Vamifeport: Monography of the First Oral Ferroportin Inhibitor
Abstract
1. Background
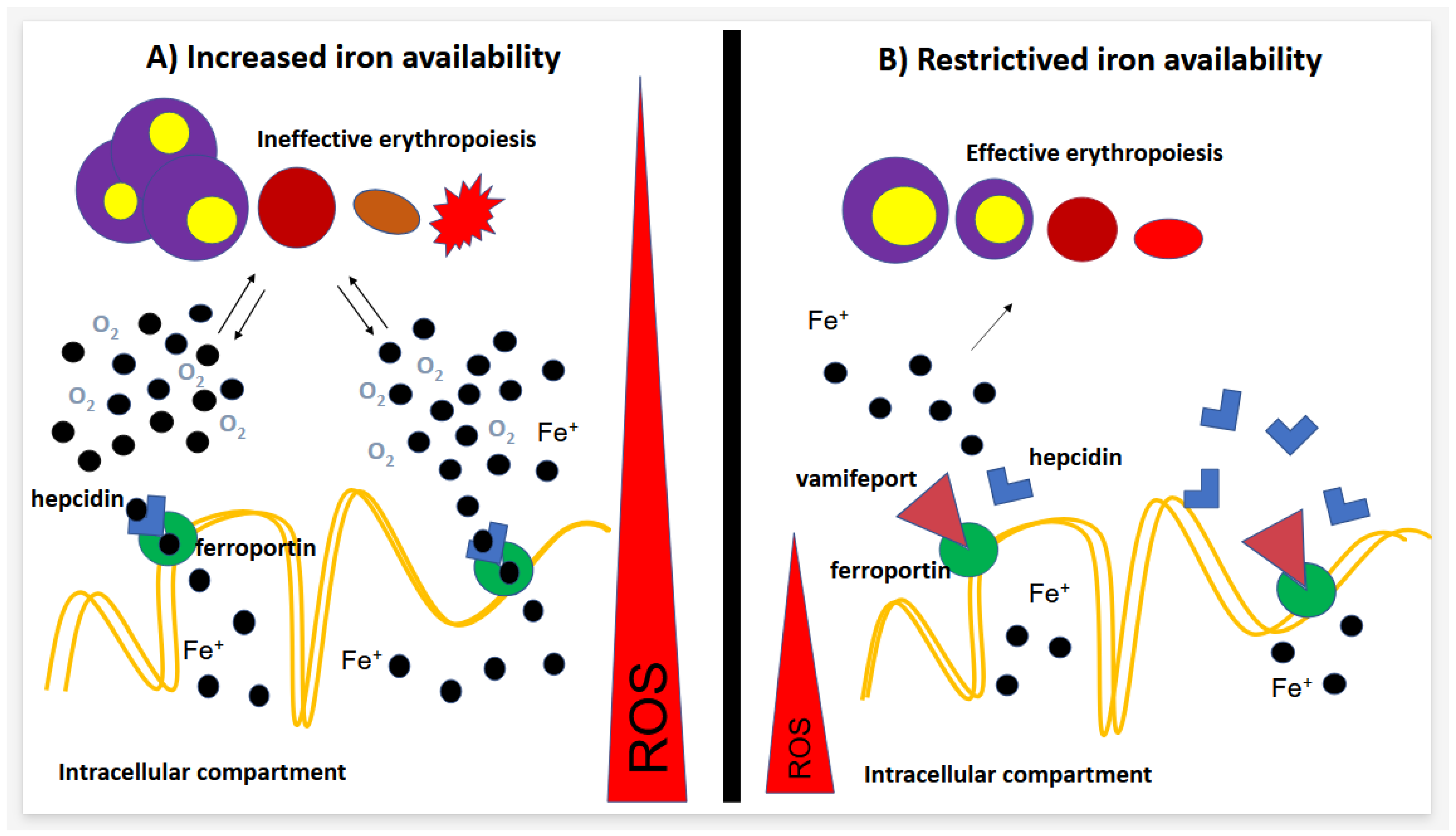
1.1. Ferroportin–Hepcidin Axis
1.2. Hepcidin–Ferroportin Axis Behavior in Dyserythropoietic Diseases
2. Vamifeport
2.1. Preclinical Studies in SCD and Thalassemia Model
2.2. Clinical Studies
3. Pharmacokinetics
4. Safety
5. Drug Interactions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Parrow, N.L.; Violet, P.-C.; George, N.A.; Ali, F.; Bhanvadia, S.; Wong, R.; Tisdale, J.F.; Fitzhugh, C.; Levine, M.; Thein, S.L.; et al. Dietary iron restriction improves markers of disease severity in murine sickle cell anemia. Blood 2021, 137, 1553–1555. [Google Scholar] [CrossRef] [PubMed]
- Nyffenegger, N.; Zennadi, R.; Kalleda, N.; Flace, A.; Ingoglia, G.; Buzzi, R.M.; Doucerain, C.; Buehler, P.W.; Schaer, D.J.; Dürrenberger, F.; et al. The oral ferroportin inhibitor vamifeport improves hemodynamics in a mouse model of sickle cell disease. Blood 2022, 140, 769–781. [Google Scholar] [CrossRef]
- Muckenthaler, M.U.; Galy, B.; Hentze, M.W. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu. Rev. Nutr. 2008, 28, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Pilo, F.; Cilloni, D.; Della Porta, M.G.; Forni, G.L.; Piperno, A.; Santini, V.; Angelucci, E. Iron-mediated tissue damage in acquired ineffective erythropoiesis disease: It’s more a matter of burden or more of exposure to toxic iron form? Leuk. Res. 2022, 114, 106792. [Google Scholar] [CrossRef]
- Drakesmith, H.; Nemeth, E.; Ganz, T. Ironing out Ferroportin. Cell Metab. 2015, 22, 777–787. [Google Scholar] [CrossRef]
- Aschemeyer, S.; Qiao, B.; Stefanova, D.; Valore, E.V.; Sek, A.C.; Ruwe, T.A.; Vieth, K.R.; Jung, G.; Casu, C.; Rivella, S.; et al. Structure-function analysis of ferroportin defines the binding site and an alternative mechanism of action of hepcidin. Blood 2018, 131, 899–910. [Google Scholar] [CrossRef]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A Red Carpet for Iron Metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [PubMed]
- Kautz, L.; Jung, G.; Valore, E.V.; Rivella, S.; Nemeth, E.; Ganz, T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat. Genet. 2014, 46, 678–684. [Google Scholar] [CrossRef]
- Coffey, R.; Ganz, T. Erythroferrone: An Erythroid Regulator of Hepcidin and Iron Metabolism. HemaSphere 2018, 2, e35. [Google Scholar] [CrossRef]
- Ganz, T. Erythropoietic regulators of iron metabolism. Free Radic. Biol. Med. 2019, 133, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Richard, F.; van Lier, J.J.; Roubert, B.; Haboubi, T.; Göhring, U.-M.; Dürrenberger, F. Oral ferroportin inhibitor VIT-2763: First-in-human, phase 1 study in healthy volunteers. Am. J. Hematol. 2020, 95, 68–77. [Google Scholar] [CrossRef]
- Manolova, V.; Nyffenegger, N.; Flace, A.; Altermatt, P.; Varol, A.; Doucerain, C.; Sundstrom, H.; Dürrenberger, F. Oral ferroportin inhibitor ameliorates ineffective erythropoiesis in a model of β-thalassemia. J. Clin. Investig. 2019, 130, 491–506. [Google Scholar] [CrossRef] [PubMed]
- Nyffenegger, N.; Flace, A.; Doucerain, C.; Dürrenberger, F.; Manolova, V. The Oral Ferroportin Inhibitor VIT-2763 Improves Erythropoiesis without Interfering with Iron Chelation Therapy in a Mouse Model of β-Thalassemia. Int. J. Mol. Sci. 2021, 22, 873. [Google Scholar] [CrossRef] [PubMed]
- Nyffenegger, N.; Flace, A.; Canclini, C.; Duerrenberger, F.; Manolova, V. Ferroportin inhibitors prevent iron loading in a mouse model of hereditary hemochromatosis. Am. J. Hematol. 2017, 92, E471. [Google Scholar]
- Mangaonkar, A.A.; Thawer, F.; Son, J.; Ajebo, G.; Xu, H.; Barrett, N.J.; Wells, L.G.; Bowman, L.; Clair, B.; Patel, N.; et al. Regulation of iron homeostasis through the erythroferrone-hepcidin axis in sickle cell disease. Br. J. Haematol. 2020, 189, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Ginzburg, Y.Z.; Glassberg, J. Inflammation, Hemolysis, and Erythropoiesis Lead to Competitive Regulation of Hepcidin and Possibly Systemic Iron Status in Sickle Cell Disease. EBioMedicine 2018, 34, 8–9. [Google Scholar] [CrossRef]
- Ezeh, C.; Ugochukwu, C.C.; Weinstein, J.; Okpala, I. Hepcidin, haemoglobin and ferritin levels in sickle cell anaemia. Eur. J. Haematol. 2005, 74, 86–88. [Google Scholar] [CrossRef]
- Wood, J.C.; Cohen, A.R.; Pressel, S.L.; Aygun, B.; Imran, H.; Luchtman-Jones, L.; Thompson, A.A.; Fuh, B.; Schultz, W.H.; Davis, B.R.; et al. TWiTCH Investigators Organ iron accumulation in chronically transfused children with sickle cell anaemia: Baseline results from the TWiTCH trial. Br. J. Haematol. 2016, 172, 122–130. [Google Scholar] [CrossRef]
- Lincoln, T.L.; Aroesty, J.; Morrison, P. Iron-deficiency anemia and sickle-cell disease: A hypothesis. Lancet 1973, 2, 260–261. [Google Scholar] [CrossRef]
- Hofrichter, J.; Ross, P.D.; Eaton, W.A. Kinetics and mechanism of deoxyhemoglobin S gelation: A new approach to understanding sickle cell disease. Proc. Natl. Acad. Sci. USA 1974, 71, 4864–4868. [Google Scholar] [CrossRef] [PubMed]
- Castro, O.; Poillon, W.N.; Finke, H.; Massac, E. Improvement of sickle cell anemia by iron-limited erythropoiesis. Am. J. Hematol. 1994, 47, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.; Taher, A.; Viprakasit, V.; Kattamis, A.; Coates, T.D.; Garbowski, M.; Dürrenberger, F.; Manolova, V.; Richard, F.; Cappellini, M.D. Oral ferroportin inhibitor vamifeport for improving iron homeostasis and erythropoiesis in β-thalassemia: Current evidence and future clinical development. Expert Rev. Hematol. 2021, 14, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Taher, A.; Kourakli-Symeonidis, A.; Tantiworawit, A.; Wong, P.; Szecsödy, P. S272: Safety and preliminary pharmacodynamic effects of the Ferroportin inhibitor Vamifeport (VIT-2763) in patients with non-transfusion-dependent Beta Thalassemia (NTDT): Results from a phase 2A study. HemaSphere 2022, 6, 173. [Google Scholar] [CrossRef]
| Trial | Phase | Target Population | Objectives | Clinicaltrial.gov ID | Status |
|---|---|---|---|---|---|
| VITHAL | Phase 2a, double-blind, randomized, placebo-controlled, parallel group, multicenter study on safety, tolerability, pharmacokinetics, pharmacodynamics and preliminary efficacy of multiple doses of VIT-2763 in subjects with non-transfusion-dependent β-thalassemia | People aged 18 and older with NTDT 12 years to 65 years | Tolerability and safety. | NCT04364269 | Completed |
| VIT-2763-THAL-203 | Phase 2b multiple-dose, double-blind, randomized, placebo-controlled, parallel-group, multicenter trial | TDT | The main purpose of this study is to evaluate the efficacy of 3 multiple doses of VIT-2763 as measured by the reduction in red blood cell (RBC) transfusion burden from week 13 to week 24, to identify the most efficacious and safe dose. | NCT04938635 | Withdrawn (strategic reasons) |
| VIT-2763-SCD-202 | Phase 2a, double-blind, randomized, placebo-controlled, efficacy, and safety study of multiple doses of VIT-2763 in subjects with sickle cell disease | SCD | The purpose of this study is to investigate the effect of VIT-2763 on markers of hemolysis (breakdown in red blood cells) in sickle cell disease (SCD). The safety, tolerability and clinical beneficial effects of VIT-2763 for the treatment of SCD are also explored. | NCT04817670 | Ongoing |
| Preclinical Results | Clinical Results | |
|---|---|---|
| Thalassemia |
|
|
| SCD |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilo, F.; Angelucci, E. Vamifeport: Monography of the First Oral Ferroportin Inhibitor. J. Clin. Med. 2024, 13, 5524. https://doi.org/10.3390/jcm13185524
Pilo F, Angelucci E. Vamifeport: Monography of the First Oral Ferroportin Inhibitor. Journal of Clinical Medicine. 2024; 13(18):5524. https://doi.org/10.3390/jcm13185524
Chicago/Turabian StylePilo, Federica, and Emanuele Angelucci. 2024. "Vamifeport: Monography of the First Oral Ferroportin Inhibitor" Journal of Clinical Medicine 13, no. 18: 5524. https://doi.org/10.3390/jcm13185524
APA StylePilo, F., & Angelucci, E. (2024). Vamifeport: Monography of the First Oral Ferroportin Inhibitor. Journal of Clinical Medicine, 13(18), 5524. https://doi.org/10.3390/jcm13185524






