Predictive Value of the D-Dimer-to-Fibrinogen Ratio for Acute Kidney Injury after Living-Donor Liver Transplantation: A Retrospective Observational Cohort Study Using Logistic Regression and Propensity Score Matching Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Liver Transplantation
2.3. Measurements of D-Dimer and Fibrinogen
2.4. Preoperative and Intraoperative Findings
2.5. Classification of Acute Kidney Injury
2.6. Postoperative Outcomes
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Comparison of Perioperative Findings
3.3. Association between Perioperative Findings and AKI Development
3.4. Comparison of AUC between Logistic Models with DFR, D-Dimer, and Fibrinogen
3.5. Comparison of Predictive Accuracy of DFR, D-Dimer, and Fibrinogen for AKI Development
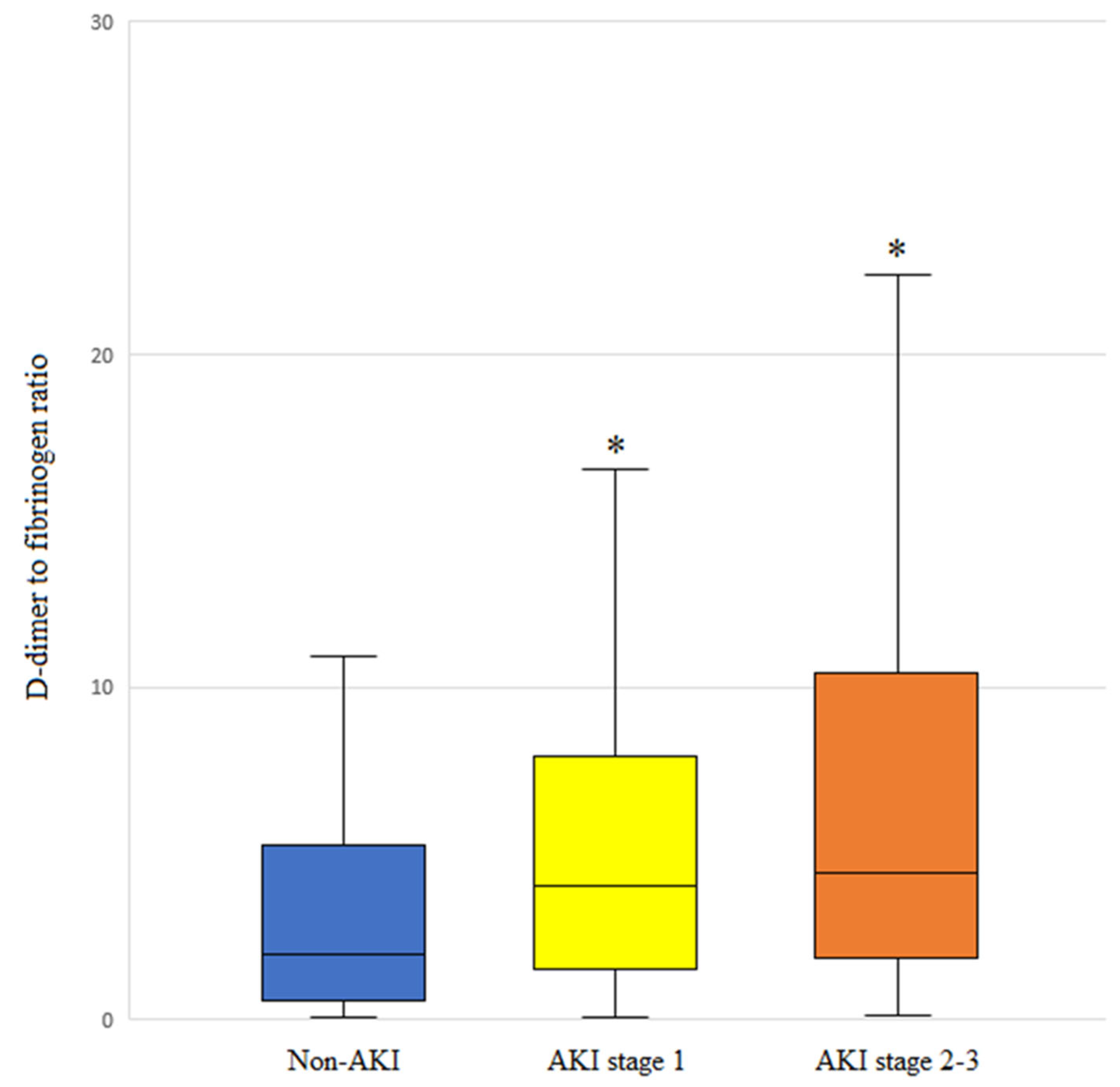
3.6. DFR Level and AKI Stage
3.7. Association of Perioperative Findings with Severe AKI (AKI Stages 2–3)
3.8. Association of DFR with Inflammatory Factors
3.9. Association of High DFR with Postoperative Complications
3.10. Perioperative Findings before and after PS Matching
3.11. Association between High DFR and AKI Occurrence in PS-Matched Patients
3.12. Prevalence of AKI between the Low and High DFR Groups across Different AKI Stages in PS-Matched Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Feng, S. Living donor liver transplantation in high Model for End-Stage Liver Disease score patients. Liver Transpl. 2017, 23, S9–S21. [Google Scholar]
- Lin, C.C.; Chuang, F.R.; Wang, C.C.; Chen, Y.S.; Chen, C.L.; Liu, Y.W.; Cheng, Y.F.; Lee, C.H.; Jawan, B. Early postoperative complications in recipients of living donor liver transplantation. Transpl. Proc. 2004, 36, 2338–2341. [Google Scholar] [PubMed]
- Hilmi, I.A.; Damian, D.; Al-Khafaji, A.; Planinsic, R.; Boucek, C.; Sakai, T.; Chang, C.C.; Kellum, J.A. Acute kidney injury following orthotopic liver transplantation: Incidence, risk factors, and effects on patient and graft outcomes. Br. J. Anaesth. 2015, 114, 919–926. [Google Scholar] [PubMed]
- Wiesen, P.; Massion, P.B.; Joris, J.; Detry, O.; Damas, P. Incidence and risk factors for early renal dysfunction after liver transplantation. World J. Transpl. 2016, 6, 220–232. [Google Scholar] [PubMed]
- Dong, V.; Nadim, M.K.; Karvellas, C.J. Post-Liver Transplant Acute Kidney Injury. Liver Transpl. 2021, 27, 1653–1664. [Google Scholar]
- Hobson, C.; Ozrazgat-Baslanti, T.; Kuxhausen, A.; Thottakkara, P.; Efron, P.A.; Moore, F.A.; Moldawer, L.L.; Segal, M.S.; Bihorac, A. Cost and Mortality Associated with Postoperative Acute Kidney Injury. Ann. Surg. 2015, 261, 1207–1214. [Google Scholar]
- Soomro, A.Y.; Guerchicoff, A.; Nichols, D.J.; Suleman, J.; Dangas, G.D. The current role and future prospects of D-dimer biomarker. Eur. Heart J. Cardiovasc. Pharmacother. 2016, 2, 175–184. [Google Scholar]
- Mori, K.; Tsujita, Y.; Yamane, T.; Eguchi, Y. Decreasing Plasma Fibrinogen Levels in the Intensive Care Unit Are Associated with High Mortality Rates in Patients with Sepsis-Induced Coagulopathy. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221101386. [Google Scholar]
- Gram, J.; Duscha, H.; Zurborn, K.H.; Bruhn, H.D. Increased levels of fibrinolysis reaction products (D-dimer) in patients with decompensated alcoholic liver cirrhosis. Scand. J. Gastroenterol. 1991, 26, 1173–1178. [Google Scholar] [PubMed]
- Shorr, A.F.; Thomas, S.J.; Alkins, S.A.; Fitzpatrick, T.M.; Ling, G.S. D-dimer correlates with proinflammatory cytokine levels and outcomes in critically ill patients. Chest 2002, 121, 1262–1268. [Google Scholar]
- Li, Y.; Qi, X.; Li, H.; Dai, J.; Deng, H.; Li, J.; Peng, Y.; Liu, X.; Sun, X.; Guo, X. D-dimer level for predicting the in-hospital mortality in liver cirrhosis: A retrospective study. Exp. Ther. Med. 2017, 13, 285–289. [Google Scholar] [PubMed]
- Arif, S.; Khan, A.S.; Khan, A.R. Changes in fibrinogen level in liver cirrhosis. J. Ayub Med. Coll. Abbottabad 2002, 14, 19–21. [Google Scholar]
- Peck-Radosavljevic, M. Review article: Coagulation disorders in chronic liver disease. Aliment. Pharmacol. Ther. 2007, 26 (Suppl. S1), 21–28. [Google Scholar] [PubMed]
- Ferro, D.; Celestini, A.; Violi, F. Hyperfibrinolysis in liver disease. Clin. Liver Dis. 2009, 13, 21–31. [Google Scholar]
- Wang, C.; Yu, X.; Wang, T.; Ding, M.; Ran, L. D-dimer/fibrinogen ratio for the prediction of deep venous thrombosis after traumatic spinal cord injury. Spinal Cord 2023, 61, 447–452. [Google Scholar] [PubMed]
- Kara, H.; Bayir, A.; Degirmenci, S.; Kayis, S.A.; Akinci, M.; Ak, A.; Celik, B.; Dogru, A.; Ozturk, B. D-dimer and D-dimer/fibrinogen ratio in predicting pulmonary embolism in patients evaluated in a hospital emergency department. Acta Clin. Belg. 2014, 69, 240–245. [Google Scholar] [PubMed]
- Bai, Y.; Zheng, Y.Y.; Tang, J.N.; Yang, X.M.; Guo, Q.Q.; Zhang, J.C.; Cheng, M.D.; Song, F.H.; Wang, K.; Zhang, Z.L.; et al. D-Dimer to Fibrinogen Ratio as a Novel Prognostic Marker in Patients after Undergoing Percutaneous Coronary Intervention: A Retrospective Cohort Study. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620948586. [Google Scholar]
- Murat, S.; Murat, B.; Dural, M.; Mert, G.O.; Cavusoglu, Y. Prognostic value of D-dimer/fibrinogen ratio on in-hospital outcomes of patients with heart failure and COVID-19. Biomark. Med. 2021, 15, 1519–1528. [Google Scholar]
- Park, J.; Kim, S.U.; Choi, H.J.; Hong, S.H.; Chae, M.S. Predictive Role of the D-Dimer Level in Acute Kidney Injury in Living Donor Liver Transplantation: A Retrospective Observational Cohort Study. J. Clin. Med. 2022, 11, 450. [Google Scholar] [CrossRef]
- Park, J.; Joo, M.A.; Choi, H.J.; Hong, S.H.; Park, C.S.; Choi, J.H.; Chae, M.S. Predictive utility of fibrinogen in acute kidney injury in living donor liver transplantation: A propensity score-matching analysis. PLoS ONE 2021, 16, e0252715. [Google Scholar]
- Stevens, P.E.; Levin, A.; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [PubMed]
- Zhang, W.R.; Garg, A.X.; Coca, S.G.; Devereaux, P.J.; Eikelboom, J.; Kavsak, P.; McArthur, E.; Thiessen-Philbrook, H.; Shortt, C.; Shlipak, M.; et al. Plasma IL-6 and IL-10 Concentrations Predict AKI and Long-Term Mortality in Adults after Cardiac Surgery. J. Am. Soc. Nephrol. 2015, 26, 3123–3132. [Google Scholar] [PubMed]
- Prochaska, J.H.; Frank, B.; Nagler, M.; Lamparter, H.; Weisser, G.; Schulz, A.; Eggebrecht, L.; Gobel, S.; Arnold, N.; Panova-Noeva, M.; et al. Age-related diagnostic value of D-dimer testing and the role of inflammation in patients with suspected deep vein thrombosis. Sci. Rep. 2017, 7, 4591. [Google Scholar]
- Marchi, R.; Linares, M.; Rojas, H.; Ruiz-Saez, A.; Meyer, M.; Casini, A.; Brennan, S.O. A novel fibrinogen mutation: FGA g. 3057 C > T (p. Arg104 > Cys) impairs fibrinogen secretion. BMC Hematol. 2017, 17, 22. [Google Scholar]
- Horoldt, B.S.; Burattin, M.; Gunson, B.K.; Bramhall, S.R.; Nightingale, P.; Hubscher, S.G.; Neuberger, J.M. Does the Banff rejection activity index predict outcome in patients with early acute cellular rejection following liver transplantation? Liver Transpl. 2006, 12, 1144–1151. [Google Scholar]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar]
- Lee, D.D.; Croome, K.P.; Shalev, J.A.; Musto, K.R.; Sharma, M.; Keaveny, A.P.; Taner, C.B. Early allograft dysfunction after liver transplantation: An intermediate outcome measure for targeted improvements. Ann. Hepatol. 2016, 15, 53–60. [Google Scholar]
- Stuart, E.A. Matching methods for causal inference: A review and a look forward. Stat. Sci. 2010, 25, 1–21. [Google Scholar]
- Bell, S.; Ross, V.C.; Zealley, K.A.; Millar, F.; Isles, C. Management of post-operative acute kidney injury. QJM 2017, 110, 695–700. [Google Scholar]
- Gameiro, J.; Fonseca, J.A.; Neves, M.; Jorge, S.; Lopes, J.A. Acute kidney injury in major abdominal surgery: Incidence, risk factors, pathogenesis and outcomes. Ann. Intensive Care 2018, 8, 22. [Google Scholar]
- Murashima, M.; Nishimoto, M.; Kokubu, M.; Hamano, T.; Matsui, M.; Eriguchi, M.; Samejima, K.I.; Akai, Y.; Tsuruya, K. Inflammation as a predictor of acute kidney injury and mediator of higher mortality after acute kidney injury in non-cardiac surgery. Sci. Rep. 2019, 9, 20260. [Google Scholar]
- Cantaluppi, V.; Quercia, A.D.; Dellepiane, S.; Ferrario, S.; Camussi, G.; Biancone, L. Interaction between systemic inflammation and renal tubular epithelial cells. Nephrol. Dial. Transpl. 2014, 29, 2004–2011. [Google Scholar]
- Dellepiane, S.; Marengo, M.; Cantaluppi, V. Detrimental cross-talk between sepsis and acute kidney injury: New pathogenic mechanisms, early biomarkers and targeted therapies. Crit. Care 2016, 20, 61. [Google Scholar]
- Zhou, J.; Zhang, X.; Lyu, L.; Ma, X.; Miao, G.; Chu, H. Modifiable risk factors of acute kidney injury after liver transplantation: A systematic review and meta-analysis. BMC Nephrol. 2021, 22, 149. [Google Scholar]
- Yao, Y.; Cao, J.; Wang, Q.; Shi, Q.; Liu, K.; Luo, Z.; Chen, X.; Chen, S.; Yu, K.; Huang, Z.; et al. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: A case control study. J. Intensive Care 2020, 8, 49. [Google Scholar]
- Fruchter, O.; Yigla, M.; Kramer, M.R. D-dimer as a prognostic biomarker for mortality in chronic obstructive pulmonary disease exacerbation. Am. J. Med. Sci. 2015, 349, 29–35. [Google Scholar] [PubMed]
- Cioni, G.; Cristani, A.; Mussini, C.; Grandi, S.; Pentore, R.; Zeneroli, M.L.; Tizzanini, W.; Zagni, G.; Ventura, E. Incidence and clinical significance of elevated fibrin(ogen) degradation product and/or D-dimer levels in liver cirrhosis patients. Ital. J. Gastroenterol. 1990, 22, 70–74. [Google Scholar]
- Rizzo, K.; Vella, K.; Zammit, D.; Gatt, P.; Grima, C.; Inguanez, M.B.; Gerada, J.; Ellul, P.; Vassallo, M.; Azzopardi, N.; et al. Fibrinogen measurement in liver disease: Validation of the functional fibrinogen thromboelastography assay and a novel mathematical predictive model. Blood Transfus. 2019, 17, 237–246. [Google Scholar]
- Cong, Y.L.; Wei, Y.X.; Zhang, L.W.; Yin, Z.J.; Bai, J. The relationship between hemostatic changes in liver cirrhosis patients with different degrees of liver lesions in reference to Child-Pugh scores. Zhonghua Gan Zang Bing Za Zhi 2005, 13, 31–34. [Google Scholar]
- Zhao, T.J.; Yang, Q.K.; Tan, C.Y.; Bi, L.D.; Li, J.; Miao, Z.L. Prognostic value of D-dimer/fibrinogen ratio in the adverse outcomes of patients hospitalized for heart failure. Biomark. Med. 2020, 14, 1733–1745. [Google Scholar]
- Kucher, N.; Kohler, H.P.; Dornhofer, T.; Wallmann, D.; Lammle, B. Accuracy of D-dimer/fibrinogen ratio to predict pulmonary embolism: A prospective diagnostic study. J. Thromb. Haemost. 2003, 1, 708–713. [Google Scholar] [PubMed]
- Nadim, M.K.; Garcia-Tsao, G. Acute Kidney Injury in Patients with Cirrhosis. N. Engl. J. Med. 2023, 388, 733–745. [Google Scholar] [PubMed]
- Qi, T.; Zhu, C.; Lu, G.; Hao, J.; He, Q.; Chen, Y.; Zhou, F.; Chen, J.; Hou, J. Elevated D-dimer is associated with increased 28-day mortality in acute-on-chronic liver failure in China: A retrospective study. BMC Gastroenterol. 2019, 19, 20. [Google Scholar]
- Miwa, T.; Utakata, Y.; Hanai, T.; Aiba, M.; Unome, S.; Imai, K.; Takai, K.; Shiraki, M.; Katsumura, N.; Shimizu, M. Acute kidney injury development is associated with mortality in Japanese patients with cirrhosis: Impact of amino acid imbalance. J. Gastroenterol. 2024, 59, 849–857. [Google Scholar]
- Chenitz, K.B.; Lane-Fall, M.B. Decreased urine output and acute kidney injury in the postanesthesia care unit. Anesthesiol. Clin. 2012, 30, 513–526. [Google Scholar]
- Patschan, D.; Muller, G.A. Acute Kidney Injury in Diabetes Mellitus. Int. J. Nephrol. 2016, 2016, 6232909. [Google Scholar]
- Park, J.; Jeong, J.; Choi, H.J.; Shim, J.W.; Lee, H.M.; Hong, S.H.; Park, C.S.; Choi, J.H.; Chae, M.S. Role of thrombocytopenia in risk stratification for acute kidney injury after living donor liver transplantation. Platelets 2021, 32, 453–462. [Google Scholar]
- Liu, S.; Wang, X.; Lu, Y.; Li, T.; Gong, Z.; Sheng, T.; Hu, B.; Peng, Z.; Sun, X. The effects of intraoperative cryoprecipitate transfusion on acute renal failure following orthotropic liver transplantation. Hepatol. Int. 2013, 7, 901–909. [Google Scholar]
- Huang, J.; Chen, J.; Yang, J.; Han, M.; Xue, Z.; Wang, Y.; Xu, M.; Qi, H.; Wang, Y. Prediction models for acute kidney injury following liver transplantation: A systematic review and critical appraisal. Intensive Crit. Care Nurs. 2024, 86, 103808. [Google Scholar]

| Group | Non-AKI | AKI | p |
|---|---|---|---|
| n | 500 | 148 | |
| Age (years) | 54 (48–60) | 53 (48–59) | 0.440 |
| Sex (male) | 341 (68.2%) | 109 (73.6%) | 0.206 |
| Body mass index (kg/m2) | 24 (22–26) | 24 (22–28) | 0.092 |
| Etiology | |||
| Alcohol | 93 (18.6%) | 38 (25.7%) | 0.309 |
| Hepatitis A | 18 (3.6%) | 9 (6.1%) | |
| Hepatitis B | 284 (56.8%) | 78 (52.7%) | |
| Hepatitis C | 37 (7.4%) | 8 (5.4%) | |
| Autoimmune | 24 (4.8%) | 4 (2.7%) | |
| Drugs and toxins | 10 (2.2%) | 3 (1.4%) | |
| Cryptogenic | 33 (6.6%) | 9 (6.1%) | |
| Comorbidity | |||
| Diabetes mellitus | 121 (24.2%) | 49 (33.1%) | 0.030 |
| Hypertension | 101 (20.2%) | 31 (20.9%) | 0.843 |
| MELD score (point) | 12 (6–23) | 17 (9–25) | 0.006 |
| Hepatic decompensation | |||
| Varix | 114 (22.8%) | 41 (27.7%) | 0.219 |
| Ascites | 224 (44.88%) | 84 (56.8%) | 0.011 |
| Cardiac function | |||
| Ejection fraction (%) | 64 (62–67) | 64 (62–67) | 0.289 |
| Diastolic dysfunction | 200 (40.0%) | 65 (43.9%) | 0.244 |
| Laboratory variables | |||
| Hematocrit (%) | 30 (25–36) | 28 (24–32) | 0.004 |
| WBC count (×109/L) | 4.4 (2.8–6.8) | 4.5 (3.1–8.2) | 0.335 |
| Albumin (g/dL) | 3.1 (2.7–3.6) | 2.9 (2.6–3.3) | <0.001 |
| Platelet count (×109/L) | 68 (47–109) | 56 (39–76) | <0.001 |
| International normalized ratio | 1.4 (1.2–2.1) | 1.6 (1.3–2.0) | 0.037 |
| DFR level | 1.7 (0.4–5.3) | 4.1 (1.6–9.2) | <0.001 |
| Total bilirubin (mg/dL) | 2.1 (0.7–11.7) | 3.6 (1.3–17.1) | 0.002 |
| Sodium (mEq/L) | 139 (135–142) | 138 (134–141) | 0.086 |
| Potassium (mEq/L) | 4 (3.7–4.3) | 4 (3.5–4.3) | 0.273 |
| Calcium (mg/dL) | 8.4 (8–8.9) | 8.4 (7.9–8.7) | 0.166 |
| Glucose (mg/dL) | 107 (92–138) | 113 (95–146) | 0.165 |
| Creatinine (mg/dL) | 0.9 (0.7–1.1) | 0.9 (0.7–1.3) | 0.299 |
| Ammonia (μg/dL) | 95 (64–147) | 100 (67–156) | 0.392 |
| Group | Non-AKI | AKI | p |
|---|---|---|---|
| n | 500 | 148 | |
| Surgical duration (min) | 495 (440–560) | 506 (456–584) | 0.103 |
| Postreperfusion syndrome | 256 (51.2%) | 87 (58.8%) | 0.104 |
| Average of vital signs | |||
| MBP (mmHg) | 75 (70–82) | 76 (68–85) | 0.520 |
| HR (beats/min) | 89 (80–99) | 93 (83–102) | 0.037 |
| CVP (mmHg) | 9 (7.3–11) | 9.3 (7–11.5) | 0.689 |
| Blood product transfusion (unit) | |||
| Packed red blood cells | 7 (4–13) | 10 (6–16) | <0.001 |
| Fresh frozen plasma | 6 (4–10) | 10 (6–12) | <0.001 |
| Platelet concentrate | 4 (0–8) | 6 (0–12) | 0.009 |
| Cryoprecipitate | 0 (0–0) | 0 (0–0) | <0.001 |
| Blood loss (L) | 2.9 (2.2–3.8) | 3.1 (2.5–4.5) | 0.004 |
| Hourly fluid infusion (mL/kg/h) | 10.5 (8.2–14.1) | 11.1 (8.2–15) | 0.273 |
| Hourly urine output (mL/kg/h) | 1.6 (0.8–2.4) | 0.9 (0.6–1.6) | <0.001 |
| Donor-graft finding | |||
| Age (years) | 35 (26–42) | 35 (26–40) | 0.751 |
| Sex (male) | 310 (62%) | 93 (63%) | 0.854 |
| GRWR (%) | 1.2 (1.0–1.5) | 1.2 (1.1–1.5) | 0.204 |
| Graft ischemic time (min) | 87 (68–105) | 96 (73–106) | 0.041 |
| Fatty change (%) | 5 (1–5) | 4 (0–5) | 0.596 |
| Univariate Analysis | Multivariate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| β | Odds Ratio | 95% CI | p | β | Odds Ratio | 95% CI | p | |
| Preoperative recipient factor | ||||||||
| Age (years) | −0.003 | 0.997 | 0.978–1.016 | 0.748 | ||||
| Sex (male vs. female) | −0.265 | 0.767 | 0.509–1.158 | 0.207 | ||||
| Body mass index (kg/m2) | 0.044 | 1.045 | 0.998–1.093 | 0.061 | ||||
| Comorbidity | ||||||||
| Diabetes mellitus | 0.438 | 1.550 | 1.040–2.310 | 0.031 | 0.433 | 1.541 | 1.003–2.368 | 0.048 |
| Hypertension | 0.046 | 1.047 | 0.666–1.645 | 0.843 | ||||
| Alcoholic liver cirrhosis | 0.378 | 1.459 | 0.944–2.254 | 0.089 | ||||
| MELD score (point) | 0.018 | 1.018 | 1.001–1.035 | 0.033 | −0.027 | 0.974 | 0.952–0.996 | 0.021 |
| Hepatic decompensation | ||||||||
| Varix | 0.260 | 1.297 | 0.856–1.967 | 0.220 | ||||
| Ascites | 0.481 | 1.617 | 1.117–2.341 | 0.011 | ||||
| Cardiac function | ||||||||
| Ejection fraction (%) | 0.029 | 1.029 | 0.988–1.073 | 0.173 | ||||
| Diastolic dysfunction | 0.161 | 1.175 | 0.811–1.702 | 0.395 | ||||
| Laboratory variables | ||||||||
| Hematocrit (%) | −0.041 | 0.960 | 0.933–0.988 | 0.005 | ||||
| WBC count (×109/L) | 0.014 | 1.015 | 0.985–1.046 | 0.318 | ||||
| Albumin (g/dL) | −0.610 | 0.543 | 0.392–0.753 | <0.001 | ||||
| Platelet count (×109/L) | −0.008 | 0.992 | 0.988–0.996 | 0.001 | −0.005 | 0.995 | 0.991–1.000 | 0.033 |
| International normalized ratio | 0.094 | 1.098 | 0.885–1.363 | 0.396 | ||||
| Total bilirubin | 0.015 | 1.015 | 1.000–1.031 | 0.056 | ||||
| High DFR (>1.05) | 1.661 | 5.267 | 3.082–9.001 | <0.001 | 1.391 | 4.020 | 2.230–7.247 | <0.001 |
| Comparative factors * High D-Dimer (>0.5 mg/L) | 1.445 | 4.243 | 1.919–9.381 | <0.001 | 0.834 | 2.302 | 0.999–5.303 | 0.050 |
| Low fibrinogen (<160 mg/dL) | 0.742 | 2.099 | 1.442–3.055 | <0.001 | 0.402 | 1.494 | 0.988–2.260 | 0.057 |
| Sodium (mEq/L) | −0.023 | 0.977 | 0.945–1.009 | 0.163 | ||||
| Potassium (mEq/L) | −0.157 | 0.854 | 0.625–1.167 | 0.323 | ||||
| Calcium (mg/dL) | −0.153 | 0.859 | 0.670–1.101 | 0.229 | ||||
| Glucose (mg/dL) | 0.001 | 1.001 | 0.998–1.005 | 0.408 | ||||
| Creatinine (mg/dL) | −0.116 | 0.890 | 0.744–1.065 | 0.205 | ||||
| Ammonia (μg/dL) | 0.001 | 1.001 | 0.999–1.003 | 0.325 | ||||
| Intraoperative recipient factor | ||||||||
| Surgical duration (min) | 0.001 | 1.001 | 0.999–1.003 | 0.105 | ||||
| Postreperfusion syndrome | 0.307 | 1.359 | 0.938–1.971 | 0.105 | ||||
| Average of vital signs | ||||||||
| MBP (mmHg) | 0.002 | 1.002 | 0.996–1.008 | 0.468 | ||||
| HR (beats/min) | 0.014 | 1.015 | 1.001–1.028 | 0.031 | 0.013 | 1.013 | 0.999–1.027 | 0.069 |
| CVP (mmHg) | 0.023 | 1.024 | 0.969–1.082 | 0.404 | ||||
| Blood product transfusion (unit) | ||||||||
| Packed red blood cells | 0.032 | 1.032 | 1.012–1.053 | 0.001 | ||||
| Fresh frozen plasma | 0.032 | 1.0333 | 1.008–1.058 | 0.008 | ||||
| Platelet concentrate | 0.002 | 1.002 | 0.990–1.014 | 0.769 | ||||
| Cryoprecipitate | 0.156 | 1.169 | 1.081–1.264 | <0.001 | 0.013 | 1.111 | 1.022–1.208 | 0.013 |
| Blood loss (L) | 0.107 | 1.113 | 1.017–1.218 | 0.020 | 0.054 | 1.055 | 0.986–1.129 | 0.121 |
| Hourly fluid infusion (mL/kg/h) | 0.012 | 1.012 | 0.995–1.030 | 0.159 | ||||
| Hourly urine output (mL/kg/h) | −0.390 | 0.677 | 0.562–0.816 | <0.001 | −0.358 | 0.699 | 0.551–0.889 | 0.003 |
| Donor-graft factor | ||||||||
| Age (years) | −0.005 | 0.995 | 0.979–1.012 | 0.586 | ||||
| Sex (male) | −0.036 | 0.965 | 0.660–1.410 | 0.854 | ||||
| GRWR (%) | 0.179 | 1.196 | 0.836–1.710 | 0.327 | ||||
| Graft ischemic time (min) | 0.008 | 1.008 | 1.000–1.015 | 0.030 | 0.007 | 1.007 | 1.000–1.015 | 0.062 |
| Fatty change (%) | 0.008 | 1.006 | 0.981–1.035 | 0.572 | ||||
| AUC | 95% CI | p | |
|---|---|---|---|
| High FDR (>1.05) | 0.646 | 0.607–0.682 | <0.001 |
| High D-Dimer (>0.5 mg/L) | 0.584 | 0.545–0.622 | <0.001 |
| Low fibrinogen (<160 mg/dL) | 0.591 | 0.552–0.630 | <0.001 |
| Group | Before Propensity Score-Matched Analysis | After Propensity Score-Matched Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| High DFR | Low DFR | p | SD | High DFR | Low DFR | p | SD | |
| n | 428 | 220 | 116 | 116 | ||||
| Preoperative finding | ||||||||
| Age (years) | 53 (47–59) | 55 (50–60) | 0.005 | −0.173 | 53 (49–60) | 60 (54–64) | 0.528 | 0.009 |
| Sex (male) | 281 (65.7%) | 169 (76.8%) | 0.003 | −0.235 | 86 (74.1%) | 79 (68.1%) | 0.311 | 0.127 |
| Body mass index (kg/m2) | 24 (22–27) | 24 (22–26) | 0.215 | 0.150 | 24 (22–26) | 24 (22–26) | 0.723 | −0.02 |
| Diabetes mellitus | 112 (26.2%) | 58 (26.4%) | 0.957 | −0.004 | 33 (28.4%) | 29 (25.0%) | 0.553 | 0.078 |
| Hypertension | 74 (17.3%) | 58 (26.4%) | 0.007 | −0.240 | 25 (21.6%) | 24 (20.7%) | 0.872 | 0.023 |
| MELD | 18 (10–27) | 6 (4–12) | <0.001 | 0.918 | 13 (6–21) | 11 (6–18) | 0.351 | 0.023 |
| Varix | 118 (27.6%) | 37 (16.8%) | 0.002 | 0.240 | 25 (21.6%) | 30 (25.9%) | 0.440 | −0.096 |
| Ascites | 259 (60.5%) | 49 (22.3%) | <0.001 | 0.781 | 53 (45.7%) | 46 (39.7%) | 0.353 | 0.123 |
| Ejection fraction | 64 (62–67) | 64 (62–66) | 0.173 | 0.075 | 64 (63–67) | 64 (62–66) | 0.302 | 0.048 |
| Diastolic dysfunction | 176 (41.1%) | 89 (40.5%) | 0.870 | 0.014 | 44 (37.9%) | 44 (37.9%) | 1.000 | <0.001 |
| Laboratory variables | ||||||||
| Hematocrit (%) | 27 (24–32) | 34 (29–39) | <0.001 | −0.878 | 30 (26–36) | 30 (25–35) | 0.517 | 0.102 |
| White blood cell count (×109/L) | 4.8 (3.0–8.9) | 4.0 (2.7–5.1) | <0.001 | 0.370 | 4.5 (2.8–8.0) | 3.7 (2.5–5.8) | 0.005 | 0.177 |
| Albumin (g/dL) | 2.9 (2.6–3.3) | 3.4 (3.0–3.9) | <0.001 | −0.890 | 3.1 (2.7–3.5) | 3.1 (2.7–3.4) | 0.775 | 0.047 |
| Platelet count (×109/L) | 57 (41–82) | 90 (60–132) | <0.001 | −0.652 | 67 (46–109) | 73 (49–103) | 0.548 | 0.093 |
| Sodium (mEq/L) | 138 (134–141) | 141 (139–142) | <0.001 | −0.512 | 140 (136–142) | 140 (136–142) | 0.584 | −0.062 |
| Potassium (mEq/L) | 4 (3.6–4.4) | 4 (3.7–4.3) | 0.898 | 0.041 | 4.0 (3.6–4.3) | 4.0 (3.7–4.3) | 0.955 | 0.049 |
| Calcium (mEq/L) | 8.4 (7.9–8.8) | 8.5 (8.1–8.9) | 0.002 | −0.112 | 8.4 (8.0–8.8) | 8.3 (7.9–8.8) | 0.421 | 0.062 |
| Glucose (mg/dL) | 114 (93–148) | 103 (91–126) | 0.003 | 0.237 | 107 (92–138) | 106 (93–139) | 0.895 | 0.026 |
| Urea nitrogen | 18 (12–34) | 13 (11–16) | <0.001 | 0.463 | 16 (10–25) | 14 (11–17) | 0.294 | 0.120 |
| Creatinine (mg/dL) | 0.9 (0.6–1.5) | 0.8 (0.7–1.0) | 0.015 | 0.251 | 0.8 (0.6–1.2) | 0.8 (0.7–1.0) | 0.774 | 0.096 |
| Total bilirubin | 4.4 (1.4–18.1) | 1.0 (0.6–2.4) | <0.001 | 0.566 | 1.9 (0.9–7.1) | 1.7 (0.9–6.8) | 0.688 | 0.008 |
| Ammonia | 100 (68–162) | 87 (62–136) | 0.006 | 0.254 | 100 (65–152) | 113 (66–162) | 0.633 | 0.089 |
| INR | 1.7 (1.4–2.3) | 1.2(1.1–1.4 | <0.001 | 0.691 | 1.5 (1.2–1.9) | 1.4 (1.2–1.6) | 0.271 | 0.193 |
| Intraoperative finding | ||||||||
| Total surgery duration (min) | 503 (450–570) | 490 (435–540) | 0.094 | 0.078 | 505 (450–595) | 500 (436–579) | 0.428 | 0.089 |
| Postreperfusion syndrome | 236 (55.1%) | 107 (48.6%) | 0.116 | 0.131 | 57 (49.1%) | 56 (48.3%) | 0.895 | 0.017 |
| Average vital signs | ||||||||
| MBP (mmHg) | 76 (70–84) | 75 (69–82) | 0.434 | 0.094 | 75 (69–83) | 77 (70–83) | 0.512 | −0.001 |
| HR (beats/min) | 91 (80–102) | 88 (80–97) | 0.031 | 0.160 | 91 (80–98) | 87 (79–97) | 0.212 | 0.164 |
| CVP (mmHg) | 9 (8–12) | 9 (7–11) | 0.001 | 0.295 | 9 (7–11) | 9 (7–11) | 0.734 | 0.085 |
| Blood product transfusion (unit) | ||||||||
| Packed red blood cells | 10 (6–16) | 4 (2–8) | <0.001 | 0.698 | 9 (5–12) | 5 (4–8) | 0.004 | 0.169 |
| Fresh frozen plasma | 10 (6–13) | 4 (3–6) | <0.001 | 0.757 | 8 (5–10) | 6 (4–10) | <0.001 | 0.135 |
| Platelet concentrate | 6 (0–12) | 0 (0–5) | <0.001 | 0.394 | 5 (0–10) | 0 (0–6) | 0.034 | 0.129 |
| Hourly fluid infusion (mL/kg/h) | 10.9 (8.1–15.2) | 10.4 (8.3–13.4) | 0.185 | 0.156 | 11.0 (7.7–14.3) | 10.4 (7.9–14.0) | 0.893 | 0.026 |
| Hourly urine output (mL/kg/h) | 1.07 (0.6–1.9) | 1.9 (1.2–2.8) | <0.001 | −0.806 | 1.4 (0.8–2.4) | 1.6 (0.9–2.4) | 0.557 | −0.075 |
| Donor-graft finding | ||||||||
| Age (years) | 35 (26–41) | 34 (26–44) | 0.970 | −0.032 | 35 (27–45) | 34 (26–44) | 0.866 | 0.013 |
| Sex (male) | 271 (63.3%) | 132 (60.0%) | 0.410 | −0.069 | 72 (62.1%) | 69 (59.5%) | 0.687 | −0.054 |
| GRWR (%) | 1.2 (1.0–1.5) | 1.2 (1.1–1.6) | 0.582 | −0.039 | 1.3 (1.0–1.5) | 1.2 (1.0–1.5) | 0.725 | −0.036 |
| Graft ischemic time (min) | 91 (69–107) | 87 (69–100) | 0.166 | 0.128 | 83 (66–100) | 85 (69–100) | 0.653 | −0.049 |
| Fatty change (%) | 5 (1–5) | 4 (0–5) | 0.010 | 0.089 | 5 (1–5), | 5 (1–5) | 0.873 | 0.005 |
| ß | Odds Ratio | 95% CI | p | |
|---|---|---|---|---|
| In the entire study population (n = 648) | ||||
| High FDR (vs. low FDR) | 1.661 | 5.267 | 3.082–9.001 | <0.001 |
| In the PS-matched study population (n = 232) | ||||
| High FDR (vs. low FDR) adjusted for PS | 1.401 | 4.059 | 1.988–8.288 | <0.001 |
| Group | Low DFR (<1.05) | High DFR (>1.05) | p |
|---|---|---|---|
| n | 116 | 116 | <0.001 |
| Non-AKI | 104 (57.1%) | 78 (42.9%) | |
| AKI stage 1 | 9 (25.0%) | 27 (75.0%) | |
| AKI stages 2–3 | 3 (21.4%) | 11 (78.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Kim, M.; Kim, J.-W.; Choi, H.J.; Hong, S.H. Predictive Value of the D-Dimer-to-Fibrinogen Ratio for Acute Kidney Injury after Living-Donor Liver Transplantation: A Retrospective Observational Cohort Study Using Logistic Regression and Propensity Score Matching Analyses. J. Clin. Med. 2024, 13, 5499. https://doi.org/10.3390/jcm13185499
Park J, Kim M, Kim J-W, Choi HJ, Hong SH. Predictive Value of the D-Dimer-to-Fibrinogen Ratio for Acute Kidney Injury after Living-Donor Liver Transplantation: A Retrospective Observational Cohort Study Using Logistic Regression and Propensity Score Matching Analyses. Journal of Clinical Medicine. 2024; 13(18):5499. https://doi.org/10.3390/jcm13185499
Chicago/Turabian StylePark, Jaesik, Minju Kim, Jong-Woan Kim, Ho Joong Choi, and Sang Hyun Hong. 2024. "Predictive Value of the D-Dimer-to-Fibrinogen Ratio for Acute Kidney Injury after Living-Donor Liver Transplantation: A Retrospective Observational Cohort Study Using Logistic Regression and Propensity Score Matching Analyses" Journal of Clinical Medicine 13, no. 18: 5499. https://doi.org/10.3390/jcm13185499
APA StylePark, J., Kim, M., Kim, J.-W., Choi, H. J., & Hong, S. H. (2024). Predictive Value of the D-Dimer-to-Fibrinogen Ratio for Acute Kidney Injury after Living-Donor Liver Transplantation: A Retrospective Observational Cohort Study Using Logistic Regression and Propensity Score Matching Analyses. Journal of Clinical Medicine, 13(18), 5499. https://doi.org/10.3390/jcm13185499






