) | 15 (12–19) | 12 (8–17) | 14 (11–18) | 11 (6–14) | 0.4013 |
| Chadler Fatigue Scale (Bimodal) | 4 (2–7) | 2 (0–6) | 3 (0–6) | 2 (1–5) | 0.949 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świątczak, M.; Raczak, A.; Świątczak, A.; Młodziński, K.; Sikorska, K.; Jaźwińska, A.; Kaufmann, D.; Daniłowicz-Szymanowicz, L. Fatigue Assessment in Patients with Hereditary Hemochromatosis: First Use of the Popular Diagnostic Tools. J. Clin. Med. 2024, 13, 5544. https://doi.org/10.3390/jcm13185544
Świątczak M, Raczak A, Świątczak A, Młodziński K, Sikorska K, Jaźwińska A, Kaufmann D, Daniłowicz-Szymanowicz L. Fatigue Assessment in Patients with Hereditary Hemochromatosis: First Use of the Popular Diagnostic Tools. Journal of Clinical Medicine. 2024; 13(18):5544. https://doi.org/10.3390/jcm13185544
Chicago/Turabian StyleŚwiątczak, Michał, Alicja Raczak, Agata Świątczak, Krzysztof Młodziński, Katarzyna Sikorska, Anna Jaźwińska, Damian Kaufmann, and Ludmiła Daniłowicz-Szymanowicz. 2024. "Fatigue Assessment in Patients with Hereditary Hemochromatosis: First Use of the Popular Diagnostic Tools" Journal of Clinical Medicine 13, no. 18: 5544. https://doi.org/10.3390/jcm13185544
APA StyleŚwiątczak, M., Raczak, A., Świątczak, A., Młodziński, K., Sikorska, K., Jaźwińska, A., Kaufmann, D., & Daniłowicz-Szymanowicz, L. (2024). Fatigue Assessment in Patients with Hereditary Hemochromatosis: First Use of the Popular Diagnostic Tools. Journal of Clinical Medicine, 13(18), 5544. https://doi.org/10.3390/jcm13185544







