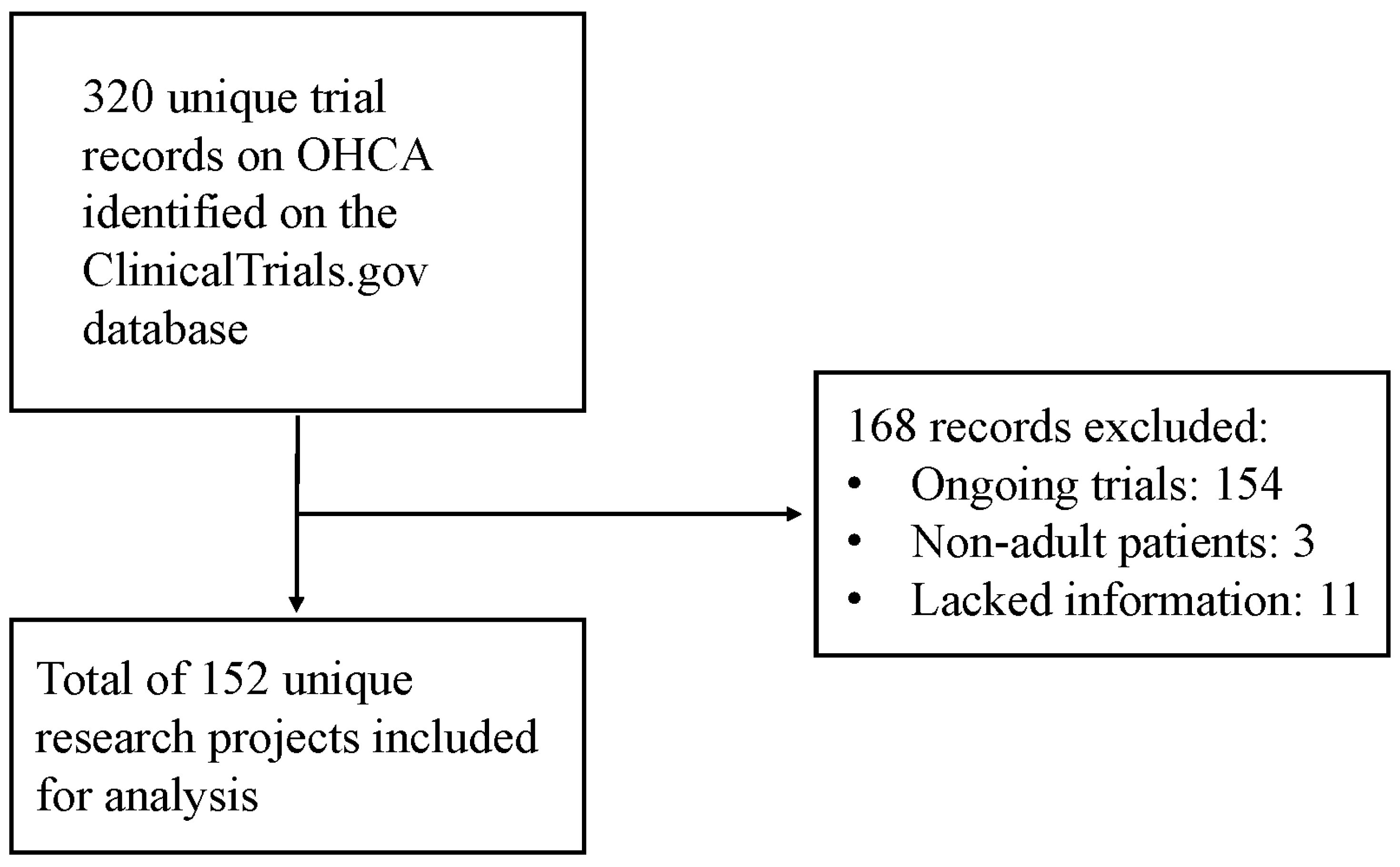
Characteristics of Out-of-Hospital Cardiac Arrest Trials Registered in ClinicalTrials.gov
Abstract
1. Introduction
2. Materials and Methods
2.1. Analysis of Data Source, Study Selection and Labeling, Ethical Considerations
2.2. Extraction of Clinical Trials’ Characteristics
2.3. Statistical Analysis
3. Results
3.1. General Characteristics of the OHCA Clinical Trials and Trends over Time
3.2. Characteristics of OHCA Interventional and Observational Trials over Time
3.2.1. Publication Characteristics
3.2.2. Comparison of Single- vs. Multi-Site Study Characteristics
4. Discussion
Study Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gräsner, J.T.; Herlitz, J.; Tjelmeland, I.B.M.; Wnent, J.; Masterson, S.; Lilja, G.; Bein, B.; Böttiger, B.W.; Rosell-Ortiz, F.; Nolan, J.P.; et al. European Resuscitation Council Guidelines 2021: Epidemiology of cardiac arrest in Europe. Resuscitation 2021, 161, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [PubMed]
- Committee on the Treatment of Cardiac Arrest: Current Status and Future Directions; Board on Health Sciences Policy; Institute of Medicine. Graham, R., McCoy, M.A., Schultz, A.M., Eds.; Strategies to Improve Cardiac Arrest Survival: A Time to Act; National Academies Press: Washington, DC, USA, 2015. [Google Scholar]
- Nishiyama, C.; Kiguchi, T.; Okubo, M.; Alihodžić, H.; Al-Araji, R.; Baldi, E.; Beganton, F.; Booth, S.; Bray, J.; Christensen, E.; et al. Three-year trends in out-of-hospital cardiac arrest across the world: Second report from the International Liaison Committee on Resuscitation (ILCOR). Resuscitation 2023, 186, 109757. [Google Scholar] [CrossRef] [PubMed]
- Kiguchi, T.; Okubo, M.; Nishiyama, C.; Maconochie, I.; Ong, M.E.H.; Kern, K.B.; Wyckoff, M.H.; McNally, B.; Christensen, E.F.; Tjelmeland, I.; et al. Out-of-hospital cardiac arrest across the World: First report from the International Liaison Committee on Resuscitation (ILCOR). Resuscitation 2020, 152, 39–49. [Google Scholar] [CrossRef] [PubMed]
- May, M. Twenty-five ways clinical trials have changed in the last 25 years. Nat. Med. 2019, 25, 2–5. [Google Scholar] [CrossRef]
- Penna, A.; Magliocca, A.; Merigo, G.; Stirparo, G.; Silvestri, I.; Fumagalli, F.; Ristagno, G. One-Year Review in Cardiac Arrest: The 2022 Randomized Controlled Trials. J. Clin. Med. 2023, 12, 2235. [Google Scholar] [CrossRef]
- Ubben, J.F.H.; Heuts, S.; Delnoij, T.S.R.; Suverein, M.M.; van de Koolwijk, A.F.; van der Horst, I.C.C.; Maessen, J.G.; Bartos, J.; Kavalkova, P.; Rob, D.; et al. Extracorporeal cardiopulmonary resuscitation for refractory OHCA: Lessons from three randomized controlled trials-the trialists’ view. Eur. Heart J. Acute Cardiovasc. Care 2023, 12, 540–547. [Google Scholar] [CrossRef]
- Tasneem, A.; Aberle, L.; Ananth, H.; Chakraborty, S.; Chiswell, K.; McCourt, B.J.; Pietrobon, R. The database for aggregate analysis of ClinicalTrials.gov (AACT) and subsequent regrouping by clinical specialty. PLoS ONE 2012, 7, e33677. [Google Scholar] [CrossRef]
- Clinical Trials.gov. Clinical Trials.gov Protocol Registration Data Element Definitions for Interventional and Observational Studies. 2019. Available online: https://prsinfo.clinicalTrials.gov/definitions.html (accessed on 1 January 2024).
- Califf, R.M.; Zarin, D.A.; Kramer, J.M.; Sherman, R.E.; Aberle, L.H.; Tasneem, A. Characteristics of clinical trials registered in ClinicalTrials.gov, 2007–2010. JAMA 2012, 307, 1838–1847. [Google Scholar] [CrossRef]
- Buffel du Vaure, C.; Dechartres, A.; Battin, C.; Ravaud, P.; Boutron, I. Exclusion of patients with concomitant chronic conditions in ongoing randomised controlled trials targeting 10 common chronic conditions and registered at ClinicalTrials.gov: A systematic review of registration details. BMJ Open 2016, 6, e012265. [Google Scholar] [CrossRef]
- Tham, T.Y.; Tran, T.L.; Prueksaritanond, S.; Isidro, J.S.; Setia, S.; Welluppillai, V. Integrated health care systems in Asia: An urgent necessity. Clin. Interv. Aging 2018, 13, 2527–2538. [Google Scholar] [CrossRef] [PubMed]
- Sibbald, B.; Roland, M. Understanding controlled trials. Why are randomised controlled trials important? BMJ 1998, 316, 201. [Google Scholar] [CrossRef] [PubMed]
- Califf, R.M.; DeMets, D.L. Principles from clinical trials relevant to clinical practice: Part I. Circulation 2002, 106, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, C.D.; Drazen, J.M.; Frizelle, F.A.; Haug, C.; Hoey, J.; Horton, R.; Kotzin, S.; Laine, C.; Marusic, A.; Overbeke, A.J.; et al. Is this clinical trial fully registered? A statement from the International Committee of Medical Journal Editors. Ann. Intern. Med. 2005, 143, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Zarin, D.A.; Tse, T.; Williams, R.J.; Rajakannan, T. Update on Trial Registration 11 Years after the ICMJE Policy Was Established. N. Engl. J. Med. 2017, 376, 383–391. [Google Scholar] [CrossRef]
- Chen, Y.P.; Liu, X.; Lv, J.W.; Li, W.F.; Zhang, Y.; Guo, Y.; Lin, A.H.; Sun, Y.; Mao, Y.P.; Ma, J. Publication status of contemporary oncology randomised controlled trials worldwide. Eur. J. Cancer 2016, 66, 17–25. [Google Scholar] [CrossRef]
- Ross, J.S.; Tse, T.; Zarin, D.A.; Xu, H.; Zhou, L.; Krumholz, H.M. Publication of NIH funded trials registered in ClinicalTrials.gov: Cross sectional analysis. BMJ 2012, 344, d7292. [Google Scholar] [CrossRef]
- Blumenthal, D.; Campbell, E.G.; Anderson, M.S.; Causino, N.; Louis, K.S. Withholding research results in academic life science. Evidence from a national survey of faculty. JAMA 1997, 277, 1224–1228. [Google Scholar] [CrossRef]
- Easterbrook, P.J.; Berlin, J.A.; Gopalan, R.; Matthews, D.R. Publication bias in clinical research. Lancet 1991, 337, 867–872. [Google Scholar] [CrossRef]
- Angell, M. Industry-sponsored clinical research: A broken system. JAMA 2008, 300, 1069–1071. [Google Scholar] [CrossRef]
- Elliott, D.B. Industry-funded research bias and conflicts of interest. Ophthalmic Physiol. Opt. 2013, 33, 1. [Google Scholar] [CrossRef] [PubMed]
- Zarin, D.A.; Tse, T.; Williams, R.J.; Califf, R.M.; Ide, N.C. The ClinicalTrials.gov results database--update and key issues. N. Engl. J. Med. 2011, 364, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Tang, L.L.; Le, Q.T.; Chua, M.L.K.; Wee, J.T.S.; Lee, N.Y.; O’Sullivan, B.; Lee, A.W.M.; Sun, Y.; et al. Characteristics of Radiotherapy Trials Compared With Other Oncological Clinical Trials in the Past 10 Years. JAMA Oncol. 2018, 4, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, B.; Zheng, Q.; Xu, J.; Li, J.; Lai, F.; Lin, B.; Peng, S.; Lv, W.; Xiao, H. The Current Landscape of Clinical Studies Focusing on Thyroid Cancer: A Comprehensive Analysis of Study Characteristics and Their Publication Status. Front. Endocrinol. 2020, 11, 575799. [Google Scholar] [CrossRef]

| Overall | Interventional | Observational | p-Value | |
|---|---|---|---|---|
| (N = 152) | (N = 107) | (N = 45) | ||
| Status, n (%) | 0.248 | |||
| Completed | 137 (90.1) | 94 (87.9) | 43 (95.6) | |
| Terminated | 15 (9.9) | 13 (12.1) | 2 (4.4) | |
| Randomized trial, n (%) | ||||
| No | 69 (45.4) | 24 (22.4) | 45 (100.0) | <0.001 |
| Yes | 83 (54.6) | 83 (77.6) | 0 (0.0) | |
| Availability of results, n (%) * | 0.006 | |||
| No | 128 (84.2) | 84 (78.5) | 44 (97.8) | |
| Yes | 24 (15.8) | 23 (21.5) | 1 (2.2) | |
| Publication status, n (%) † | 0.113 | |||
| No | 58 (38.2) | 36 (33.6) | 22 (48.9) | |
| Yes | 94 (61.8) | 71 (66.4) | 23 (51.1) | |
| Primary focus, n (%) | <0.001 | |||
| Procedures | 63 (41.4) | 46 (43.0) | 17 (37.8) | |
| Devices | 35 (23.0) | 26 (24.3) | 9 (20.0) | |
| Drugs | 22 (14.5) | 22 (20.6) | 0 (0.0) | |
| Diagnostic | 10 (6.6) | 4 (3.7) | 6 (13.3) | |
| Biomarkers | 9 (5.9) | 2 (1.9) | 7 (15.6) | |
| Other | 13 (8.6) | 7 (6.5) | 6 (13.3) | |
| Median enrollment size [IQR], n | 150 [57.75, 724] | 200 [60, 801] | 112 [50, 460] | 0.235 |
| Planned sample size ≥100 patients, n (%) | 0.551 | |||
| Yes | 95 (62.5) | 69 (64.5) | 26 (57.8) | |
| No | 57 (37.5) | 38 (35.5) | 19 (42.2) | |
| Median follow-up [IQR], days | 10 [0, 180] | 12 [0, 180] | 5 [0, 210] | 0.547 |
| Follow-up categories, n (%) | 0.013 | |||
| 0–30 days | 116 (76.3) | 85 (79.4) | 31 (68.9) | |
| 31–365 days | 12 (7.9) | 4 (3.7) | 8 (17.8) | |
| >365 days | 24 (15.8) | 18 (16.8) | 6 (13.3) | |
| Funder type, n (%) | 0.651 | |||
| Academic/university | 122 (80.3) | 87 (81.3) | 35 (77.8) | |
| Government | 18 (11.8) | 11 (10.3) | 7 (15.6) | |
| Industry | 10 (6.6) | 7 (6.5) | 3 (6.7) | |
| No-profit | 2 (1.3) | 2 (1.9) | 0 (0.0) | |
| Median start date [IQR], year | 2016 [2012, 2018] | 2015 [2011, 2017.50] | 2016 [2013, 2018] | 0.150 |
| Study period, n (%) | 0.338 | |||
| Early | 23 (15.1) | 18 (16.8) | 5 (11.1) | |
| Interim | 71 (46.7) | 52 (48.6) | 19 (42.2) | |
| Late | 58 (38.2) | 37 (34.6) | 21 (46.7) | |
| Median number of sites involved [IQR], n | 1 [1, 2] | 1 [1, 2.25] | 1 [1, 1.50] | 0.209 |
| Participating sites, n (%) | 0.203 | |||
| Single-center | 93 (61.2) | 64 (59.8) | 29 (64.4) | |
| Multi-center | 46 (30.3) | 36 (33.6) | 10 (22.2) | |
| N/A | 13 (8.6) | 7 (6.5) | 6 (13.3) | |
| Site location, n (%) | 0.092 | |||
| Europe | 92 (60.5) | 59 (55.1) | 33 (73.3) | |
| Outside of Europe | 52 (34.2) | 41 (38.3) | 11 (24.4) | |
| Both within and outside of Europe | 6 (3.9) | 6 (5.6) | 0 (0.0) | |
| N/A | 2 (1.3) | 1 (0.9) | 1 (2.2) | |
| Chairman’s office—primary location (country-detailed), n (%) | 0.272 | |||
| France | 25 (16.4) | 16 (15.0) | 9 (20.0) | |
| United States | 24 (15.8) | 20 (18.7) | 4 (8.9) | |
| Korea, Republic of | 18 (11.8) | 14 (13.1) | 4 (8.9) | |
| Sweden | 16 (10.5) | 12 (11.2) | 4 (8.9) | |
| Denmark | 11 (7.2) | 8 (7.5) | 3 (6.7) | |
| Norway | 6 (3.9) | 4 (3.7) | 2 (4.4) | |
| Slovenia | 6 (3.9) | 2 (1.9) | 4 (8.9) | |
| Taiwan | 6 (3.9) | 5 (4.7) | 1 (2.2) | |
| Canada | 5 (3.3) | 4 (3.7) | 1 (2.2) | |
| Finland | 4 (2.6) | 2 (1.9) | 2 (4.4) | |
| Germany | 4 (2.6) | 3 (2.8) | 1 (2.2) | |
| Spain | 4 (2.6) | 4 (3.7) | 0 (0.0) | |
| Netherlands | 4 (2.6) | 3 (2.8) | 1 (2.2) | |
| Italy | 3 (2.0) | 1 (0.9) | 2 (4.4) | |
| United Kingdom | 3 (2.0) | 0 (0.0) | 3 (6.7) | |
| Australia | 2 (1.3) | 2 (1.9) | 0 (0.0) | |
| Belgium | 2 (1.3) | 2 (1.9) | 0 (0.0) | |
| Other | 9 (5.9) | 5 (5.7) | 4 (8.9) | |
| Chairman’s office—primary location (continent) (%) | 0.104 | |||
| Europe | 97 (63.8) | 62 (57.9) | 35 (77.8) | |
| North America | 27 (17.8) | 23 (21.5) | 4 (8.9) | |
| Asia | 26 (17.1) | 20 (18.7) | 6 (13.3) | |
| Oceania | 2 (1.3) | 2 (1.9) | 0 (0.0) |
| Early Period | Interim Period | Late Period | Early Period | Interim Period | Late Period | p-Value | |
|---|---|---|---|---|---|---|---|
| (N = 18) | (N = 52) | (N = 37) | (N = 5) | (N = 19) | (N = 21) | ||
| Interventional Trials | Observational Trials | ||||||
| Trial status, n (%) | 0.337 | ||||||
| Completed | 16 (88.9) | 47 (90.4) | 31 (83.8) | 5 (100) | 19 (100) | 19 (90.5) | |
| Terminated | 2 (11.1) | 5 (9.6) | 6 (16.2) | 0 (0) | 0 (0) | 2 (9.5) | |
| Randomized trial, n (%) | |||||||
| No | 3 (16.7) | 13 (25.0) | 8 (21.6) | 5 (100.0) | 19 (100.0) | 21 (100.0) | 0.759 |
| Yes | 15 (83.3) | 39 (75.0) | 29 (78.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Published as of 31 December 2023, n (%) | 0.363 | ||||||
| No | 3 (16.7) | 18 (34.6) | 15 (40.5) | 3 (60.0) | 8 (42.1) | 11 (52.4) | |
| Yes | 15 (83.3) | 34 (65.4) | 22 (59.5) | 2 (40.0) | 11 (57.9) | 10 (47.6) | |
| Trial status and publication, n (%) | 0.594 | ||||||
| Completed and published | 13 (72.2) | 31 (59.6) | 18 (48.6) | 2 (40.0) | 11 (57.9) | 10 (47.6) | |
| Terminated and published | 2 (11.1) | 3 (5.8) | 4 (10.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Completed and unpublished | 3 (16.7) | 16 (30.8) | 13 (35.1) | 3 (60.0) | 8 (42.1) | 9 (42.9) | |
| Uncompleted and unpublished | 0 (0.0) | 2 (3.8) | 2 (5.4) | 0 (0.0) | 0 (0.0) | 2 (9.5) | |
| Availability of results, n (%) | 0.474 | ||||||
| No | 14 (77.8) | 39 (75.0) | 31 (83.8) | 5 (100.0) | 18 (94.7) | 21 (100.0) | |
| Yes | 4 (22.2) | 13 (25.0) | 6 (16.2) | 0 (0.0) | 1 (5.3) | 0 (0.0) | |
| Primary focus, n (%) | 0.256 | ||||||
| Procedures | 7 (38.9) | 28 (53.8) | 11 (29.7) | 1 (20.0) | 7 (36.8) | 9 (42.9) | |
| Devices | 5 (27.8) | 9 (17.3) | 12 (32.4) | 1 (20.0) | 4 (21.1) | 4 (19.0) | |
| Drugs | 5 (27.8) | 9 (17.3) | 8 (21.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Biomarkers | 1 (5.6) | 1 (1.9) | 0 (0.0) | 2 (40.0) | 3 (15.8) | 2 (9.5) | |
| Diagnostic test | 0 (0.0) | 1 (1.9) | 3 (8.1) | 0 (0.0) | 2 (10.5) | 4 (19) | |
| Other | 0 (0.0) | 4 (7.7) | 3 (8.1) | 1 (20.0) | 3 (15.8) | 2 (9.5) | |
| Median enrollment size [IQR], n | 685 [299.25, 1579.50] | 238.50 [85.50, 625] | 65 [34, 397] | 1662 [350, 2332] | 112 [40.5, 232] | 100 [50, 460] | 0.495 |
| Planned sample size ≥100 patients, n (%) | 0.005 | ||||||
| No | 2 (11.1) | 16 (30.8) | 20 (54.1) | 1 (20.0) | 8 (42.1) | 10 (47.6) | |
| Yes | 16 (88.9) | 36 (69.2) | 17 (45.9) | 4 (80.0) | 11 (57.9) | 11 (52.4) | |
| Median follow-up duration [IQR], days | 28 [1, 90] | 28 [0.50, 180] | 3 [0, 105] | 4 [2.5, 184.5] | 5 [0, 180] | 28 [0, 365] | 0.099 |
| Follow-up categories, n (%) | 0.740 | ||||||
| 0–30 days | 13 (72.2) | 42 (80.8) | 30 (81.1) | 1 (20.0) | 15 (78.9) | 15 (71.4) | |
| 31–365 days | 0 (0.0) | 2 (3.8) | 2 (5.4) | 1 (20.0) | 2 (10.5) | 5 (23.8) | |
| >365 days | 5 (27.8) | 8 (15.4) | 5 (13.5) | 3 (60.0) | 2 (10.5) | 1 (4.8) | |
| Funder type, n (%) | 0.044 | ||||||
| Academic/university | 9 (50.0) | 46 (88.5) | 32 (86.5) | 5 (100.0) | 15 (78.9) | 15 (71.4) | |
| Government | 5 (27.8) | 2 (3.8) | 4 (10.8) | 0 (0.0) | 2 (10.5) | 5 (23.8) | |
| Industry | 4 (22.2) | 3 (5.8) | 0 (0.0) | 0 (0.0) | 2 (10.5) | 1 (4.8) | |
| Non-profit | 0 (0.0) | 1 (1.9) | 1 (2.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Median number of sites [IQR], n | 1 [1, 5] | 1 [1, 3] | 1 [1, 2] | 1 [1, 2.5] | 1 [1, 1.75] | 1 [1, 1] | 0.530 |
| Number of participating sites, n (%) | 0.030 | ||||||
| Single-center | 11 (61.1) | 29 (55.8) | 24 (64.9) | 3 (60.0) | 10 (52.6) | 16 (76.2) | |
| Multi-center | 6 (33.3) | 17 (32.7) | 13 (35.1) | 1 (20.0) | 4 (21.1) | 5 (23.8) | |
| N/A | 1 (5.6) | 6 (11.5) | 0 (0.0) | 1 (20.0) | 5 (26.3) | 0 (0.0) | |
| Participating center’s location, n (%) | 0.083 | ||||||
| Europe | 11 (61.1) | 24 (46.2) | 24 (64.9) | 4 (80.0) | 13 (68.4) | 16 (76.2) | |
| Outside of Europe | 4 (22.2) | 25 (48.1) | 12 (32.4) | 1 (20.0) | 5 (26.3) | 5 (23.8) | |
| Both within and outside of Europe | 3 (16.7) | 2 (3.8) | 1 (2.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| N/A | 0 (0.0) | 1 (1.9) | 0 (0.0) | 0 (0.0) | 1 (5.3) | 0 (0.0) | |
| Chairman’s office—primary location (country-detailed), n (%) | 0.035 | ||||||
| France | 6 (33.3) | 6 (11.5) | 4 (10.8) | 2 (40.0) | 4 (21.1) | 3 (14.3) | |
| United States | 5 (27.8) | 9 (17.3) | 6 (16.2) | 0 (0.0) | 2 (10.5) | 2 (9.5) | |
| Korea, Republic of | 0 (0.0) | 11 (21.2) | 3 (8.1) | 0 (0.0) | 2 (10.5) | 2 (9.5) | |
| Sweden | 0 (0.0) | 6 (11.5) | 6 (16.2) | 0 (0.0) | 1 (5.3) | 3 (14.3) | |
| Denmark | 0 (0.0) | 2 (3.8) | 6 (16.2) | 0 (0.0) | 1 (5.3) | 2 (9.5) | |
| Norway | 0 (0.0) | 2 (3.8) | 2 (5.4) | 0 (0.0) | 1 (5.3) | 1 (4.8) | |
| Slovenia | 0 (0.0) | 1 (1.9) | 1 (2.7) | 0 (0.0) | 2 (10.5) | 2 (9.5) | |
| Taiwan | 2 (11.1) | 1 (1.9) | 2 (5.4) | 0 (0.0) | 0 (0.0) | 1 (4.8) | |
| Canada | 0 (0.0) | 3 (5.8) | 1 (2.7) | 1 (20.0) | 0 (0.0) | 0 (0.0) | |
| Finland | 1 (5.6) | 1 (1.9) | 0 (0.0) | 0 (0.0) | 1 (5.3) | 1 (4.8) | |
| Germany | 2 (11.1) | 1 (1.9) | 0 (0.0) | 1 (20.0) | 0 (0.0) | 0 (0.0) | |
| Spain | 1 (5.6) | 3 (5.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Netherlands | 0 (0.0) | 1 (1.9) | 2 (5.4) | 0 (0.0) | 1 (5.3) | 0 (0.0) | |
| Italy | 0 (0.0) | 0 (0.0) | 1 (2.7) | 0 (0.0) | 2 (10.5) | 0 (0.0) | |
| United Kingdom | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (14.3) | |
| Australia | 0 (0.0) | 2 (3.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Belgium | 1 (5.6) | 0 (0.0) | 1 (2.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Other | 0 (0.0) | 3 (5.8) | 2 (5.4) | 1 (20) | 2 (10.5) | 1 (4.8) | |
| Chairman’s office—primary location (continent), n (%) | 0.351 | ||||||
| Europe | 11 (61.1) | 25 (48.1) | 26 (70.3) | 5 (100.0) | 14 (73.7) | 16 (76.2) | |
| North America | 5 (27.8) | 12 (23.1) | 6 (16.2) | 0 (0.0) | 2 (10.5) | 2 (9.5) | |
| Asia | 2 (11.1) | 13 (25.0) | 5 (13.5) | 0 (0.0) | 3 (15.8) | 3 (14.3) | |
| Oceania | 0 (0.0) | 2 (3.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| (a) | ||||
|---|---|---|---|---|
| Overall | Unpublished | Published | p-Value | |
| (N = 107) | (N = 36) | (N = 71) | ||
| Trial status, n (%) | 1.00 | |||
| Completed | 94 (87.9) | 32 (88.9) | 62 (87.3) | |
| Terminated | 13 (12.1) | 4 (11.1) | 9 (12.7) | |
| Randomized trial, n (%) | 0.030 | |||
| No | 24 (22.4) | 13 (36.1) | 11 (15.5) | |
| Yes | 83 (77.6) | 23 (63.9) | 60 (84.5) | |
| Availability of results, n (%) | 0.906 | |||
| No | 84 (78.5) | 29 (80.6) | 55 (77.5) | |
| Yes | 23 (21.5) | 7 (19.4) | 16 (22.5) | |
| Primary focus, n (%) | 0.310 | |||
| Procedures | 46 (43.0) | 15 (41.7) | 31 (43.7) | |
| Devices | 26 (24.3) | 12 (33.3) | 14 (19.7) | |
| Drugs | 22 (20.6) | 4 (11.1) | 18 (25.4) | |
| Diagnostic test | 4 (3.7) | 2 (5.6) | 2 (2.8) | |
| Biomarkers | 2 (1.9) | 0 (0.0) | 2 (2.8) | |
| Other | 7 (6.5) | 3 (8.3) | 4 (5.6) | |
| Median enrollment size [IQR], n | 200 [60, 801] | 73 [46, 544] | 336 [100, 1000] | 0.003 |
| Planned sample size ≥100 patients, n (%) | <0.001 | |||
| No | 38 (35.5) | 21 (58.3) | 17 (23.9) | |
| Yes | 69 (64.5) | 15 (41.7) | 54 (76.1) | |
| Median follow-up duration [IQR], days | 12 [0, 180] | 1.50 [0, 112.50] | 28 [1, 180] | 0.192 |
| Follow-up categories, n (%) | 0.801 | |||
| 0–30 days | 85 (79.4) | 29 (80.6) | 56 (78.9) | |
| 31–365 days | 4 (3.7) | 3 (8.3) | 1 (1.4) | |
| >365 days | 18 (16.8) | 4 (11.1) | 14 (19.7) | |
| Funder type, n (%) | 0.648 | |||
| Academic/university | 87 (81.3) | 31 (86.1) | 56 (78.9) | |
| Government | 11 (10.3) | 2 (5.6) | 9 (12.7) | |
| Industry | 7 (6.5) | 2 (5.6) | 5 (7.0) | |
| Non-profit | 2 (1.9) | 1 (2.8) | 1 (1.4) | |
| Median starting date [IQR], year | 2015 [2011, 2017.50] | 2016 [2012, 2019] | 2015 [2010, 2017] | 0.073 |
| Study period, n (%) | 0.209 | |||
| early | 18 (16.8) | 3 (8.3) | 15 (21.1) | |
| interim | 52 (48.6) | 18 (50.0) | 34 (47.9) | |
| late | 37 (34.6) | 15 (41.7) | 22 (31.0) | |
| Median number of sites [IQR], n | 1 [1, 2.25] | 1 [1, 1] | 1 [1, 5.25] | 0.039 |
| Number of participating sites, n (%) | 0.054 | |||
| Single-center | 64 (59.8) | 25 (69.4) | 39 (54.9) | |
| Multi-center | 36 (33.6) | 7 (19.4) | 29 (40.8) | |
| N/A | 7 (6.5) | 4 (11.1) | 3 (4.2) | |
| Participating center’s location, n (%) | 0.012 | |||
| Europe only | 59 (55.1) | 15 (41.7) | 44 (62.0) | |
| Outside of Europe only | 41 (38.3) | 20 (55.6) | 21 (29.6) | |
| Both within and outside of Europe | 6 (5.6) | 0 (0.0) | 6 (8.5) | |
| N/A | 1 (0.9) | 1 (2.8) | 0 (0.0) | |
| Chairman’s office—primary location (country-detailed), n (%) | 0.055 | |||
| France | 16 (15.0) | 6 (16.7) | 10 (14.1) | |
| United States | 20 (18.7) | 5 (13.9) | 15 (21.1) | |
| Korea, Republic of | 14 (13.1) | 10 (27.8) | 4 (5.6) | |
| Sweden | 12 (11.2) | 3 (8.3) | 9 (12.7) | |
| Denmark | 8 (7.5) | 1 (2.8) | 7 (9.9) | |
| Norway | 4 (3.7) | 2 (5.6) | 2 (2.8) | |
| Slovenia | 2 (1.9) | 0 (0.0) | 2 (2.8) | |
| Taiwan | 5 (4.7) | 4 (11.1) | 1 (1.4) | |
| Canada | 4 (3.7) | 1 (2.8) | 3 (4.2) | |
| Finland | 2 (1.9) | 0 (0.0) | 2 (2.8) | |
| Germany | 3 (2.8) | 0 (0.0) | 3 (4.2) | |
| Spain | 4 (3.7) | 0 (0.0) | 4 (5.6) | |
| Netherlands | 3 (2.8) | 1 (2.8) | 2 (2.8) | |
| Italy | 1 (0.9) | 0 (0.0) | 1 (1.4) | |
| United Kingdom | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Australia | 2 (1.9) | 0 (0.0) | 2 (2.8) | |
| Belgium | 2 (1.9) | 1 (2.8) | 1 (1.4) | |
| Other | 5 (4.7) | 2 (5.6) | 3 (4.2) | |
| Chairman’s office—primary location (continent), n (%) | <0.001 | |||
| Europe | 62 (57.9) | 15 (41.7) | 46 (64.8) | |
| North America | 23 (21.5) | 6 (16.7) | 18 (25.4) | |
| Asia | 20 (18.7) | 15 (41.7) | 5 (7.0) | |
| Oceania | 2 (1.9) | 0 (0.0) | 2 (2.8) | |
| (b) | ||||
| Overall | Unpublished | Published | p-Value | |
| (N = 45) | (N = 22) | (N = 23) | ||
| Trial status, n (%) | ||||
| Completed | 43 (95.6) | 20 (90.9) | 23 (100.0) | 0.450 |
| Terminated | 2 (4.4) | 2 (9.1) | 0 (0.0) | |
| Availability of results, n (%) | ||||
| No | 44 (97.8) | 22 (100.0) | 22 (95.7) | 1.000 |
| Yes | 1 (2.2) | 0 (0.0) | 1 (4.3) | |
| Primary focus, n (%) | ||||
| Procedures | 17 (37.8) | 7 (31.8) | 10 (43.5) | 0.746 |
| Devices | 9 (20.0) | 5 (22.7) | 4 (17.4) | |
| Drugs | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Diagnostic test | 6 (13.3) | 2 (9.1) | 4 (17.4) | |
| Biomarkers | 7 (15.6) | 4 (18.2) | 3 (13.0) | |
| Other | 6 (13.3) | 4 (18.2) | 2 (8.7) | |
| Median enrollment size [IQR], n | 112 [50, 460] | 106.50 [56.50, 553] | 150 [50, 282] | 0.856 |
| Planned sample size ≥100 patients, n (%) | 0.899 | |||
| No | 19 (42.2) | 10 (45.5) | 9 (39.1) | |
| Yes | 26 (57.8) | 12 (54.5) | 14 (60.9) | |
| Median follow-up duration [IQR], days | 5 [0, 210] | 30 [0, 365] | 2 [0.25, 142.50] | 0.400 |
| Follow-up categories, n (%) | 0.248 | |||
| 0–30 days | 31 (68.9) | 13 (59.1) | 18 (78.3) | |
| 31–365 days | 8 (17.8) | 6 (27.3) | 2 (8.7) | |
| >365 days | 6 (13.3) | 3 (13.6) | 3 (13.0) | |
| Funder type, n (%) | 0.552 | |||
| Academic/university | 35 (77.8) | 18 (81.8) | 17 (73.9) | |
| Government | 7 (15.6) | 2 (9.1) | 5 (21.7) | |
| Industry | 3 (6.7) | 2 (9.1) | 1 (4.3) | |
| Non-profit | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Median starting date [IQR], year | 2016 [2013, 2018] | 2016.50 [2012.25, 2018.75] | 2016 [2015, 2018] | 0.900 |
| Study period, n (%) | 0.705 | |||
| Early | 5 (11.1) | 3 (13.6) | 2 (8.7) | |
| Interim | 19 (42.2) | 8 (36.4) | 11 (47.8) | |
| Late | 21 (46.7) | 11 (50.0) | 10 (43.5) | |
| Median number of sites [IQR], n | 1 [1, 1.50] | 1 [1, 2] | 1 [1, 1] | 0.840 |
| Number of participating sites, n (%) | 0.602 | |||
| Single-center | 29 (64.4) | 14 (63.6) | 15 (65.2) | |
| Multi-center | 10 (22.2) | 6 (27.3) | 4 (17.4) | |
| N/A | 6 (13.3) | 2 (9.1) | 4 (17.4) | |
| Participating center’s location, n (%) | 1.000 | |||
| Europe only | 33 (73.3) | 16 (72.7) | 17 (73.9) | |
| Outside of Europe only | 11 (24.4) | 6 (27.3) | 5 (21.7) | |
| Both within and outside of Europe | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| N/A | 1 (2.2) | 0 (0.0) | 1 (4.3) | |
| Chairman’s office—primary location (country-detailed), n (%) | 0.020 | |||
| France | 9 (20.0) | 7 (31.8) | 2 (8.7) | |
| United States | 4 (8.9) | 1 (4.5) | 3 (13.0) | |
| Korea, Republic of | 4 (8.9) | 4 (18.2) | 0 (0.0) | |
| Sweden | 4 (8.9) | 1 (4.5) | 3 (13.0) | |
| Denmark | 3 (6.7) | 1 (4.5) | 2 (8.7) | |
| Norway | 2 (4.4) | 1 (4.5) | 1 (4.3) | |
| Slovenia | 4 (8.9) | 0 (0.0) | 4 (17.4) | |
| Taiwan | 1 (2.2) | 1 (4.5) | 0 (0.0) | |
| Canada | 1 (2.2) | 0 (0.0) | 1 (4.3) | |
| Finland | 2 (4.4) | 2 (9.1) | 0 (0.0) | |
| Germany | 1 (2.2) | 0 (0.0) | 1 (4.3) | |
| Spain | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Netherlands | 1 (2.2) | 0 (0.0) | 1 (4.3) | |
| Italy | 2 (4.4) | 0 (0.0) | 2 (8.7) | |
| United Kingdom | 3 (6.7) | 1 (4.5) | 2 (8.7) | |
| Australia | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Belgium | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Other | 4 (8.9) | 3 (13.5) | 1 (4.3) | |
| Chairman’s office—primary location (continent), n (%) | 0.187 | |||
| Europe | 35 (77.8) | 16 (72.7) | 19 (82.6) | |
| North America | 4 (8.9) | 1 (4.5) | 3 (13.0) | |
| Asia | 6 (13.3) | 5 (22.7) | 1 (4.3) | |
| Oceania | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| (a) | |||||
|---|---|---|---|---|---|
| Overall | Single-Center | Multi-Center | N/A | p-Value | |
| (N = 107) | (N = 64) | (N = 36) | (N = 7) | ||
| Trial status, n (%) | 0.199 | ||||
| Completed | 94 (87.9) | 58 (90.6) | 29 (80.6) | 7 (100.0) | |
| Terminated | 13 (12.1) | 6 (9.4) | 7 (19.4) | 0 (0.0) | |
| Randomized trial, n (%) | |||||
| No | 24 (22.4) | 18 (28.1) | 4 (11.1) | 2 (28.6) | 0.136 |
| Yes | 83 (77.6) | 46 (71.9) | 32 (88.9) | 5 (71.4) | |
| Availability of results, n (%) | 0.890 | ||||
| Yes | 84 (78.5) | 50 (78.1) | 28 (77.8) | 6 (85.7) | |
| No | 23 (21.5) | 14 (21.9) | 8 (22.2) | 1 (14.3) | |
| Published as of 31 December 2023, n (%) | 0.054 | ||||
| No | 36 (33.6) | 25 (39.1) | 7 (19.4) | 4 (57.1) | |
| Yes | 71 (66.4) | 39 (60.9) | 29 (80.6) | 3 (42.9) | |
| Status and publication, n (%) | 0.130 | ||||
| Completed and published | 62 (57.9) | 36 (56.2) | 23 (63.9) | 3 (42.9) | |
| Terminated and published | 9 (8.4) | 3 (4.7) | 6 (16.7) | 0 (0.0) | |
| Completed and unpublished | 32 (29.9) | 22 (34.4) | 6 (16.7) | 4 (57.1) | |
| Uncompleted and unpublished | 4 (3.7) | 3 (4.7) | 1 (2.8) | 0 (0.0) | |
| Primary focus, n (%) | 0.522 | ||||
| Procedures | 46 (43.0) | 28 (43.8) | 15 (41.7) | 3 (42.9) | |
| Devices | 26 (24.3) | 16 (25.0) | 10 (27.8) | 0 (0.0) | |
| Drugs | 22 (20.6) | 13 (20.3) | 7 (19.4) | 2 (28.6) | |
| Diagnostic test | 4 (3.7) | 2 (3.1) | 2 (5.6) | 0 (0.0) | |
| Biomarkers | 2 (1.9) | 1 (1.6) | 1 (2.8) | 0 (0.0) | |
| Other | 7 (6.5) | 4 (6.2) | 1 (2.8) | 2 (28.6) | |
| Median enrollment size [IQR], n | 200 [60, 801] | 133.50 [56.50, 797] | 291 [103, 912.50] | 90 [37, 537.50] | 0.250 |
| Planned sample size ≥100 patients, n (%) | 0.085 | ||||
| No | 38 (35.5) | 26 (40.6) | 8 (22.2) | 4 (57.1) | |
| Yes | 69 (64.5) | 38 (59.4) | 28 (77.8) | 3 (42.9) | |
| Median follow-up duration [IQR], days | 12 [0, 180] | 3 [0, 90] | 30 [1, 180] | 180 [14, 180] | 0.165 |
| Follow-up categories, n (%) | 0.693 | ||||
| 0–30 days | 85 (79.4) | 50 (78.1) | 28 (77.8) | 7 (100) | |
| 31–365 days | 4 (3.7) | 3 (4.7) | 1 (2.8) | 0 (0.0) | |
| >365 days | 18 (16.8) | 11 (17.2) | 7 (19.4) | 0 (0.0) | |
| Funder type, n (%) | 0.462 | ||||
| Academic/university | 87 (81.3) | 52 (81.2) | 29 (80.6) | 6 (85.7) | |
| Government | 11 (10.3) | 9 (14.1) | 2 (5.6) | 0 (0.0) | |
| Industry | 7 (6.5) | 2 (3.1) | 4 (11.1) | 1 (14.3) | |
| Non-profit | 2 (1.9) | 1 (1.6) | 1 (2.8) | 0 (0.0) | |
| Median starting date [IQR], year | 2015 [2011, 2017.50] | 2015.50 [2012, 2018] | 2015 [2011, 2017] | 2014 [2012, 2015] | 0.352 |
| Study period, n (%) | 0.311 | ||||
| Early | 18 (16.8) | 11 (17.2) | 6 (16.7) | 1 (14.3) | |
| Interim | 52 (48.6) | 29 (45.3) | 17 (47.2) | 6 (85.7) | |
| Late | 37 (34.6) | 24 (37.5) | 13 (36.1) | 0 (0.0) | |
| Median number of sites [IQR], n | 1 [1, 2.25] | 1 [1, 1] | 6 [2, 10] | NA [NA, NA] | <0.001 |
| Participating center’s location, n (%) | <0.001 | ||||
| Within Europe only | 59 (55.1) | 38 (59.4) | 18 (50.0) | 3 (42.9) | |
| Outside of Europe only | 1 (0.9) | 0 (0.0) | 0 (0.0) | 1 (14.3) | |
| Both within and outside of Europe | 6 (5.6) | 0 (0.0) | 6 (16.7) | 0 (0.0) | |
| N/A | 41 (38.3) | 26 (40.6) | 12 (33.3) | 3 (42.9) | |
| Chairman’s office-primary location (country-detailed), n (%) | 0.023 | ||||
| France | 16 (15.0) | 13 (20.3) | 3 (8.3) | 0 (0.0) | |
| United States | 20 (18.7) | 9 (14.1) | 11 (30.6) | 0 (0.0) | |
| Korea, Republic of | 14 (13.1) | 10 (15.6) | 1 (2.8) | 3 (42.9) | |
| Sweden | 12 (11.2) | 6 (9.4) | 5 (13.9) | 1 (14.3) | |
| Denmark | 8 (7.5) | 5 (7.8) | 3 (8.3) | 0 (0.0) | |
| Norway | 4 (3.7) | 3 (4.7) | 1 (2.8) | 0 (0.0) | |
| Slovenia | 2 (1.9) | 2 (3.1) | 0 (0.0) | 0 (0.0) | |
| Taiwan | 5 (4.7) | 5 (7.8) | 0 (0.0) | 0 (0.0) | |
| Canada | 4 (3.7) | 1 (1.6) | 3 (8.3) | 0 (0.0) | |
| Finland | 2 (1.9) | 0 (0.0) | 2 (5.6) | 0 (0.0) | |
| Germany | 3 (2.8) | 2 (3.1) | 1 (2.8) | 0 (0.0) | |
| Spain | 4 (3.7) | 2 (3.1) | 1 (2.8) | 1 (14.3) | |
| Netherlands | 3 (2.8) | 2 (3.1) | 1 (2.8) | 0 (0.0) | |
| Italy | 1 (0.9) | 0 (0.0) | 1 (2.8) | 0 (0.0) | |
| United Kingdom | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Australia | 2 (1.9) | 1 (1.6) | 1 (2.8) | 0 (0.0) | |
| Belgium | 2 (1.9) | 0 (0.0) | 1 (2.8) | 1 (14.3) | |
| Other | 5 (4.7) | 3 (4.7) | 1 (2.8) | 1 (14.3) | |
| Chairman’s office—primary location (continent), n (%) | 0.006 | ||||
| Europe | 62 (57.9) | 38 (59.4) | 21 (58.3) | 3 (42.9) | |
| North America | 23 (21.5) | 10 (15.6) | 13 (36.1) | 0 (0.0) | |
| Asia | 20 (18.7) | 15 (23.4) | 1 (2.8) | 4 (57.1) | |
| Oceania | 2 (1.9) | 1 (1.6) | 1 (2.8) | 0 (0.0) | |
| (b) | |||||
| Overall | Single-Center | Multi-Center | N/A | p-Value | |
| (N = 45) | (N = 29) | (N = 10) | (N = 6) | ||
| Trial status, n (%) | |||||
| Completed | 43 (95.6) | 29 (100) | 8 (80) | 6 (100) | 0.026 |
| Terminated | 2 (4.4) | 0 (0.0) | 2 (20) | 0 (0.0) | |
| Availability of results, n (%) | |||||
| Yes | 44 (97.8) | 28 (96.6) | 10 (100.0) | 6 (100.0) | 0.754 |
| No | 1 (2.2) | 1 (3.4) | 0 (0.0) | 0 (0.0) | |
| Published as of 31 December 2023, n (%) | |||||
| No | 22 (48.9) | 14 (48.3) | 6 (60.0) | 2 (33.3) | 0.583 |
| Yes | 23 (51.1) | 15 (51.7) | 4 (40.0) | 4 (66.7) | |
| Status and publication, n (%) | 0.583 | ||||
| Completed and published | 23 (51.1) | 15 (51.7) | 4 (40.0) | 4 (66.7) | |
| Terminated and published | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Completed and unpublished | 20 (44.4) | 14 (48.3) | 4 (40.0) | 2 (33.3) | |
| Uncompleted and unpublished | 2 (4.4) | 0 (0.0) | 2 (20.0) | 0 (0.0) | |
| Primary focus, n (%) | 0.532 | ||||
| Procedures | 17 (37.8) | 11 (37.9) | 4 (40.0) | 2 (33.3) | |
| Devices | 9 (20.0) | 5 (17.2) | 3 (30.0) | 1 (16.7) | |
| Drugs | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Diagnostic test | 6 (13.3) | 3 (10.3) | 2 (20.0) | 1 (16.7) | |
| Biomarkers | 7 (15.6) | 7 (24.1) | 0 (0.0) | 0 (0.0) | |
| Other | 6 (13.3) | 3 (10.3) | 1 (10.0) | 2 (33.3) | |
| Median enrollment size [IQR], n | 112 [50, 460] | 150 [50, 300] | 105 [59.50, 290.50] | 357 [55.75, 896] | 0.846 |
| Planned sample size ≥100 patients, n (%) | 0.863 | ||||
| No | 19 (42.2) | 13 (44.8) | 4 (40.0) | 2 (33.3) | |
| Yes | 26 (57.8) | 16 (55.2) | 6 (60.0) | 4 (66.7) | |
| Median follow-up duration [IQR], days | 5.00 [0.00, 210.00] | 7.50 [0.00, 365.00] | 3.00 [0.00, 210.00] | 7.50 [0.25, 138.50] | 0.773 |
| Follow-up categories, n (%) | 0.513 | ||||
| 0–30 days | 31 (68.9) | 18 (62.1) | 7 (70.0) | 6 (100.0) | |
| 31–365 days | 8 (17.8) | 7 (24.1) | 1 (10.0) | 0 (0.0) | |
| >365 days | 6 (13.3) | 4 (13.8) | 2 (20.0) | 0 (0.0) | |
| Funder type, n (%) | 0.476 | ||||
| Academic/university | 35 (77.8) | 23 (79.3) | 7 (70.0) | 5 (83.3) | |
| Government | 7 (15.6) | 5 (17.2) | 1 (10.0) | 1 (16.7) | |
| Industry | 3 (6.7) | 1 (3.4) | 2 (20.0) | 0 (0.0) | |
| Non-profit | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Median starting date [IQR], year | 2016 [2013, 2018] | 2017 [2015, 2018] | 2016.50 [2013.25, 2018.75] | 2014 [2010.75, 2015] | 0.107 |
| Study period, n (%) | 0.178 | ||||
| Early | 5 (11.1) | 3 (10.3) | 1 (10.0) | 1 (16.7) | |
| Interim | 19 (42.2) | 10 (34.5) | 4 (40.0) | 5 (83.3) | |
| Late | 21 (46.7) | 16 (55.2) | 5 (50.0) | 0 (0.0) | |
| Median number of sites [IQR], n | 1.00 [1.00, 1.50] | 1.00 [1.00, 1.00] | 5.50 [2.00, 7.75] | NA [NA, NA] | <0.001 |
| Participating center’s location, n (%) | 0.527 | ||||
| Within Europe only | 33 (73.3) | 22 (75.9) | 6 (60.0) | 5 (83.3) | |
| Outside of Europe only | 11 (24.4) | 7 (24.1) | 4 (40.0) | 0 (0.0) | |
| Both within and outside of Europe | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| N/A | 1 (2.2) | 0 (0.0) | 0 (0.0) | 1 (16.7) | |
| Chairman’s office—primary location (country-detailed), n (%) | 0.275 | ||||
| France | 9 (20.0) | 5 (17.2) | 3 (30.0) | 1 (16.7) | |
| United States | 4 (8.9) | 2 (6.9) | 2 (20.0) | 0 (0.0) | |
| Korea, Republic of | 4 (8.9) | 3 (10.3) | 1 (10) | 0 (0.0) | |
| Sweden | 4 (8.9) | 2 (6.9) | 2 (20.0) | 0 (0.0) | |
| Denmark | 3 (6.7) | 2 (6.9) | 0 (0.0) | 1 (16.7) | |
| Norway | 2 (4.4) | 2 (6.9) | 0 (0.0) | 0 (0.0) | |
| Slovenia | 4 (8.9) | 4 (13.8) | 0 (0.0) | 0 (0.0) | |
| Taiwan | 1 (2.2) | 1 (3.4) | 0 (0.0) | 0 (0.0) | |
| Canada | 1 (2.2) | 0 (0.0) | 1 (10) | 0 (0.0) | |
| Finland | 2 (4.4) | 1 (3.4) | 1 (10) | 0 (0.0) | |
| Germany | 1 (2.2) | 0 (0.0) | 0 (0.0) | 1 (16.7) | |
| Spain | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Netherlands | 1 (2.2) | 1 (3.4) | 0 (0.0) | 0 (0.0) | |
| Italy | 2 (4.4) | 0 (0.0) | 0 (0.0) | 2 (33.3) | |
| United Kingdom | 3 (6.7) | 3 (10.3) | 0 (0.0) | 0 (0.0) | |
| Australia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Belgium | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Other | 4 (8.8) | 3 (10.3) | 0 (0.0) | 1 (16.6) | |
| Chairman’s office—primary location (continent), n (%) | 0.502 | ||||
| Europe | 35 (77.8) | 22 (75.9) | 7 (70.0) | 6 (100.0) | |
| North America | 4 (8.9) | 2 (6.9) | 2 (20.0) | 0 (0.0) | |
| Asia | 6 (13.3) | 5 (17.2) | 1 (10.0) | 0 (0.0) | |
| Oceania | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Andria Ursoleo, J.; Bugo, S.; Losiggio, R.; Bottussi, A.; Agosta, V.T.; Monaco, F. Characteristics of Out-of-Hospital Cardiac Arrest Trials Registered in ClinicalTrials.gov. J. Clin. Med. 2024, 13, 5421. https://doi.org/10.3390/jcm13185421
D’Andria Ursoleo J, Bugo S, Losiggio R, Bottussi A, Agosta VT, Monaco F. Characteristics of Out-of-Hospital Cardiac Arrest Trials Registered in ClinicalTrials.gov. Journal of Clinical Medicine. 2024; 13(18):5421. https://doi.org/10.3390/jcm13185421
Chicago/Turabian StyleD’Andria Ursoleo, Jacopo, Samuele Bugo, Rosario Losiggio, Alice Bottussi, Viviana Teresa Agosta, and Fabrizio Monaco. 2024. "Characteristics of Out-of-Hospital Cardiac Arrest Trials Registered in ClinicalTrials.gov" Journal of Clinical Medicine 13, no. 18: 5421. https://doi.org/10.3390/jcm13185421
APA StyleD’Andria Ursoleo, J., Bugo, S., Losiggio, R., Bottussi, A., Agosta, V. T., & Monaco, F. (2024). Characteristics of Out-of-Hospital Cardiac Arrest Trials Registered in ClinicalTrials.gov. Journal of Clinical Medicine, 13(18), 5421. https://doi.org/10.3390/jcm13185421






