Investigation of Glucose Metabolism by Continuous Glucose Monitoring and Validation of Dipeptidyl Peptidase 4 Inhibitor Use in Patients with Myotonic Dystrophy Type 1
Abstract
1. Introduction
2. Methods
2.1. Subjects
2.2. Background Information
2.3. Continuous Glucose Level Measurements by CGM
2.4. OGTT and Incretin Secretion Analysis
2.5. Investigation of the Effects of DPP-4 Inhibitor
2.6. Statistical Analysis
3. Results
3.1. Sensor Glucose Profiles Recorded by CGM and Its Parameters in DM1 Patients
3.2. Correlation of CGM Parameters
3.3. Incretin Secretion in DM1 Patients
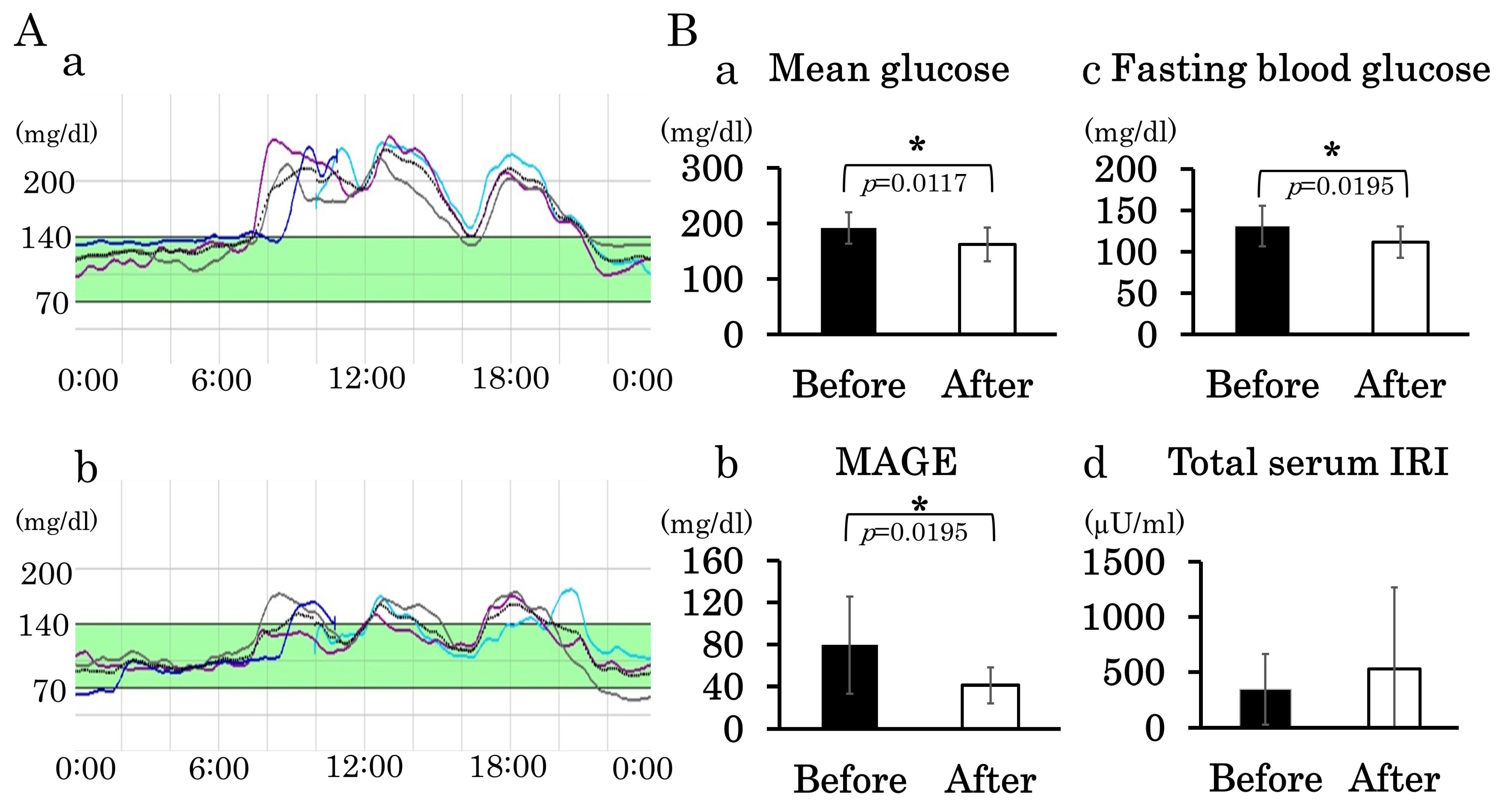
3.4. Administration of DPP-4 Inhibitor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Harper, P. Myotonic Dystrophy; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Sznajder, L.J.; Swanson, M.S. Short tandem repeat expansions and RNA-mediated pathogenesis in myotonic dystrophy. Int. J. Mol. Sci. 2019, 20, 3365. [Google Scholar] [CrossRef] [PubMed]
- Udd, B.; Krahe, R. The myotonic dystrophies: Molecular, clinical, and therapeutic challenges. Lancet Neurol. 2012, 11, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.C.; Turtle, J.R.; Miller, S.; Mcleod, J.G. Abnormalities of insulin secretion in dystrophia myotonica. Brain 1970, 93, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuis, S.; Okkersen, K.; Widomska, J.; Blom, P.; t Hoen, P.A.C.; van Engelen, B.; Glennon, J.C. Insulin signaling as a key moderator in myotonic dystrophy type 1. Front. Neurol. 2019, 10, 1229. [Google Scholar] [CrossRef]
- Matsumura, T.; Iwahashi, H.; Funahashi, T.; Takahashi, M.P.; Saito, T.; Yasui, K.; Saito, T.; Iyama, A.; Toyooka, K.; Fujimura, H.; et al. A cross-sectional study for glucose intolerance of myotonic dystrophy. J. Neurol. Sci. 2009, 276, 60–65. [Google Scholar] [CrossRef]
- Takada, H.; Goto, T.; Kon, S.; Oyama, Y. Day profile of plasma glucose in diabetic-complicated myotonic dystrophy type 1. Neuromuscul. Disord. 2013, 23, 834. [Google Scholar] [CrossRef]
- Takada, H.; Oyama, Y.; Kon, S.; Goto, T. Glucose intolerance in myotonic dystrophy type 1. Rinsho Shinkeigaku 2012, 52, 1259–1260. [Google Scholar] [CrossRef]
- Abe, H.; Mita, T.; Kudo, K.; Funayama, T.; Tokoro, M.; Kaga, H.; Ikeda, F.; Kanazawa, A.; Hirose, T.; Kawamori, R.; et al. Dramatic improvement of blood glucose control after pioglitazone treatment in poorly controlled over-weight diabetic patients with myotonic dystrophy. Endocr. J. 2009, 56, 911–913. [Google Scholar] [CrossRef]
- Yamauchi, K.; Matsumura, T.; Takada, H.; Kuru, S.; Kobayashi, M.; Kubota, T.; Kimura, E.; Nakamura, H.; Takahashi, M.P. The current status of medical care for myotonic dystrophy type 1 in the national registry of Japan. Muscle Nerve 2023, 67, 387–393. [Google Scholar] [CrossRef]
- Qu, Y.M.; Jacober, S.J.; Zhang, Q.Y.; Wolka, L.L.; DeVries, J.H. Rate of hypoglycemia in insulin-treated patients with type 2 diabetes can be predicted from glycemic variability data. Diabetes Technol. Ther. 2012, 14, 1008–1012. [Google Scholar] [CrossRef]
- Sartore, G.; Chilelli, N.C.; Burlina, S.; Di Stefano, P.; Piarulli, F.; Fedele, D.; Mosca, A.; Lapolla, A. The importance of HbA1c and glucose variability in patients with type 1 and type 2 diabetes: Outcome of continuous glucose monitoring (CGM). Acta Diabetol. 2012, 49, S153–S160. [Google Scholar] [CrossRef] [PubMed]
- Kusunoki, Y.; Katsuno, T.; Nakae, R.; Watanabe, K.; Akagami, T.; Ochi, F.; Tokuda, M.; Murai, K.; Miuchi, M.; Miyagawa, J.-I.; et al. Evaluation of blood glucose fluctuation in Japanese patients with type 1 diabetes mellitus by self-monitoring of blood glucose and continuous glucose monitoring. Diabetes Res. Clin. Pract. 2015, 108, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef]
- Buschur, E.O.; Faulds, E.; Dungan, K. CGM in the hospital: Is it ready for prime time? Curr. Diabetes Rep. 2022, 22, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Kouki, T.; Takasu, N.; Nakachi, A.; Tamanaha, T.; Komiya, I.; Tawata, M. Low-dose metformin improves hyperglycaemia related to myotonic dystrophy. Diabet. Med. 2005, 22, 346–347. [Google Scholar] [CrossRef]
- Laustriat, D.; Gide, J.; Barrault, L.; Chautard, E.; Benoit, C.; Auboeuf, D.; Boland, A.; Battail, C.; Artiguenave, F.; Deleuze, J.-F.; et al. In vitro and in vivo modulation of alternative splicing by the biguanide metformin. Mol. Ther.-Nucleic Acids 2015, 4, e262. [Google Scholar] [CrossRef]
- Bassez, G.; Audureau, E.; Hogrel, J.Y.; Arrouasse, R.; Baghdoyan, S.; Bhugaloo, H.; Gourlay-Chu, M.-L.; Le Corvoisier, P.; Peschanski, M. Improved mobility with metformin in patients with myotonic dystrophy type 1: A randomized controlled trial. Brain 2018, 141, 2855–2865. [Google Scholar] [CrossRef]
- Karagiannis, T.; Boura, P.; Tsapas, A. Safety of dipeptidyl peptidase 4 inhibitors: A perspective review. Ther. Adv. Drug Saf. 2014, 5, 138–146. [Google Scholar] [CrossRef]
- Bergman, M.; Abdul-Ghani, M.; DeFronzo, R.A.; Manco, M.; Sesti, G.; Fiorentino, T.V.; Ceriello, A.; Rhee, M.; Phillips, L.S.; Chung, S.; et al. Review of methods for detecting glycemic disorders. Diabetes Res. Clin. Pract. 2020, 165, 108233. [Google Scholar] [CrossRef]
- Mazze, R.S.; Strock, E.; Wesley, D.; Borgman, S.; Morgan, B.; Bergenstal, R.; Cuddihy, R. Characterizing glucose exposure for individuals with normal glucose tolerance using continuous glucose monitoring and ambulatory glucose profile analysis. Diabetes Technol. Ther. 2008, 10, 149–159. [Google Scholar] [CrossRef]
- Huff, T.A.; Horton, E.S.; Lebovitz, H.E. Abnormal insulin secretion in myotonic dystrophy. N. Engl. J. Med. 1967, 277, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Moxley, R.T.; Griggs, R.C.; Goldblatt, D. Decreased insulin sensitivity of forearm muscle in myotonic dystrophy. J. Clin. Investig. 1978, 62, 857–867. [Google Scholar] [CrossRef]
- Festoff, B.W.; Moore, W.V. Evaluation of insulin receptor in myotonic dystrophy. Ann. Neurol. 1979, 6, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Seaquist, E.R.; Anderson, J.; Childs, B.; Cryer, P.; Dagogo-Jack, S.; Fish, L.; Heller, S.R.; Rodriguez, H.; Rosenzweig, J.; Vigersky, R. Hypoglycemia and diabetes: A report of a workgroup of the American Diabetes Association and The Endocrine Society. Diabetes Care 2013, 36, 1384–1395. [Google Scholar] [CrossRef]
- Gallwitz, B. Clinical Use of DPP-4 Inhibitors. Front. Endocrinol. 2019, 10, 389. [Google Scholar] [CrossRef]
- Saini, K.; Sharma, S.; Khan, Y. DPP-4 inhibitors for treating T2DM-hype or hope? an analysis based on the current literature. Front. Mol. Biosci. 2023, 10, 1130625. [Google Scholar] [CrossRef]
- Xing, D.Y.; Kollman, C.; Beck, R.W.; Tamborlane, W.V.; Laffel, L.; Buckingham, B.A.; Wilson, D.M.; Weinzimer, S.; Fiallo-Scharer, R.; Ruedy, K.J.; et al. Optimal sampling intervals to assess long-term glycemic control using continuous glucose monitoring. Diabetes Technol. Ther. 2011, 13, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Riddlesworth, T.D.; Beck, R.W.; Gal, R.L.; Connor, C.G.; Bergenstal, R.M.; Lee, S.; Willi, S.M. Optimal sampling duration for continuous glucose monitoring to determine long-term glycemic control. Diabetes Technol. Ther. 2018, 20, 314–316. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef]



| DM1 | Control | ||||
|---|---|---|---|---|---|
| Total | NGT | IGT | DM | ||
| Age (years) | 48.9 ± 13.7 | 54.0 ± 13.3 | 50.4 ± 13.3 | 40.7 ± 13.8 | 65.4 ± 5.8 |
| Sex (men/women) | 20/9 | 4/3 | 12/3 | 4/3 | 3/2 |
| The number of CTG repeat | 1228 ± 660 | 1061 ± 618 | 1197 ± 455 | 1500 ± 993 | |
| Body mass index (kg/m2) | 22.1 ± 4.5 | 18.2 ± 2.7 | 23.1 ± 4.5 | 23.8 ± 3.4 | 18.8 ± 1.8 |
| HbA1c (%) | 5.8 ± 0.8 | 5.5 ± 0.3 | 5.5 ± 0.4 | 6.8 ± 1.0 | 5.5 ± 0.5 |
| Fasting blood glucose (mg/dL) | 94.4 ± 23.8 | 82.9 ± 8.5 | 88.2 ± 12.8 | 119 ± 33.0 | 113 ± 38.9 |
| Cystatin C (mg/L) | 1.04 ± 0.25 | 1.08 ± 0.33 | 1.04 ± 0.25 | 1.01 ± 0.20 | 0.98 ± 0.21 |
| Low-density lipoprotein cholesterol (mg/dL) | 110 ± 35.5 | 96.7 ± 21.7 | 116 ± 42.8 | 113 ± 23.7 | 84.0 ± 36.4 |
| High-density lipoprotein cholesterol (mg/dL) | 45.3 ± 9.7 | 43.9 ± 7.2 | 45.6 ± 11.0 | 46.1 ± 8.5 | 48.0 ± 19.6 |
| Triglyceride (mg/dL) | 166 ± 124 | 101 ± 27.2 | 151 ± 113 | 263 ± 166 | 216.8 ± 124.5 |
| HbA1c (%) | FBS (mg/dL) | 120BS (mg/dL) | CGM | ||||
|---|---|---|---|---|---|---|---|
| Lowest (mg/dL) | % < 70 mg/dL | % < 60 mg/dL | |||||
| NGT | 5.3 | 77 | 91 | 55 | 9.44 | 2.62 | After breakfast |
| (6/7) | 6.0 | 100 | 103 | 66 | 2.43 | 0.00 | Midnight |
| 5.6 | 82 | 119 | 57 | 1.04 | 0.52 | Before dinner | |
| 5.3 | 79 | 92 | 68 | 1.04 | 0.00 | Midnight | |
| 5.6 | 79 | 91 | 68 | 1.04 | 0.00 | After breakfast | |
| 5.6 | 73 | 53 | 65 | 9.01 | 0.00 | After dinner | |
| IGT | 5.1 | 78 | 192 | 68 | 3.29 | 0.00 | After dinner |
| (4/15) | 5.3 | 75 | 175 | 40 | 11.09 | 9.19 | Midnight |
| 5.7 | 82 | 163 | 59 | 8.15 | 0.52 | Midnight | |
| 5.8 | 89 | 129 | 62 | 2.95 | 0.00 | After dinner |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takada, H.; Matsumura, T.; Shimamura, H.; Matsui, M.; Kon, S.; Fukumoto, A.; Kubota, T.; Yoshida, K.; Iwahashi, H.; Takahashi, M.P. Investigation of Glucose Metabolism by Continuous Glucose Monitoring and Validation of Dipeptidyl Peptidase 4 Inhibitor Use in Patients with Myotonic Dystrophy Type 1. J. Clin. Med. 2024, 13, 5252. https://doi.org/10.3390/jcm13175252
Takada H, Matsumura T, Shimamura H, Matsui M, Kon S, Fukumoto A, Kubota T, Yoshida K, Iwahashi H, Takahashi MP. Investigation of Glucose Metabolism by Continuous Glucose Monitoring and Validation of Dipeptidyl Peptidase 4 Inhibitor Use in Patients with Myotonic Dystrophy Type 1. Journal of Clinical Medicine. 2024; 13(17):5252. https://doi.org/10.3390/jcm13175252
Chicago/Turabian StyleTakada, Hiroto, Tsuyoshi Matsumura, Haruna Shimamura, Misa Matsui, Seiko Kon, Aono Fukumoto, Tomoya Kubota, Kosuke Yoshida, Hiromi Iwahashi, and Masanori P. Takahashi. 2024. "Investigation of Glucose Metabolism by Continuous Glucose Monitoring and Validation of Dipeptidyl Peptidase 4 Inhibitor Use in Patients with Myotonic Dystrophy Type 1" Journal of Clinical Medicine 13, no. 17: 5252. https://doi.org/10.3390/jcm13175252
APA StyleTakada, H., Matsumura, T., Shimamura, H., Matsui, M., Kon, S., Fukumoto, A., Kubota, T., Yoshida, K., Iwahashi, H., & Takahashi, M. P. (2024). Investigation of Glucose Metabolism by Continuous Glucose Monitoring and Validation of Dipeptidyl Peptidase 4 Inhibitor Use in Patients with Myotonic Dystrophy Type 1. Journal of Clinical Medicine, 13(17), 5252. https://doi.org/10.3390/jcm13175252





