Added Value of Histological Evaluation of Muscle Biopsies in Porcine Vascularized Composite Allografts
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Measurements
2.3. Specimen Procurement
2.4. Histological Analysis
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Clinical Data
3.3. Histopathology
3.4. Blood Gas Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Rahmel, A. Vascularized Composite Allografts: Procurement, Allocation, and Implementation. Curr. Transplant. Rep. 2014, 1, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Iske, J.; Nian, Y.; Maenosono, R.; Maurer, M.; Sauer, I.M.; Tullius, S.G. Composite tissue allotransplantation: Opportunities and challenges. Cell Mol. Immunol. 2019, 16, 343–349. [Google Scholar] [CrossRef]
- Charles, A.L.; Guilbert, A.S.; Guillot, M.; Talha, S.; Lejay, A.; Meyer, A.; Kindo, M.; Wolff, V.; Bouitbir, J.; Zoll, J.; et al. Muscles Susceptibility to Ischemia-Reperfusion Injuries Depends on Fiber Type Specific Antioxidant Level. Front. Physiol. 2017, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Blaisdell, F.W. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: A review. Cardiovasc. Surg. 2002, 10, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Kruit, A.S.; Brouwers, K.; van Midden, D.; Zegers, H.; Koers, E.; van Alfen, N.; Hummelink, S.; Ulrich, D.J.O. Successful 18-h acellular extracorporeal perfusion and replantation of porcine limbs—Histology versus nerve stimulation. Transpl. Int. 2021, 34, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Krezdorn, N.; Macleod, F.; Tasigiorgos, S.; Turk, M.D.M.; Wo, L.; Kiwanuka, B.A.H.; Lopdrup, B.I.D.R.; Kollar, B.; Edelman, E.R.; Pomahac, B. Twenty-Four-Hour Ex Vivo Perfusion with Acellular Solution Enables Successful Replantation of Porcine Forelimbs. Plast. Reconstr. Surg. 2019, 144, 608e–618e. [Google Scholar] [CrossRef]
- Ozer, K.; Rojas-Pena, A.; Mendias, C.L.; Bryner, B.S.; Toomasian, C.; Bartlett, R.H. The Effect of Ex Situ Perfusion in a Swine Limb Vascularized Composite Tissue Allograft on Survival up to 24 Hours. J. Hand Surg. Am. 2016, 41, 3–12. [Google Scholar] [CrossRef]
- Kueckelhaus, M.; Dermietzel, A.; Alhefzi, M.; Aycart, M.A.; Fischer, S.; Krezdorn, N.; Wo, L.; Maarouf, O.H.; Riella, L.V.; Abdi, R.; et al. Acellular Hypothermic Extracorporeal Perfusion Extends Allowable Ischemia Time in a Porcine Whole Limb Replantation Model. Plast. Reconstr. Surg. 2017, 139, 922e–932e. [Google Scholar] [CrossRef]
- Constantinescu, M.A.; Knall, E.; Xu, X.; Kiermeir, D.M.; Jenni, H.; Gygax, E.; Rieben, R.; Banič, A.; Vögelin, E. Preservation of amputated extremities by extracorporeal blood perfusion; a feasibility study in a porcine model. J. Surg. Res. 2011, 171, 291–299. [Google Scholar] [CrossRef]
- Muller, S.; Constantinescu, M.A.; Kiermeir, D.M.; Gajanayake, T.; Bongoni, A.K.; Vollbach, F.H.; Meoli, M.; Plock, J.; Jenni, H.; Banic, A.; et al. Ischemia/reperfusion injury of porcine limbs after extracorporeal perfusion. J. Surg. Res. 2013, 181, 170–182. [Google Scholar] [CrossRef]
- Werner, N.L.; Alghanem, F.; Rakestraw, S.L.; Sarver, D.C.; Nicely, B.; Pietroski, R.E.; Lange, P.; Rudich, S.M.; Mendias, C.L.; Rojas-Pena, A.; et al. Ex Situ Perfusion of Human Limb Allografts for 24 Hours. Transplantation 2017, 101, e68–e74. [Google Scholar] [CrossRef]
- Rezaei, M.; Ordenana, C.; Figueroa, B.A.; Said, S.A.; Fahradyan, V.; Dalla Pozza, E.; Orfahli, L.M.; Annunziata, M.J.; Rohde, E.; Madajka, M.; et al. Ex Vivo Normothermic Perfusion of Human Upper Limbs. Transplantation 2022, 106, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, J.M.; Morgan, R.D.; Knight, S.R.; Morris, P.J. Systematic review and meta-analysis of hypothermic machine perfusion versus static cold storage of kidney allografts on transplant outcomes. Br. J. Surg. 2013, 100, 991–1001. [Google Scholar] [CrossRef]
- Titford, M. The long history of hematoxylin. Biotech. Histochem. 2005, 80, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Wittekind, D. Traditional staining for routine diagnostic pathology including the role of tannic acid. 1. Value and limitations of the hematoxylin-eosin stain. Biotech. Histochem. 2003, 78, 261–270. [Google Scholar] [CrossRef]
- Nowak, L.; Reyes, P.F. Muscle Biopsy: A Diagnostic Tool in Muscle Diseases. J. Histotechnol. 2008, 31, 101–108. [Google Scholar] [CrossRef]
- Lehninger, A.; Nelson, D.; Cox, M. Principles of Biochemistry; Worth Publishers: New York, NY, USA, 1973. [Google Scholar]
- Carmo-Araújo, E.M.; Dal-Pai-Silva, M.; Dal-Pai, V.; Cecchini, R.; Anjos Ferreira, A.L. Ischaemia and reperfusion effects on skeletal muscle tissue: Morphological and histochemical studies. Int. J. Exp. Pathol. 2007, 88, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Rus, H.G.; Niculescu, F.I.; Shin, M.L. Role of the C5b-9 complement complex in cell cycle and apoptosis. Immunol. Rev. 2001, 180, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, K.; Thijssen, M.F.; Kruit, A.S.; van Midden, D.; Koers, E.J.; Zegers, H.J.H.; Hummelink, S.; Ulrich, D.J.O. 24-hour Perfusion of Porcine Myocutaneous Flaps Mitigates Reperfusion Injury: A 7-day Follow-up Study. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4123. [Google Scholar] [CrossRef]
- Pasut, G.; Panisello, A.; Folch-Puy, E.; Lopez, A.; Castro-Benitez, C.; Calvo, M.; Carbonell, T.; Garcia-Gil, A.; Adam, R.; Rosello-Catafau, J. Polyethylene glycols: An effective strategy for limiting liver ischemia reperfusion injury. World J. Gastroenterol. 2016, 22, 6501–6508. [Google Scholar] [CrossRef]
- Prem, J.T.; Eppinger, M.; Lemmon, G.; Miller, S.; Nolan, D.; Peoples, J. The role of glutamine in skeletal muscle ischemia/reperfusion injury in the rat hind limb model. Am. J. Surg. 1999, 178, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Durante, W. The Emerging Role of l-Glutamine in Cardiovascular Health and Disease. Nutrients 2019, 11, 2092. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, K.; Kruit, A.S.; van Midden, D.; Zegers, H.J.H.; Doorduin, J.; Koers, E.; Hummelink, S.; Ulrich, D.J.O. 24 Hours Ex-vivo Hypothermic Acellular Perfusion of Porcine Forelimb: A 7-day Follow-up Study. Plast. Reconstr. Surg. 2024. [Google Scholar] [CrossRef] [PubMed]
- Michel, S.G.; La Muraglia, G.M., 2nd; Madariaga, M.L.; Titus, J.S.; Selig, M.K.; Farkash, E.A.; Allan, J.S.; Anderson, L.M.; Madsen, J.C. Twelve-Hour Hypothermic Machine Perfusion for Donor Heart Preservation Leads to Improved Ultrastructural Characteristics Compared to Conventional Cold Storage. Ann. Transplant. 2015, 20, 461–468. [Google Scholar] [CrossRef]
- Turóczi, Z.; Arányi, P.; Lukáts, Á.; Garbaisz, D.; Lotz, G.; Harsányi, L.; Szijártó, A. Muscle fiber viability, a novel method for the fast detection of ischemic muscle injury in rats. PLoS ONE 2014, 9, e84783. [Google Scholar] [CrossRef]
- Garbaisz, D.; Turoczi, Z.; Aranyi, P.; Fulop, A.; Rosero, O.; Hermesz, E.; Ferencz, A.; Lotz, G.; Harsanyi, L.; Szijarto, A. Attenuation of skeletal muscle and renal injury to the lower limb following ischemia-reperfusion using mPTP inhibitor NIM-811. PLoS ONE 2014, 9, e101067. [Google Scholar] [CrossRef]
- Homer-Vanniasinkam, S.; Rowlands, T.E.; Hardy, S.C.; Gough, M.J. Skeletal muscle ischaemia-reperfusion injury: Further characterisation of a rodent model. Eur. J. Vasc. Endovasc. Surg. 2001, 22, 523–527. [Google Scholar] [CrossRef][Green Version]
- Girela-López, E.; Beltran-Aroca, C.M.; Jimena, I.; Pérez-Jorge, P.; Ramos-Medina, V.; Ruz-Caracuel, I.; Gill, J.R.; Peña-Amaro, J. Structural abnormalities in the human diaphragm in drowning and hanging deaths: Preliminary results. Forensic Sci. Med. Pathol. 2020, 16, 265–271. [Google Scholar] [CrossRef]
- Cong, L.; Pu, C.Q.; Shi, Q.; Wang, Q.; Lu, X.H. Complement membrane attack complex is related with immune-mediated necrotizing myopathy. Int. J. Clin. Exp. Pathol. 2014, 7, 4143–4149. [Google Scholar]
- Fechner, G.; Bajanowski, T.; Brinkmann, B. Immunohistochemical alterations after muscle trauma. Int. J. Legal Med. 1993, 105, 203–207. [Google Scholar] [CrossRef]
- Brinkmann, B.; Sepulchre, M.A.; Fechner, G. The application of selected histochemical and immunohistochemical markers and procedures to the diagnosis of early myocardial damage. Int. J. Legal Med. 1993, 106, 135–141. [Google Scholar] [CrossRef]
- Thomsen, H.; Held, H. Susceptibility of C5b-9(m) to postmortem changes. Int. J. Legal Med. 1994, 106, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Ortmann, C.; Pfeiffer, H.; Brinkmann, B. A comparative study on the immunohistochemical detection of early myocardial damage. Int. J. Legal Med. 2000, 113, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Sabatasso, S.; Mangin, P.; Fracasso, T.; Moretti, M.; Docquier, M.; Djonov, V. Early markers for myocardial ischemia and sudden cardiac death. Int. J. Legal Med. 2016, 130, 1265–1280. [Google Scholar] [CrossRef] [PubMed]







| Interstitial edema |
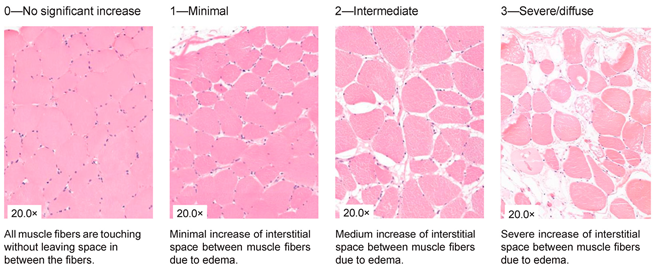 |
| Inflammation |
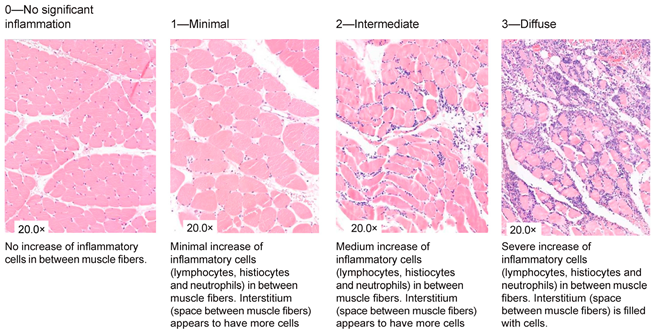 |
| Variation in shape and size |
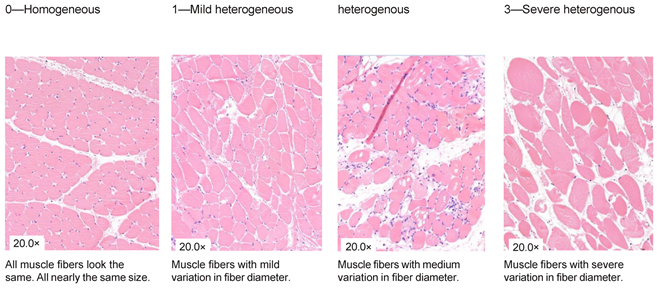 |
| Damaged muscle fibers |
 |
| Unclassifiable | 0—Normal NADH Staining (<5 Abnormal Fibers) | 1—Minimal Alterations (5–14 Abnormal Fibers) | 2—Intermediate Alterations (15–49 Abnormal Fibers) | 3—Severe/Diffuse Alterations (>50 Abnormal Fibers) |
|---|---|---|---|---|
 | ||||
| Negative | Positive |
|---|---|
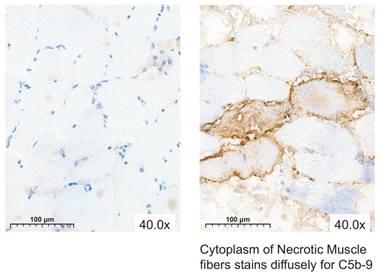 | |
| SCS (n = 8) | MP (n = 8) | p-Value | |
|---|---|---|---|
| Characteristics | Mean (SD) | Mean (SD) | |
| Harvest (min) | 92 (17) | 87 (14) | 0.636 |
| Ex vivo storage time (h) | 4 (0) | 24 (0) | <0.001 |
| WIT before storage (min) | 2.6 (1.1) | 12.9 (7.4) | 0.005 |
| WIT before reperfusion (min) | 52 (27) | 58 (16) | 0.172 |
| Limb weight before intervention (g) | 2580 (285) | 2554 (395) | 0.916 |
| Limb weight after intervention (g) | 2542 (274) | 3676 (312) | <0.001 |
| Weight difference (%) | −2% | 44% | <0.001 |
| Temperature before intervention (°C) | 35.4 (1.0) | 34.6 (1.1) | 0.171 |
| Temperature after intervention (°C) | 6.5 (1.5) | 12.0 (1.4) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brouwers, K.; van Geel, S.R.W.M.; van Midden, D.; Kruit, A.S.; Kusters, B.; Hummelink, S.; Ulrich, D.J.O. Added Value of Histological Evaluation of Muscle Biopsies in Porcine Vascularized Composite Allografts. J. Clin. Med. 2024, 13, 5167. https://doi.org/10.3390/jcm13175167
Brouwers K, van Geel SRWM, van Midden D, Kruit AS, Kusters B, Hummelink S, Ulrich DJO. Added Value of Histological Evaluation of Muscle Biopsies in Porcine Vascularized Composite Allografts. Journal of Clinical Medicine. 2024; 13(17):5167. https://doi.org/10.3390/jcm13175167
Chicago/Turabian StyleBrouwers, Kaj, Shannen R. W. M. van Geel, Dominique van Midden, Anne Sophie Kruit, Benno Kusters, Stefan Hummelink, and Dietmar J. O. Ulrich. 2024. "Added Value of Histological Evaluation of Muscle Biopsies in Porcine Vascularized Composite Allografts" Journal of Clinical Medicine 13, no. 17: 5167. https://doi.org/10.3390/jcm13175167
APA StyleBrouwers, K., van Geel, S. R. W. M., van Midden, D., Kruit, A. S., Kusters, B., Hummelink, S., & Ulrich, D. J. O. (2024). Added Value of Histological Evaluation of Muscle Biopsies in Porcine Vascularized Composite Allografts. Journal of Clinical Medicine, 13(17), 5167. https://doi.org/10.3390/jcm13175167








