The Efficacy and Safety of CollaSel Pro® Hydrolyzed Collagen Peptide Supplementation without Addons in Improving Skin Health in Adult Females: A Double Blind, Randomized, Placebo-Controlled Clinical Study Using Biophysical and Skin Imaging Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Manufacturing and Quality Evaluation of Collagen
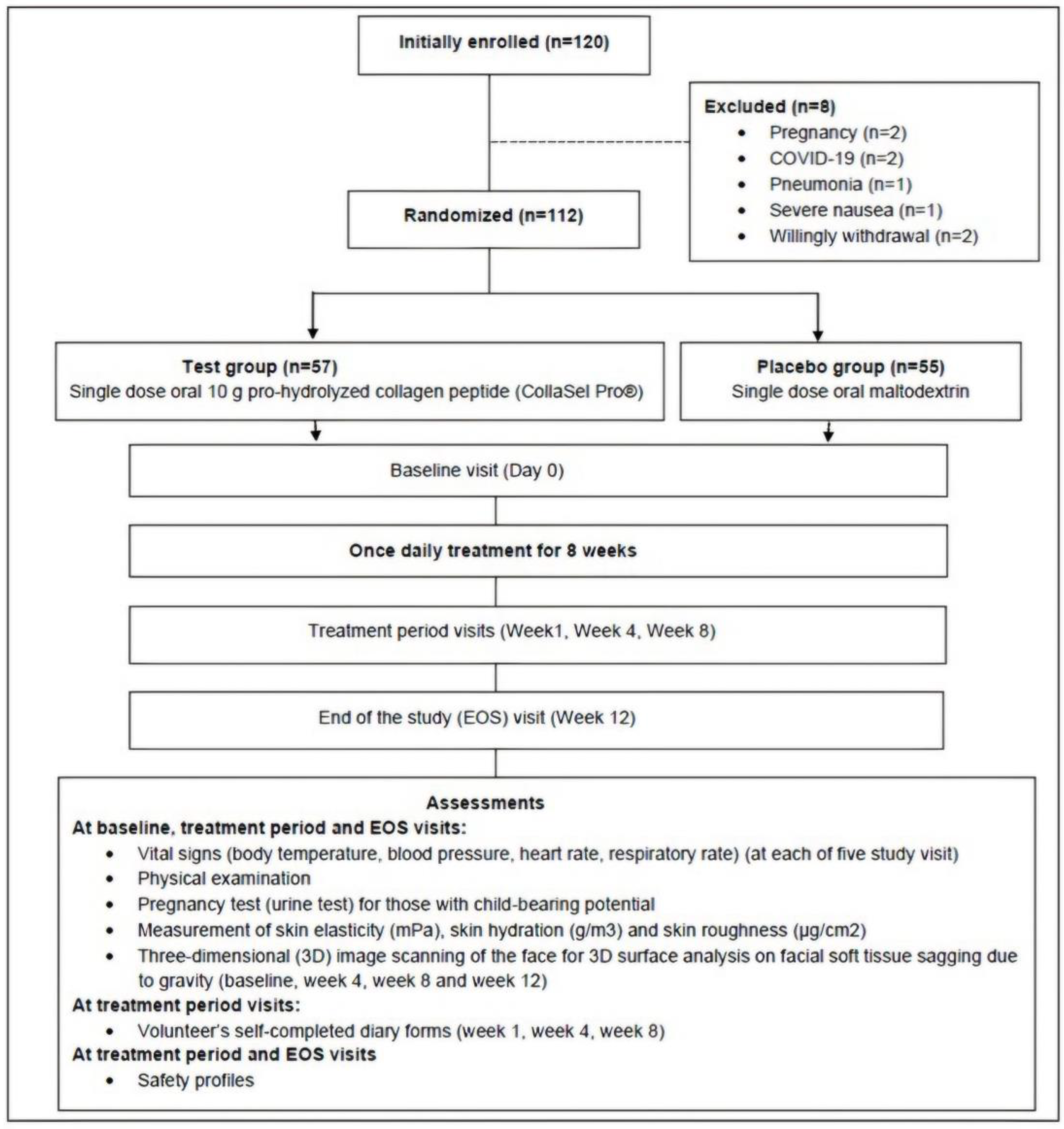
2.2. Study Design
2.3. Data Collection
2.4. Test Product and Placebo
2.5. Measurement of Skin Elasticity, Skin Hydration, and Skin Roughness
2.6. 3D Facial Scanning and Image Processing
2.7. Surface Analysis for Facial Soft Tissue Sagging
2.8. Statistical Analysis
3. Results
3.1. Baseline Characteristics (n = 112)
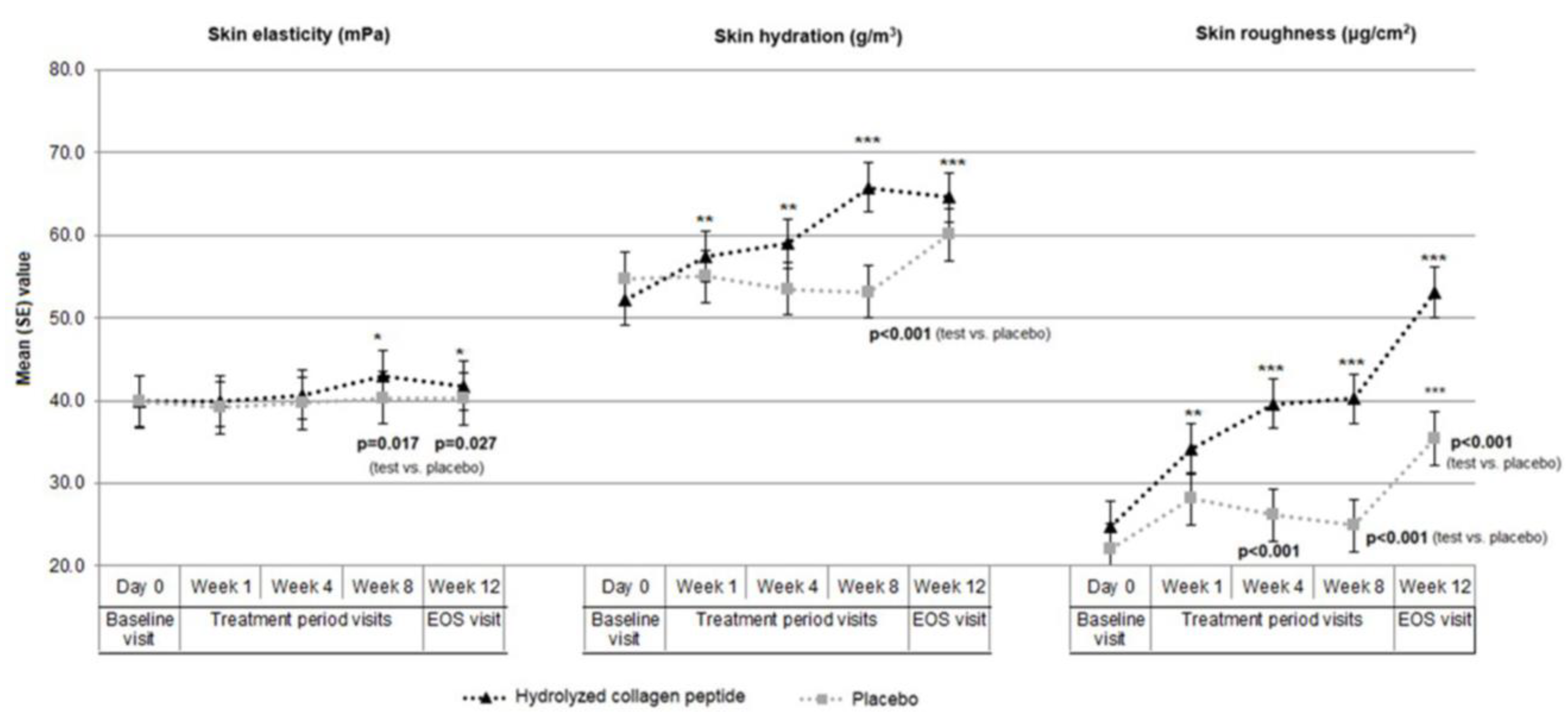
3.2. Skin Elasticity in Test Product and Placebo Groups
3.3. Skin Hydration in Test Product and Placebo Groups
3.4. Skin Roughness in Test Product and Placebo Groups
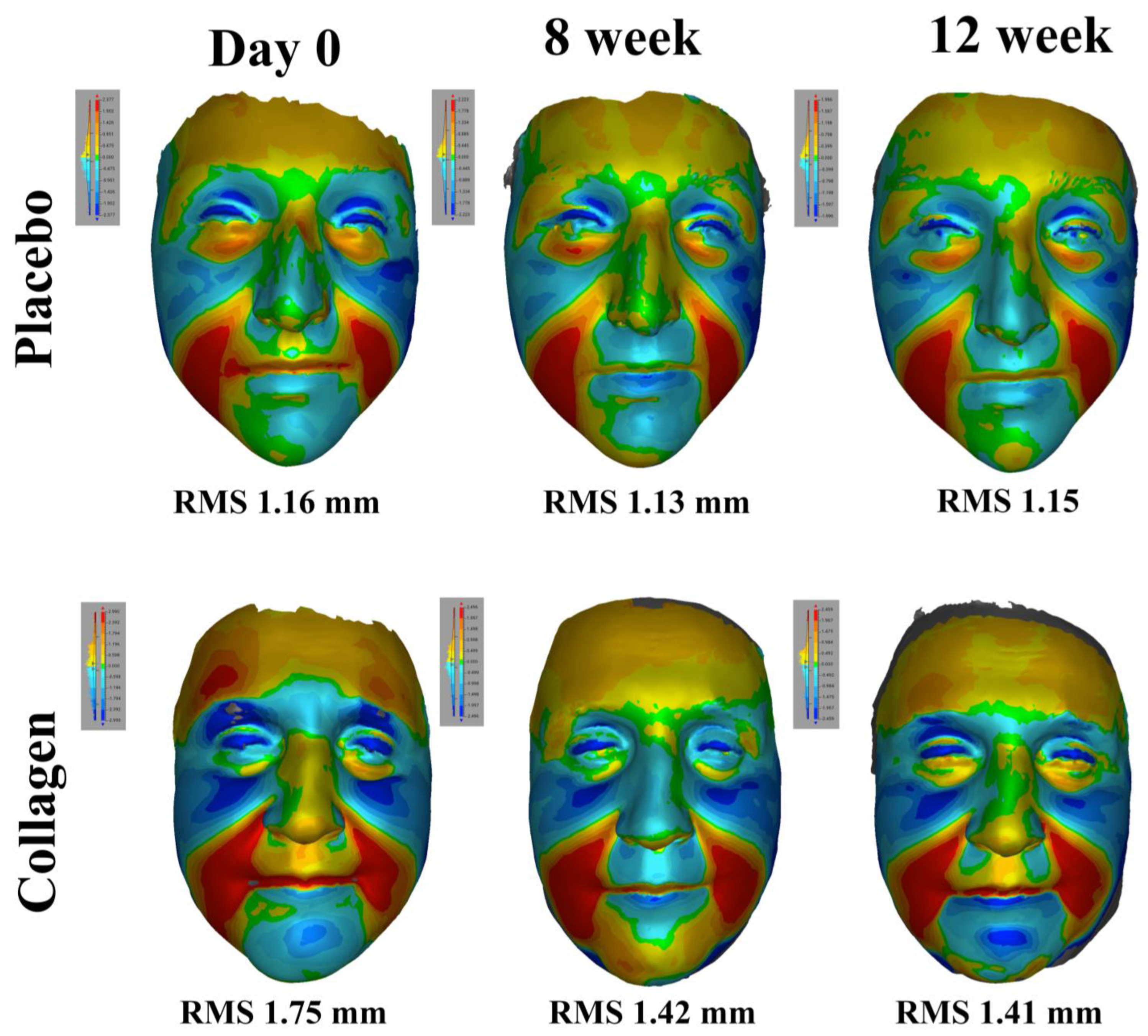
3.5. 3D Surface Analysis for Facial Configuration in Test Product and Placebo Groups
3.6. Safety Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lupu, M.A.; Gradisteanu Pircalabioru, G.; Chifiriuc, M.C.; Albulescu, R.; Tanase, C. Beneficial Beneficial effects of food supplements based on hydrolyzed collagen for skin care (Review). Exp. Ther. Med. 2020, 20, 12–17. [Google Scholar] [CrossRef]
- Justyna, Z. Oral collagen supplements intake on improving skin structure and function. J. Educ. Health Sport 2022, 12, 434–440. [Google Scholar]
- Al-Atif, H. Collagen Supplements for Aging and Wrinkles: A Paradigm Shift in the Fields of Dermatology and Cosmetics. Dermatol. Pract. Concept. 2022, 12, e2022018. [Google Scholar] [CrossRef]
- Patrícia Maia Campos, M.B.G.; Meloo, M.O.; Calixto, L.S.; Fossa, M.M. An oral supplementation based on hydrolyzed collagen and vitamins improves skin elasticity and dermis echogenicity: A clinical placebo-controlled study. Clin. Pharmacol. Biopharm. 2015, 4, 142. [Google Scholar]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- Naylor, E.C.; Watson, R.E.; Sherratt, M.J. Molecular aspects of skin ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.G.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef]
- Lee, D.H.; Oh, J.-H.; Chung, J.H. Glycosaminoglycan and proteoglycan in skin aging. J. Dermatol. Sci. 2016, 83, 174–181. [Google Scholar] [CrossRef]
- McCabe, M.C.; Hill, R.C.; Calderone, K.; Cui, Y.; Yan, Y.; Quan, T.; Fisher, G.J.; Hansen, K.C. Alterations in extracellular matrix composition during aging and photoaging of the skin. Matrix Biol. Plus 2020, 8, 100041. [Google Scholar] [CrossRef]
- Genovese, L.; Corbo, A.; Sibilla, S. An insight into the changes in skin texture and properties following dietary intervention with a nutricosmeceutical containing a blend of collagen bioactive peptides and antioxidants. Skin Pharmacol. Physiol. 2017, 30, 146–158. [Google Scholar] [CrossRef]
- Sibilla, S.; Godfrey, M.; Brewer, S.; Budh-Raja, A.; Genovese, L. An overview of the beneficial effects of hydrolysed collagen as a nutraceutical on skin properties: Scientific background and clinical studies. Open Nutraceuticals J. 2015, 8, 29–42. [Google Scholar] [CrossRef]
- Aguirre-Cruz, G.; León-López, A.; Cruz-Gómez, V.; Jiménez-Alvarado, R.; Aguirre-Alvarez, G. Collagen hydrolysates for skin protection: Oral administration and topical formulation. Antioxidants 2020, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Reilly, D.M.; Lozano, J. Skin collagen through the lifestages: Importance for skin health and beauty. Plast. Aesthet. Res. 2021, 8, 2–24. [Google Scholar] [CrossRef]
- Evans, M.; Lewis, E.D.; Zakaria, N.; Pelipyagina, T.; Guthrie, N. A randomized, triple-blind, placebo-controlled, parallel study to evaluate the efficacy of a freshwater marine collagen on skin wrinkles and elasticity. J. Cosmet. Dermatol. 2021, 20, 825–834. [Google Scholar] [CrossRef]
- de Miranda, R.B.; Weimer, P.; Rossi, R.C. Effects of hydrolyzed collagen supplementation on skin aging: A systematic review and meta-analysis. Int. J. Dermatol. 2021, 60, 1449–1461. [Google Scholar] [CrossRef]
- Asserin, J.; Lati, E.; Shioya, T.; Prawitt, J. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: Evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J. Cosmet. Dermatol. 2015, 14, 291–301. [Google Scholar] [CrossRef]
- Czajka, A.; Kania, E.M.; Genovese, L.; Corbo, A.; Merone, G.; Luci, C.; Sibilla, S. Daily oral supplementation with collagen peptides combined with vitamins and other bioactive compounds improves skin elasticity and has a beneficial effect on joint and general wellbeing. Nutr. Res. 2018, 57, 97–108. [Google Scholar] [CrossRef]
- Borumand, M.; Sibilla, S. Effects of a nutritional supplement containing collagen peptides on skin elasticity, hydration and wrinkles. J. Med. Nutr. Nutraceutic. 2015, 4, 47–53. [Google Scholar]
- Proksch, E.; Segger, D.; Degwert, J.; Schunck, M.; Zague, V.; Oesser, S. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: A double-blind, placebocontrolled study. Skin Pharmacol. Physiol. 2014, 27, 47–55. [Google Scholar] [CrossRef]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef]
- Sionkowska, A.; Skrzyński, S.; Śmiechowski, K.; Kołodziejczak, A. The review of versatile application of collagen. Polym. Adv. Technol. 2017, 28, 4–9. [Google Scholar] [CrossRef]
- Avila Rodríguez, M.I.; Rodríguez Barroso, L.G.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Tak, Y.J.; Shin, D.K.; Kim, A.H.; Kim, J.I.; Lee, Y.L.; Ko, H.C.; Kim, Y.W.; Lee, S.Y. Effect of collagen tripeptide and adjusting for climate change on skin hydration in middle-aged women: A randomized, double-blind, placebo-controlled trial. Front. Med. 2021, 11, 608903. [Google Scholar] [CrossRef]
- Samadi, A.; Movaffaghi, M.; Kazemi, F.; Yazdanparast, T.; Nasrollahi, S.A.; Firooz, A. Tolerability and efficacy assessment of an oral collagen supplement for the improvement of biophysical and ultrasonographic parameters of skin in middle eastern consumers. J. Cosmet. Dermatol. 2023, 22, 2252–2258. [Google Scholar] [CrossRef] [PubMed]
- Guadanhim, L.R.S.; Miot, H.A.; Soares, J.L.M.; Silva, S.A.M.; Leonardi, G.R.; Lopes, R.D.; Bagatin, E. Efficacy and safety of topical or oral hydrolyzed collagen in women with dermatoporosis: A randomized, double-blind, factorial design study. Dermatol. Ther. 2023, 13, 523–534. [Google Scholar] [CrossRef]
- Yamamoto, K.; Igawa, K.; Sugimoto, K.; Yoshizawa, Y.; Yanagiguchi, K.; Ikeda, T.; Yamada, S.; Hayashi, Y. Biological safety of fish (tilapia) collagen. Biomed Res Int. 2014, 2014, 630757. [Google Scholar] [CrossRef]
- Bianchi, F.M.; Angelinetta, C.; Rizzi, G.; Pratico, A.; Villa, R. Evaluation of the Efficacy of a Hydrolyzed Collagen Supplement for Improving Skin Moisturization, Smoothness, and Wrinkles. J. Clin. Aesthet. Dermatol. 2022, 15, 48–52. [Google Scholar]
- GME Collagen Peptides Monograph Standardised Methods for the Testing of Edible Collagen peptides, Final Version 1–October. 2020. Available online: https://www.gelatine.org/fileadmin/user_upload/gme_content/GME_Statements/GME_CP_Monograph-version_1_-_public.pdf (accessed on 19 August 2024).
- Toma, A.M.; Zhurov, A.; Playle, R.; Ong, E.; Richmond, S. Reproducibility of facial soft tissue landmarks on 3D laser-scanned facial images. Orthod. Craniofac. Res. 2009, 12, 33–42. [Google Scholar] [CrossRef]
- Ozsoy, U.; Sekerci, R.; Ogut, E. Effect of sitting, standing, and supine body positions on facial soft tissue: Detailed 3D analysis. Int. J. Oral Maxillofac. Surg. 2015, 44, 1309–1316. [Google Scholar] [CrossRef]
- Choi, S.Y.; Ko, E.J.; Lee, Y.H.; Kim, B.G.; Shin, H.J.; Seo, D.B.; Lee, S.J.; Kim, B.J.; Kim, M.N. Effects of collagen tripeptide supplement on skin properties: A prospective, randomized, controlled study. J. Cosmet. Laser Ther. 2014, 16, 132–137. [Google Scholar] [CrossRef]
- Yoon, H.S.; Cho, H.H.; Cho, S.; Lee, S.R.; Shin, M.H.; Chung, J.H. Supplementating with dietary astaxanthin combined with collagen hydrolysate improves facial elasticity and decreases matrix metalloproteinase-1 and -12 expression: A comparative study with placebo. J. Med. Food 2014, 17, 810–816. [Google Scholar] [CrossRef]
- Ito, N.; Seki, S.; Ueda, F. Effects of composite supplement containing collagen peptide and ornithine on skin conditions and plasma IGF-1 levels—A randomized, double-blind, placebo-controlled trial. Mar. Drugs 2018, 16, 482. [Google Scholar] [CrossRef] [PubMed]
- Kalil, C.L.P.V.; Campos, V.; Cignachi, S.; Izidoro, J.F.; Reinehr, C.P.H.; Chaves, C. Evaluation of cutaneous rejuvenation associated with the use of ortho-silicic acid stabilized by hydrolyzed marine collagen. J. Cosmet. Dermatol. 2018, 17, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.U.; Chung, H.C.; Choi, J.; Sakai, Y.; Lee, B.Y. Oral intake of low-molecular-weight collagen peptide improves hydration, elasticity, and wrinkling in human skin: A randomized, double-blind, placebo-controlled study. Nutrients 2018, 10, 826. [Google Scholar] [CrossRef]
- Addor, F.A.S.; Cotta Vieira, J.; Abreu Melo, C.S. Improvement of dermal parameters in aged skin after oral use of a nutrient supplement. Clin. Cosmet. Investig. Dermatol. 2018, 11, 195–201. [Google Scholar] [CrossRef]
- Choi, F.D.; Sung, C.T.; Juhasz, M.L.; Mesinkovska, N.A. Oral Collagen Supplementation: A Systematic Review of Dermatological Applications. J. Drugs Dermatol. 2019, 18, 9–16. [Google Scholar]
- Bonnet, C. Introduction to Naticol® Marine Collagen Peptides for Anti-aging and Overview of Clinical Studies. Available online: https://pdfs.semanticscholar.org/fed1/fa27de3676b4626343d35ea1d1567ca3f216.pdf (accessed on 10 September 2023).
- Ohara, H.; Ito, K.; Iida, H.; Matsumoto, H. Improvement in the moisture content of the stratum corneum following 4 weeks of collagen hydrolysate ingestion. Nippon. Shokuhin Kagaku Kogaku Kaishi 2009, 59, 137–145. [Google Scholar] [CrossRef]
- Geng, R.; Kang, S.G.; Huang, K.; Tong, T. Boosting the Photoaged Skin: The Potential Role of Dietary Components. Nutrients 2021, 13, 1691. [Google Scholar] [CrossRef]
- Schwartz, S.R.; Park, J. Ingestion of BioCell Collagen(®), a novel hydrolyzed chicken sternal cartilage extract; enhanced blood microcirculation and reduced facial aging signs. Clin. Interv. Aging 2012, 7, 267–273. [Google Scholar]
- Proksch, E.; Schunck, M.; Zague, V.; Segger, D.; Degwert, J.; Oesser, S. Oral intake of specific bioactive collagen peptides reduces skin wrinkles and increases dermal matrix synthesis. Skin Pharmacol. Physiol. 2014, 27, 113–119. [Google Scholar] [CrossRef]
- Imokawa, G.; Ishida, K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging I: Reduced skin elasticity, highly associated with enhanced dermal elastase activity, triggers wrinkling and sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef]
- Matsumoto, H. Clinical effects of fish type I collagen hydrolysate on skin properties. ITE Lett. Batter. New Technol. Med. 2006, 7, 386–390. [Google Scholar]
- Inoue, N.; Sugihara, F.; Wang, X. Ingestion of bioactive collagen hydrolysates enhance facial skin moisture and elasticity and reduce facial ageing signs in a randomised double-blind placebo-controlled clinical study. J. Sci. Food Agric. 2016, 96, 4077–4081. [Google Scholar] [CrossRef]
- Maia Campos, P.M.B.G.; Franco, R.S.B.; Kakuda, L.; Cadioli, G.F.; Costa, G.M.D.; Bouvret, E. Oral supplementation with hydrolyzed fish cartilage improves the morphological and structural characteristics of the skin: A double-blind, placebo-controlled clinical study. Molecules 2021, 26, 4880. [Google Scholar] [CrossRef] [PubMed]
- Motwani, M.S.; Khan, K.; Pai, A.; Joshi, R. Efficacy of a collagen hydrolysate and antioxidants-containing nutraceutical on metrics of skin health in Indian women. J. Cosmet. Dermatol. 2020, 19, 3371–3382. [Google Scholar] [CrossRef] [PubMed]
- Gniadecka, M. Effects of ageing on dermal echogenicity. Skin Res. Technol. 2001, 7, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Borumand, M.; Sibilla, S. Daily consumption of the collagen supplement Pure Gold Collagen® reduces visible signs of aging. Clin. Interv. Aging 2014, 9, 1747–1758. [Google Scholar] [CrossRef]
- López-Morales, C.A.; Vázquez-Leyva, S.; Vallejo-Castillo, L.; Carballo-Uicab, G.; Muñoz-García, L.; Herbert-Pucheta, J.E.; Zepeda-Vallejo, L.G.; Velasco-Velázquez, M.; Pavón, L.; Pérez-Tapia, S.M.; et al. Determination of Peptide Profile Consistency and Safety of Collagen Hydrolysates as Quality Attributes. J. Food Sci. 2019, 84, 430–439. [Google Scholar] [CrossRef]
- Marcos-Garcés, V.; Molina Aguilar, P.; Bea Serrano, C.; Garcia Bustos, V.; Benavent Segui, J.; Ferrandez Izquierdo, A.; Ruiz-Sauri, A. Age-related dermal collagen changes during development, maturation and ageing—A morphometric and comparative study. J. Anat. 2014, 225, 98–108. [Google Scholar] [CrossRef]
- Asai, T.T.; Oikawa, F.; Yoshikawa, K.; Inoue, N.; Sato, K. Food-derived collagen peptides, prolyl-hydroxyproline (Pro-Hyp), and hydroxyprolyl-glycine (Hyp-Gly) enhance growth of primary cultured mouse skin fibroblast using fetal bovine serum free from hydroxyprolyl peptide. Int. J. Mol. Sci. 2019, 21, 229. [Google Scholar] [CrossRef]
- Zague, V.; de Freitas, V.; da Costa Rosa, M.; de Castro, G.A.; Jaeger, R.G.; Machado-Santelli, G.M. Collagen hydrolysate intake increases skin collagen expression and suppresses matrix metalloproteinase 2 activity. J. Med. Food 2011, 14, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y.; Song, H.; He, J.; Li, G.; Zheng, Y.; Li, B. Collagen peptides promote photoaging skin cell repair by activating the TGF-β/Smad pathway and depressing collagen degradation. Food Funct. 2019, 10, 6121–6134. [Google Scholar] [CrossRef] [PubMed]
- Barati, M.; Jabbari, M.; Navekar, R.; Farahmand, F.; Zeinalian, R.; Salehi-Sahlabadi, A.; Abbaszadeh, N.; Mokari-Yamchi, A.; Davoodi, S.H. Collagen supplementation for skin health: A mechanistic systematic review. J. Cosmet. Dermatol. 2020, 19, 2820–2829. [Google Scholar] [CrossRef] [PubMed]
| Test Product (Hydrolyzed Collagen Peptide) Group (n = 57) | Placebo Group (n = 55) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Visit | Treatment Period Visits | EOS Visit | Baseline Visit | Treatment Period Visits | EOS Visit | ||||||
| Skin Measurements | Day 0 | Week 1 | Week 4 | Week 8 | Week 12 | Day 0 | Week 1 | Week 4 | Week 8 | Week 12 | |
| Skin elasticity (mPa) | n | 57 | 57 | 57 | 57 | 56 | 55 | 54 | 55 | 55 | 55 |
| mean(SD) | 39.9 (4.7) | 39.9 (5.2) | 40.7 (6.0) | 43.0 (7.4) | 41.8 (4.3) | 39.9 (6.3) | 39.1 (6.0) | 39.7 (5.0) | 40.3 (3.3) | 40.2 (3.3) | |
| p-value | vs. baseline | 0.883 | 0.222 | 0.009 | 0.001 | 0.251 | 0.753 | 0.571 | 0.693 | ||
| vs. week 1 | 0.237 | 0.006 | 0.01 | 0.218 | 0.078 | 0.066 | |||||
| vs. week 4 | 0.049 | 0.14 | 0.298 | 0.392 | |||||||
| vs. week 8 | 0.175 | 0.754 | |||||||||
| p-value vs. placebo | 0.971 | 0.494 | 0.328 | 0.017 | 0.027 | ||||||
| Skin hydration (g/m3) | n | 57 | 57 | 57 | 57 | 56 | 55 | 54 | 55 | 55 | 55 |
| mean(SD) | 52.2 (16.1) | 57.4 (17.8) | 59.0 (18.2) | 65.8 (18.9) | 64.6 (14.4) | 54.8 (14.2) | 55.0 (12.5) | 53.5 (14.1) | 53.1 (14.9) | 60.1 (15.8) | |
| p-value | vs. baseline | 0.003 | 0.002 | <0.001 | <0.001 | 0.846 | 0.47 | 0.467 | 0.017 | ||
| vs. week 1 | 0.47 | <0.001 | <0.001 | 0.329 | 0.285 | 0.03 | |||||
| vs. week 4 | 0.001 | 0.004 | 0.835 | 0.005 | |||||||
| vs. week 8 | 0.336 | 0.002 | |||||||||
| p-value vs. placebo | 0.367 | 0.412 | 0.076 | <0.001 | 0.115 | ||||||
| Skin roughness (μg/cm2) | n | 53 | 56 | 56 | 56 | 56 | 53 | 51 | 55 | 55 | 55 |
| mean(SD) | 24.8 (18.2) | 34.1 (21.8) | 39.6 (15.8) | 40.2 (20.4) | 53.1 (24.1) | 22.0 (13.8) | 28.1 (16.3) | 26.1 (16.3) | 24.9 (20.9) | 35.4 (25.6) | |
| p-value | vs. baseline | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 | 0.044 | 0.235 | <0.001 | ||
| vs. week 1 | 0.08 | 0.033 | <0.001 | 0.024 | 0.115 | 0.076 | |||||
| vs. week 4 | 0.447 | <0.001 | 0.687 | 0.002 | |||||||
| vs. week 8 | 0.001 | <0.001 | |||||||||
| p-value vs. placebo | 0.371 | 0.111 | <0.001 | <0.001 | <0.001 | ||||||
| Test Product Group | Placebo Group | ||||||
|---|---|---|---|---|---|---|---|
| Baseline Visit | Treatment Visit | EOS Visit | Baseline Visit | Treatment Visit | EOS Visit | ||
| 3D Surface Analysis | Day 0 | Week 8 | Week 12 | Day 0 | Week 8 | Week 12 | |
| RMS (mm) | n | 28 | 28 | 28 | 25 | 23 | 25 |
| mean(SD) | 1.10 (0.21) | 1.02 (0.21) | 1.09 (0.20) | 1.08 (0.25) | 1.03 (0.15) | 1.11 (0.24) | |
| p-value | vs. baseline | 0.012 | 0.546 | 0.659 | 0.493 | ||
| vs. week 8 | 0.017 | 0.605 | |||||
| p-value vs. placebo | 0.493 | 0.532 | 0.894 | ||||
| RMS (mm) * | n | 23 | 23 | 23 | 20 | 18 | 20 |
| mean(SD) | 1.12 (0.22) | 1.03 (0.22) | 1.06 (0.21) | 1.1 (0.27) | 1.03 (0.15) | 1.06 (0.24) | |
| p-value | vs. baseline | 0.009 | 0.027 | 0.94 | 0.48 | ||
| vs. week 8 | 0.23 | 0.47 | |||||
| Patients with adverse events (N) | 19 |
| Number of SAEs (n) | 0 |
| Number of AEs (n) | 43 |
| Type of AEs, n(%) | |
| Nausea | 9 (20.9) |
| Constipation | 4 (9.3) |
| Mild itching | 2 (4.7) |
| Menstrual bleeding increase | 2 (4.7) |
| Oedema | 2 (4.7) |
| Dry mouth | 2 (4.7) |
| Bloating | 2 (4.7) |
| Skin tension increased | 2 (4.7) |
| Weight increase | 2 (4.7) |
| Vomiting | 2 (4.7) |
| Halitosis | 1 (2.3) |
| Dry skin | 1 (2.3) |
| Fatigue | 1 (2.3) |
| Heat | 1 (2.3) |
| Diarrhea | 1 (2.3) |
| Abdominal pain | 1 (2.3) |
| Thirst | 1 (2.3) |
| Mild tingling | 1 (2.3) |
| Headache | 1 (2.3) |
| Rash | 1 (2.3) |
| Drowsiness | 1 (2.3) |
| Acne | 1 (2.3) |
| Cracked nail | 1 (2.3) |
| State of repletion | 1 (2.3) |
| Total | 43 (100.0) |
| Relation to study drug, n(%) | |
| Certain | - |
| Probable/Likely | - |
| Possible | 32 (74.4) |
| Unlikely | 11 (25.6) |
| Severity | |
| Mild | 41 (95.3) |
| Moderate | 2 (4.7) |
| Severe | 0 (0.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demir-Dora, D.; Ozsoy, U.; Yildirim, Y.; Yilmaz, O.; Aytac, P.; Yilmaz, B.; Dogan Kurtoglu, E.; Akman, A.; Tezman, S.; Inaloz, H.S.; et al. The Efficacy and Safety of CollaSel Pro® Hydrolyzed Collagen Peptide Supplementation without Addons in Improving Skin Health in Adult Females: A Double Blind, Randomized, Placebo-Controlled Clinical Study Using Biophysical and Skin Imaging Techniques. J. Clin. Med. 2024, 13, 5370. https://doi.org/10.3390/jcm13185370
Demir-Dora D, Ozsoy U, Yildirim Y, Yilmaz O, Aytac P, Yilmaz B, Dogan Kurtoglu E, Akman A, Tezman S, Inaloz HS, et al. The Efficacy and Safety of CollaSel Pro® Hydrolyzed Collagen Peptide Supplementation without Addons in Improving Skin Health in Adult Females: A Double Blind, Randomized, Placebo-Controlled Clinical Study Using Biophysical and Skin Imaging Techniques. Journal of Clinical Medicine. 2024; 13(18):5370. https://doi.org/10.3390/jcm13185370
Chicago/Turabian StyleDemir-Dora, Devrim, Umut Ozsoy, Yilmaz Yildirim, Oguz Yilmaz, Peri Aytac, Beste Yilmaz, Emel Dogan Kurtoglu, Ayse Akman, Selim Tezman, Huseyin Serhat Inaloz, and et al. 2024. "The Efficacy and Safety of CollaSel Pro® Hydrolyzed Collagen Peptide Supplementation without Addons in Improving Skin Health in Adult Females: A Double Blind, Randomized, Placebo-Controlled Clinical Study Using Biophysical and Skin Imaging Techniques" Journal of Clinical Medicine 13, no. 18: 5370. https://doi.org/10.3390/jcm13185370
APA StyleDemir-Dora, D., Ozsoy, U., Yildirim, Y., Yilmaz, O., Aytac, P., Yilmaz, B., Dogan Kurtoglu, E., Akman, A., Tezman, S., Inaloz, H. S., & Erenmemisoglu, A. (2024). The Efficacy and Safety of CollaSel Pro® Hydrolyzed Collagen Peptide Supplementation without Addons in Improving Skin Health in Adult Females: A Double Blind, Randomized, Placebo-Controlled Clinical Study Using Biophysical and Skin Imaging Techniques. Journal of Clinical Medicine, 13(18), 5370. https://doi.org/10.3390/jcm13185370





